What is unique about Candida auris?
The emerging fungal pathogen Candida auris is causing outbreaks of invasive disease in healthcare facilities around the world [1–4]. Isolates often exhibit resistance to multiple drug classes, and invasive disease carries an astonishingly high mortality rate, approaching 60% [5]. The initial description of C. auris arose following the isolation of a novel species from the ear canal of a patient in a Japanese hospital [2]. Since this report in 2009, cases have subsequently appeared in hospitals in South Korea, India, the Middle East, South Africa, and South America [6–10]. More recent reports reveal the spread of C. auris to North American and European healthcare facilities [3, 11]. Genomic analysis shows that the circulating strains cluster into distinct clades, which appear to have emerged independently [5]. Retrospective analyses of Candida isolates collected from international sites in the decades leading to 2009 uncovered only rare cases of C. auris, confirming the recent emergence of this species [5, 10].
C. auris is the first fungal pathogen categorized as a public health threat due to its ability to readily colonize skin, spread rapidly among patients, and cause severe disease [3–5, 12]. The efficient person-to-person transmission observed for C. auris is striking, because candidiasis caused by other species typically arises from the patient’s own microbiome, often from the gastrointestinal tract. However, unlike other Candida spp., C. auris does not appear to efficiently colonize the gastrointestinal tract, presumably due to its poor growth under anaerobic conditions [13]. An additional factor contributing to the spread of C. auris is the propensity of the species to persist on the surfaces of hospital rooms and on medical devices [14, 15]. With these barriers to nosocomial control, C. auris continues to spread. In an area where it first emerged, C. auris now accounts for nearly 20% of Candida bloodstream isolates, surpassing that of C. albicans, which is typically the most common species [16].
Who develops C. auris infection?
C. auris can cause invasive disease involving a variety of clinical niches [3, 5]. The most frequently reported site is the bloodstream, with isolation from the urinary or respiratory tract close behind [5]. Despite the high frequency of most Candida species to cause oral or esophageal disease, C. auris is not frequently reported at these sites. A study by Pathirana and colleagues shed light on one reason for this apparent void. They examined activity of a salivary cationic peptide, histatin 5, against C. auris and found the vast majority of isolates to be highly sensitive, particularly those exhibiting antifungal resistance [17]. Another intriguing observation is that a common risk factor for invasive candidiasis, neutropenia, has not been reported for C. auris infection, because this is a common risk for other Candida spp. [5, 6]. Invasive disease in the absence of neutropenia suggests that the host neutrophil response may not be adequate for control of C. auris.
Pursuits to uncover the traits of patients with invasive C. auris disease have revealed a common theme. These patients have often undergone multiple medical interventions, including surgical procedures, mechanical ventilation, vascular catheterization, and gastrostomy tube placement [5, 18]. The widespread use of artificial devices evokes the question of surface-associated biofilms. Although isolates of C. auris can form biofilms in vitro, they appear to grow to a biomass less than that of C. albicans [19, 20]. However, similar to biofilms formed by other Candida spp., C. auris biofilms exhibit drug resistance beyond that observed during planktonic growth [19]. How biofilm formation by C. auris may influence immunity is unknown. However, biofilms formed by other Candida species resist phagocytic killing [21–24].
How do animal models mimic C. auris infection in patients?
Several groups have explored the pathogenicity of C. auris, employing a variety of model systems [11, 19, 25–28]. Initial reports utilized an invertebrate moth larvae (Galleria mellonella) model of invasive candidiasis [11, 19]. These studies examined C. auris strains collected in the United Kingdom and found them to be considerably more virulent than other nonfilamentous Candida isolates. A subset of C. auris exhibited pathogenicity similar to even the most virulent species, C. albicans and Candida tropicalis [11]. Of note, the more virulent C. auris isolates grew in nonaggregative forms, in contrast to the less pathogenic strains that formed large aggregates. As these nonaggregative forms also tended toward more robust biofilm formation, it is intriguing to speculate clinical relevance for this phenotype [19].
Investigation of C. auris in murine models of invasive candidiasis have included animals with both compromised and intact immunity [25–27]. Using BALB/c mice with cyclophosamide-induced neutropenia, Ben-Ami and colleagues examined the virulence of a C. auris strain collected in Israel [25]. This strain exhibited virulence beyond that of the closely related C. haemulonii, which was nonvirulent. However, C. auris was significantly less virulent than C. albicans, in which animals succumbed more rapidly (1 versus 4 days), and 10-fold higher fungal burdens were noted in the kidney [25]. Examination of the kidneys of mice that had succumbed to C. auris infection revealed the formation of aggregates, which the authors propose may serve as a mode of immune evasion [25]. If and how these renal aggregates relate to the aggregative forms found in vitro remains unclear [11, 19]. In contrast, a second investigation examining 2 C. auris isolates from India in an immunocompetent IRC murine model of disseminated candidiasis revealed survival similar to C. albicans [27]. In a third investigation using BALB/c mice, Wang and colleagues compared the virulence of an isolate of C. auris from China to C. albicans and found C. albicans to be more virulent [26]. In this invasive candidiasis model, mice infected with C. albicans died by day 6 whereas the C. auris-infected animals survived to 14 days.
