Key Teaching Points.
-
•
Loperamide abuse can present with severe cardiotoxicity. Profound electrocardiogram (ECG) abnormalities (sinus bradycardia, wide QRS, prolonged PR, markedly prolonged QTc, Brugada-like ECG pattern), malignant ventricular arrhythmias, and ventricular dysfunction have all been reported.
-
•
There is wide interindividual variability in toxic dose, ECG and clinical abnormalities, and duration of loperamide toxicity.
-
•
The determinants of this variability have not been identified.
-
•
QTc prolongation can persist for weeks to months after the last reported intake in loperamide abusers. The duration for which these patients need to be monitored is unclear but it should at least be for a few weeks.
-
•
Loperamide is not detected on routine blood and urine toxicity screens. A special assay, which is not readily available, is required for detection of loperamide in the serum.
Introduction
The United States is currently in the midst of a prescription opioid abuse epidemic. Loperamide, which is expected to be safe when used appropriately, has emerged as an over-the-counter substitute for opioids during the last few years, and its abuse is increasingly being reported. Serious cardiovascular side effects, including malignant ventricular arrhythmias such as torsades de pointes (TdP), Brugada-like electrocardiographic changes, and marked QTc prolongation, have been reported with loperamide abuse.
We report a 26-year-old white man who had marked QTc prolongation and multiple episodes of TdP-associated circulatory arrest following loperamide abuse. In addition, we review the data on all the reported patients with loperamide-associated cardiotoxicity to determine the toxic dose range and serum concentration, duration of cardiotoxicity following cessation of abuse, and reported symptoms.
Case report
A 26-year-old man presented to our emergency room (ER) with a history of 4 episodes of abrupt-onset syncope over the preceding 8 months. His past medical history was significant for intravenous opiate (heroin) abuse; he, however denied abusing heroin during the past 18 months. An electrocardiogram (ECG) was obtained in the ER and showed marked QTc prolongation and coved-type Brugada type 1 ST elevation in leads V1 and V2 (Figure 1). He was therefore admitted to the inpatient unit, where he admitted to abusing loperamide (150 pills per day, each containing 2 mg of loperamide) over the past 8 months. His urine and blood drug screens were negative. Serum loperamide concentration, sent approximately 24 hours after the last reported ingestion, measured 70 ng/mL (normal <5 ng/mL). The onset of his syncopal episodes correlated with loperamide abuse. He denied any cardiovascular symptoms prior to loperamide abuse and the family history was negative. An echocardiogram was obtained immediately after admission and showed normal intracardiac anatomy and biventricular size and function. Brugada type 1 changes in leads V1 and V2 resolved 48 hours after the admission; however, the QTc continued to be markedly prolonged (>500 ms). On day 6 of admission, at 4:20 AM, he had multiple runs of TdP leading to cardiorespiratory arrest (Figure 2). Cardiopulmonary resuscitation was initiated and he was successfully revived. Labs revealed normal serum electrolytes. Ventricular pacing was initiated at a rate of 90 beats per minute (bpm) via a quadripolar catheter placed through his internal jugular vein and electrolytes were maintained within the normal range. The pacing catheter was removed 10 days after the last reported ingestion, as no further runs of TdP were noted. His corrected QT interval, however, remained markedly prolonged 16 days after the last reported ingestion, raising suspicion of congenital long QT syndrome (LQTS), which had been unmasked by loperamide abuse (Figure 3). He therefore underwent an exercise stress test, which showed normal QTc shortening during exercise and a delta QTc of <30 ms during recovery. Only minimal prolongation of QTc was noted upon standing from a supine position during the resting phase of the exercise stress test (Viskin test).1 Given the persistence of prolonged QTc, a loop recorder was placed and he was discharged home with a life vest, which he was instructed to wear at all times.