One potential factor that may account for the different observations among these studies is genetic variability among the strains, which had been collected from diverse geographic locations. Another contributing factor includes the differences in immunosuppression among the model systems. Taken together, it appears that a subset of C. auris exhibits a high level of virulence. Typically, the virulence of Candida in noncompromised infection models is blunted. The unexpected fitness of C. auris in several immunocompetent models suggests that this species may withstand host immune responses that are typically sufficient to prevent invasive disease caused by other Candida spp.
Is the immune response to C. auris effective?
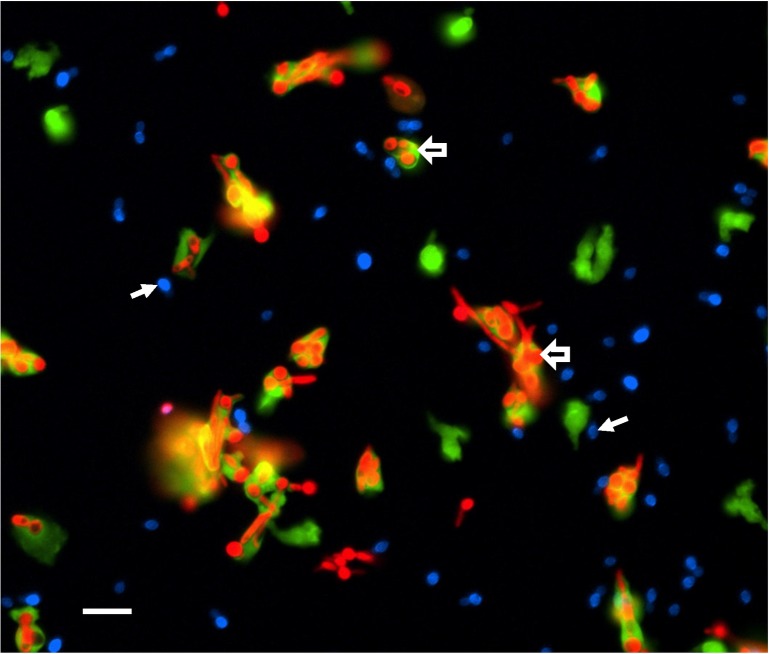
A recent report questioned the efficacy of the phagocytic response against a C. auris strain collected from India [28]. Neutrophils, key leukocytes for the control of invasive candidiasis, kill fungi through phagocytosis and the delivery of antimicrobial contents during neutrophil extracellular trap (NET) formation [29, 30]. However, human neutrophils were found to lack effective activity against C. auris. They failed to engage C. auris, phagocytose the yeast, or release NETs. When presented with both C. albicans and C. auris, human neutrophil exhibited a strong preference for engaging and killing C. albicans (Fig 1) [28]. The phagocytes functioned normally against C. albicans but appeared to disregard C. auris. In vivo analysis of neutrophils in a zebrafish model of invasive candidiasis mimicked the phenotype.
Fig 1. Human neutrophils fail to engage Candida auris.
Calcein AM-labeled human neutrophils (green) were cocultured with red fluorescent protein-tagged C. albicans (red) and calcofluor white-stained C. auris (blue) for 30 min. Neutrophils preferentially engaged C. albicans, ignoring C. auris. Open arrows point to neutrophils phagocytosing C. albicans. Closed arrows show C. auris cells. Few neutrophils engage C. auris in the presence or absence of C. albicans. Measurement bar represents 10 μm.
This impaired innate immune response may help to explain why mortality rates remain high even for patients treated with appropriate antifungals [5]. However, little is known regarding the mechanism underlying this phenomenon or how the genetic diversity of C. auris may influence evasion of neutrophil engulfment. It is intriguing that a similar pattern of phagocyte evasion has been observed for C. lusitaniae, a species phylogenetically close to C. auris [31]. Ex vivo studies employing a macrophage cell line found that C. lusitaniae avoids phagocytosis, in contrast to C. albicans and C. glabrata, which are more readily engulfed [32]. The similarity of the phagocytic responses to C. auris and C. lusitaniae prompts the question of an altered fungal component that is shared for the two species but divergent from more distantly related Candida species. Investigations are also exploring the recognition of C. auris by mononuclear cells. Peripheral blood mononuclear cells appear to elicit unique cytokine responses upon encounter with C. auris and C. albicans, suggesting exposure to different fungal components [33].