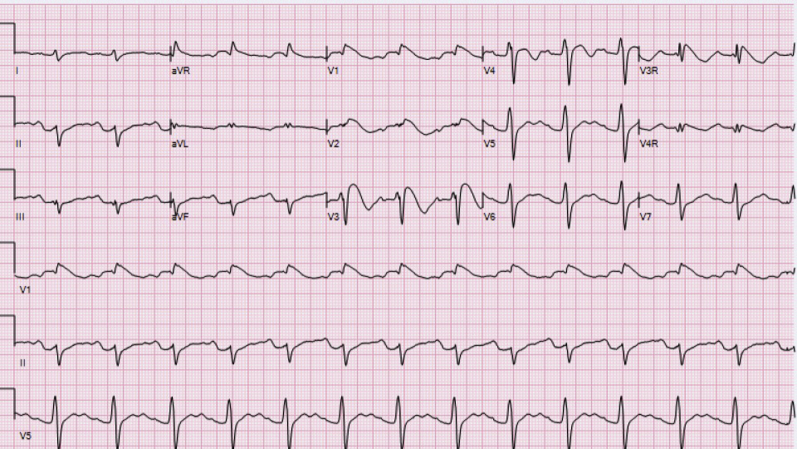
Figure 1.
Electrocardiogram (ECG) at presentation shows markedly prolonged QTc, Brugada type 1 ECG changes in leads V1 and V2, prolonged PR interval, and abnormal T waves.
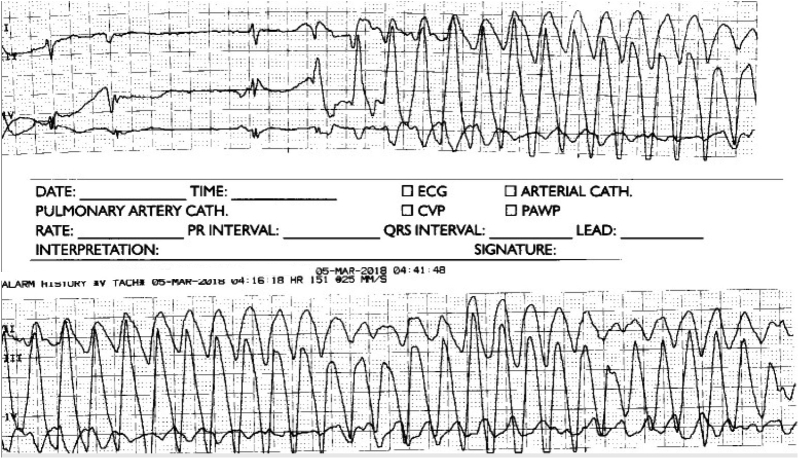
Figure 2.
Torsades de pointes.
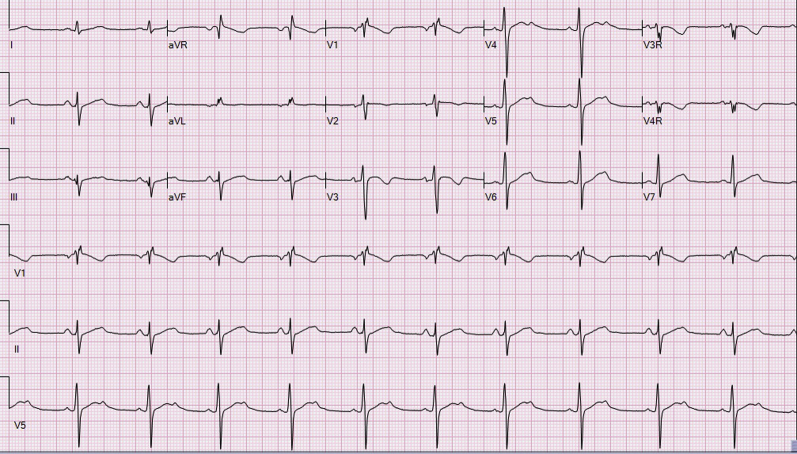
Figure 3.
Borderline prolonged QTc 16 days after the last reported ingestion.
He presented for a follow-up visit a month after hospital discharge, at which time he reported no symptoms. Interrogation of his loop recorder did not reveal any arrhythmias; however, ECG continued to show borderline prolonged QTc of 450 ms. Genetic testing was therefore sent and revealed a variant of unknown significance in the RYR2 gene (pY2743C). No polymorphisms or mutations were found in the long QT (LQT) genes. He denied abusing loperamide at this visit.
At the next follow-up visit 2 months later, he reported a few brief episodes of dizziness. Interrogation of his loop recorder showed 3 short runs of fast tachycardia in the 2 weeks prior to the visit. An ECG was obtained and showed markedly prolonged QTc and Brugada type 1 changes in leads V1 and V2. He denied loperamide abuse initially but later admitted to taking 100 loperamide pills per day, each containing 2 mg of loperamide over the past month. He also admitted to not wearing his life vest. Given the history of continued loperamide abuse, concerning symptoms, and ECG changes, a subcutaneous implantable cardioverter-defibrillator was offered. The family, however, declined and the patient is currently undergoing rehabilitation and is doing well, without any further episodes of dizziness or syncope.