Why are future investigations of C. auris needed?
Unlike other Candida species, C. auris has emerged as a nosocomial threat, exhibiting rapid person-to-person transmission and causing critical invasive disease. Recent investigations are just beginning to shed light on the mechanisms of pathogenicity for this deadly infection. In several models of invasive disease, strains of C. auris display virulence similar to that of the most virulent Candida species. The observation that neutrophils exhibit reduced activity against C. auris may contribute to poor outcomes for patients with invasive disease. However, the high mortality for invasive candidiasis likely also reflects the comorbidities and prolonged hospitalizations for this cohort. One promising finding is the efficacy of the C. albicans NDV-3A vaccine for protection against C. auris in mice [34]. Prevention would be ideal for the vulnerable population of hospitalized patients.
In light of the considerable diversity among the circulating C. auris strains, investigations to understand the influence of genetic diversity on immunity will be valuable. Furthermore, recent studies have revealed a filamentous form of C. auris, which appears to be triggered by a heritable phenotypic switch generated in vivo [35]. With inducing conditions and filamentation regulators distinct from C. albicans, the impact of this morphological transition on immune cell recognition is a mystery. As the tools for genetic manipulation of C. auris are becoming available, it will be fascinating to see how these genetic and phenotypic variations influence virulence and immunity [36].
Funding Statement
The author is supported by the National Institutes of Health (K08 AI108727), the Burroughs Wellcome Fund (1012299) and the Doris Duke Charitable Foundation (112580130). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rhodes J, Abdolrasouli A, Farrer RA, Cuomo CA, Aanensen DM, Armstrong-James D, et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg Microbes Infect. 2018;7(1):43 10.1038/s41426-018-0045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiology and immunology. 2009;53(1):41–4. 10.1111/j.1348-0421.2008.00083.x . [DOI] [PubMed] [Google Scholar]
- 3.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35 10.1186/s13756-016-0132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudramurthy SM, Chakrabarti A, Paul RA, Sood P, Kaur H, Capoor MR, et al. Candida auris candidaemia in Indian ICUs: analysis of risk factors. The Journal of antimicrobial chemotherapy. 2017;72(6):1794–801. 10.1093/jac/dkx034 . [DOI] [PubMed] [Google Scholar]
- 5.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64(2):134–40. 10.1093/cid/ciw691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis. 2014;33(6):919–26. 10.1007/s10096-013-2027-1 . [DOI] [PubMed] [Google Scholar]
- 7.Magobo RE, Corcoran C, Seetharam S, Govender NP. Candida auris-associated candidemia, South Africa. Emerging infectious diseases. 2014;20(7):1250–1. 10.3201/eid2007.131765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emara M, Ahmad S, Khan Z, Joseph L, Al-Obaid I, Purohit P, et al. Candida auris candidemia in Kuwait, 2014. Emerging infectious diseases. 2015;21(6):1091–2. 10.3201/eid2106.150270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, et al. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. The Journal of infection. 2016;73(4):369–74. 10.1016/j.jinf.2016.07.008 . [DOI] [PubMed] [Google Scholar]
- 10.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, et al. First three reported cases of nosocomial fungemia caused by Candida auris. Journal of clinical microbiology. 2011;49(9):3139–42. 10.1128/JCM.00319-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borman AM, Szekely A, Johnson EM. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere. 2016;1(4). 10.1128/mSphere.00189-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamoth F, Kontoyiannis DP. The Candida auris Alert: Facts and Perspectives. The Journal of infectious diseases. 2018;217(4):516–20. 10.1093/infdis/jix597 . [DOI] [PubMed] [Google Scholar]
- 13.Day AM, McNiff MM, da Silva Dantas A, Gow NAR, Quinn J. Hog1 regulates stress tolerance and virulence in the emerging fungal pathogen Candida auris. mSphere. 2018;3(5). 10.1128/mSphere.00506-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, et al. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. Journal of clinical microbiology. 2017;55(10):2996–3005. 10.1128/JCM.00921-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, et al. A Candida auris outbreak and its control in an intensive care setting. The New England journal of medicine. 2018;379(14):1322–31. 10.1056/NEJMoa1714373 . [DOI] [PubMed] [Google Scholar]
- 16.Mathur P, Hasan F, Singh PK, Malhotra R, Walia K, Chowdhary A. Five-year profile of candidaemia at an Indian trauma centre: High rates of Candida auris blood stream infections. Mycoses. 2018. 10.1111/myc.12790 . [DOI] [PubMed] [Google Scholar]