Discussion
Loperamide, an over-the-counter antidiarrheal agent, works by a number of different mechanisms of action that decrease peristalsis and fluid secretion, resulting in longer gastrointestinal transit time and increased absorption of fluids and electrolytes from the gastrointestinal tract. It is a phenylpiperidine derivative with a chemical structure similar to opiate receptor agonists such as diphenoxylate and haloperidol and was designed to maintain the antidiarrheal activity of these drugs but minimize the negative aspects associated with their effects on the opiate μ-receptor. It was previously thought to have low abuse potential.2 However, it is increasingly being used extramedically to self-treat opioid withdrawal symptoms, with typical doses in the range of 70–100 mg per day, which is several times higher than the recommended dose of 16 mg per day.3 Data from the National Poison Data System showed a 91% increase in reported exposures from 2010 to 2015, of which half were single-agent loperamide use only.4
Loperamide has been shown to inhibit human cardiac sodium channels in vitro, and QRS prolongation in the overdose setting suggests that this interaction likely occurs in vivo.5, 6 Additional effects include potent blockade of hERG channels in vitro and marked action potential prolongation in isolated porcine ventricular myocytes.7 Loperamide also inhibits delayed-rectifier potassium currents in vitro.5 Xenobiotics that inhibit delayed-rectifier potassium currents can prolong the QTc duration and increase the risk for polymorphic ventricular tachycardia (VT), both of which have been reported after oral loperamide overdose.5, 8 An alternative mechanism of drug-induced torsades is increased late sodium current9; however, effects of loperamide on late sodium current have not been systematically evaluated. Additionally, loperamide is known to inhibit calcium channels, which may contribute to cardiac toxicity in overdose.2
Profound ECG abnormalities and malignant arrhythmias have been reported with loperamide abuse over the past few years. The reported abnormalities include nonsustained and sustained VT, premature ventricular contractions, polymorphic VT, TdP, increase in QRS width, sinus arrest, first-degree heart block, nonspecific T-wave abnormality, Brugada-like pattern, and markedly prolonged QTc.5, 10, 11 In addition, reversible depression of cardiac function has been reported after loperamide abuse in humans.10
As per data from the National Poison Data System (n = 179), cardiac conduction disturbances were reported in 13% of patients, ventricular tachycardia or fibrillation in 9% of patients, and other dysrhythmias in 6% of patients with a history of loperamide abuse. The average loperamide dose was 196.5 mg (range: 2–1200 mg).12 A search of the U.S. Food and Drug Administration Adverse Event Reporting System database revealed 48 cases of serious cardiac adverse events associated with loperamide use since December 28, 1976 (U.S. drug approval date). The most frequently reported cardiac adverse events were syncope (n = 24), cardiac arrest (n = 13), QT-interval prolongation (n = 13), ventricular tachycardia (n = 10), and TdP (n = 7). Out of these 48 cases, 10 cases resulted in death. The most commonly reported reasons for use were drug abuse (n = 22) and diarrhea treatment (n = 17). More than one half of the 48 cases were reported after 2010. Of the 22 drug abuse cases, the median daily dose was 250 mg (range 70–1600 mg) and events occurred as early as 6 hours after a dose and as long as 18 months after initiation of loperamide.13
Loperamide is primarily metabolized by CYP 3A4 and CYP 2C81 and it is also a P-glycoprotein efflux transporter substrate.14 The therapeutic pharmacokinetic half-life of loperamide is approximately 9–14 hours.15 Extremely high doses may saturate the metabolic pathway and the excretory efflux transport, allowing it to exert central nervous system and other physiologic effects. The half-life can be quite prolonged in supratherapeutic doses.11 Eggleston and colleagues11 reported a half-life of 34.8 hours in a patient with QRS and QTc prolongation and ventricular dysrhythmia following loperamide ingestions. The decreased intestinal motility seen with loperamide may also contribute to delayed and ongoing absorption.
Overdrive pacing to prevent pause-dependent TdP, intravenous lipid emulsion, intravenous sodium bicarbonates, and extracorporeal membrane oxygenation have been shown to be helpful in management of loperamide toxicity.16 Our patient responded well to ventricular pacing at 90 bpm without any further episodes of TdP. This is consistent with findings that have been reported in other studies.