- 17.Pathirana RU, Friedman J, Norris HL, Salvatori O, McCall AD, Kay J, et al. Fluconazole-resistant Candida auris is susceptible to salivary histatin 5 killing and to intrinsic host defenses. Antimicrob Agents Chemother. 2018;62(2). 10.1128/AAC.01872-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams E, Quinn M, Tsay S, Poirot E, Chaturvedi S, Southwick K, et al. Candida auris in Healthcare Facilities, New York, USA, 2013–2017. Emerging infectious diseases. 2018;24(10):1816–24. 10.3201/eid2410.180649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, et al. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerging infectious diseases. 2017;23(2):328–31. 10.3201/eid2302.161320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother. 2017;61(5). 10.1128/AAC.02396-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katragkou A, Simitsopoulou M, Chatzimoschou A, Georgiadou E, Walsh TJ, Roilides E. Effects of interferon-gamma and granulocyte colony-stimulating factor on antifungal activity of human polymorphonuclear neutrophils against Candida albicans grown as biofilms or planktonic cells. Cytokine. 2011;55(3):330–4. Epub 2011/06/07. S1043-4666(11)00158-X [pii] 10.1016/j.cyto.2011.05.007 . [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, et al. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. The Journal of infectious diseases. 2012;206(12):1936–45. Epub 2012/10/04. jis607 [pii] 10.1093/infdis/jis607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson CJ, Kernien JF, Hoyer AR, Nett JE. Mechanisms involved in the triggering of neutrophil extracellular traps (NETs) by Candida glabrata during planktonic and biofilm growth. Sci Rep. 2017;7(1):13065 10.1038/s41598-017-13588-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson CJ, Cabezas-Olcoz J, Kernien JF, Wang SX, Beebe DJ, Huttenlocher A, et al. The Extracellular matrix of Candida albicans biofilms impairs formation of neutrophil extracellular traps. PLoS Pathog. 2016;12(9):e1005884 10.1371/journal.ppat.1005884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, et al. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerging infectious diseases. 2017;23(1). 10.3201/eid2302.161486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Bing J, Zheng Q, Zhang F, Liu J, Yue H, et al. The first isolate of Candida auris in China: clinical and biological aspects. Emerg Microbes Infect. 2018;7(1):93 10.1038/s41426-018-0095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakhim H, Vaezi A, Dannaoui E, Chowdhary A, Nasiry D, Faeli L, et al. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses. 2018;61(6):377–82. 10.1111/myc.12754 . [DOI] [PubMed] [Google Scholar]
- 28.Johnson CJ, Davis JM, Huttenlocher A, Kernien JF, Nett JE. Emerging fungal pathogen Candida auris evades neutrophil attack. mBio. 2018;9(4). 10.1128/mBio.01403-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cellular microbiology. 2006;8(4):668–76. Epub 2006/03/22. CMI659 [pii] 10.1111/j.1462-5822.2005.00659.x . [DOI] [PubMed] [Google Scholar]
- 30.Fidel PL Jr., Immunity to Candida. Oral Dis. 2002;8 Suppl 2:69–75. Epub 2002/08/08. . [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee S, Alampalli SV, Nageshan RK, Chettiar ST, Joshi S, Tatu US. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC genomics. 2015;16:686 10.1186/s12864-015-1863-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dementhon K, El-Kirat-Chatel S, Noel T. Development of an in vitro model for the multi-parametric quantification of the cellular interactions between Candida yeasts and phagocytes. PloS one. 2012;7(3):e32621 10.1371/journal.pone.0032621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruno M JM, Kruppa MG, Zuchao M, Ning Jiao Y, Lowman DW, Giamarellos-Bourboulis EJ, Williams DL, Chowdhary A, Meis JF, Netea MG. Candida auris: understanding the mechanisms of host immune response. 20th Congress of the International Society for Human and Animal Mycology, Amsterdam, the Netherlands. 2018.
- 34.Singh S, Uppuluri P, Alqarihi A, Elhassan H, French S, Lockhart SR, et al. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. bioRxiv. 2018:465096 10.1101/465096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yue H, Bing J, Zheng Q, Zhang Y, Hu T, Du H, et al. Filamentation in Candida auris, an emerging fungal pathogen of humans: passage through the mammalian body induces a heritable phenotypic switch. Emerg Microbes Infect. 2018;7(1):188 10.1038/s41426-018-0187-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grahl N, Demers EG, Crocker AW, Hogan DA. Use of RNA-Protein Complexes for Genome Editing in Non-albicans Candida Species. mSphere. 2017;2(3). 10.1128/mSphere.00218-17 [DOI] [PMC free article] [PubMed] [Google Scholar]