It is difficult to get a good sense of the extent of loperamide abuse from isolated case reports; however, given the opiate abuse epidemic that we are in the midst of, it is likely that the abuse is widespread, with only a few patients who manifest serious cardiovascular toxicity being reported. In addition, there are reports of chronic long-term loperamide abuse without any cardiovascular effects.17 Even among patients who manifest serious cardiovascular toxicity following chronic loperamide abuse, there appears to be wide interindividual variability in loperamide dose, duration, and cardiotoxic effects (Supplemental Table 1). However, mechanisms that underlie this variability have not been elucidated. It is possible that polymorphisms in the LQT genes (KCNQ1, KCNH2, and SCN5A, among several others) or cytochrome p450 CYP 3A4 and CYP 2C8/P-glycoprotein genes could explain this variability. Our patient had QTc prolongation for more than 14 days after the last reported ingestion, which made us think about unmasking of congenital LQTS by loperamide. We therefore obtained an exercise stress test, which did not show findings suggestive of inherited long QTc type 1. In addition, genetic testing, which is positive in approximately 70% of patients with inherited LQTS, was also performed and was negative for variants in the long QT genes. An alternative mechanism for the long duration of QTc prolongation after loperamide ingestion could be disruption of transport of new channels to the membrane, as is seen with other drugs that induce LQTS.
Conclusion
Loperamide can lead to severe cardiotoxicity; however, there is wide interindividual variability in cardiotoxic manifestations of loperamide, the basis of which is poorly understood. Further systematic studies are needed to determine the factors that underlie this variability and identify at-risk patients.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2019.01.004.
Appendix. Supplementary data
References
- 1.Viskin S., Postema P.G., Bhiyan Z.A. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55:1955–1961. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker D.E. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7:S11–18. [PubMed] [Google Scholar]
- 3.Lasoff D.R., Koh C.H., Corbett B. Loperamide trends in abuse and misuse over 13 years: 2002-2015. Pharmacotherapy. 2017;37:249–253. doi: 10.1002/phar.1885. [DOI] [PubMed] [Google Scholar]
- 4.Vakkalanka J.P., Charlton N.P., Holstege C.P. Epidemiologic trends in loperamide abuse and misuse. Ann Emerg Med. 2017;69:73–78. doi: 10.1016/j.annemergmed.2016.08.444. [DOI] [PubMed] [Google Scholar]
- 5.Eggleston W., Clark K.H., Marraffa J.M. Loperamide abuse associated with cardiac dysrhythmia and death. Ann Emerg Med. 2017;69:83–86. doi: 10.1016/j.annemergmed.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Harmer A.R., Valentin J.-P., Pollard C.E. On the relationship between block of the cardiac Na(+) channel and drug-induced prolongation of the QRS complex. Br J Pharmacol. 2011;164:260–273. doi: 10.1111/j.1476-5381.2011.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klien M.G., Haigney M.C.P., Mehler P.S., Fatima N., Flagg T.P., Krantz M.J. Potent inhibition of herg channels by the over-the-counter antidiarrheal agent loperamide. JACC Clin Electrophysiol. 2016;2:784–789. doi: 10.1016/j.jacep.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Nattel S. An emerging malignant arrhythmia epidemic due to loperamide abuse: underlying mechanisms and clinical relevance. JACC Clin Electrophysiol. 2016;2:790–792. doi: 10.1016/j.jacep.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Yang T., Chun Y.W., Stroud D.M. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation. 2014;130:224–234. doi: 10.1161/CIRCULATIONAHA.113.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A., Freeland S., Steinberg L., Prystowksky E.N. Wide complex tachycardia and cardiomyopathy: What would you do? J Cardiovasc Electrophysiol. 2018;29:1171–1173. doi: 10.1111/jce.13642. [DOI] [PubMed] [Google Scholar]
- 11.Eggleston W., Nacca N., Marraffa J.M. Loperamide toxicokinetics: serum concentrations in the overdose setting. Clin Toxicol Phila. 2015;53:495–496. doi: 10.3109/15563650.2015.1026971. [DOI] [PubMed] [Google Scholar]
- 12.Eggleston W., Marraffa J.M., Stork C.M. Notes from the field: cardiac dysrhythmias after loperamide abuse - New York, 2008-2016. MMWR Morb Mortal Wkly Rep. 2016;65:1276–1277. doi: 10.15585/mmwr.mm6545a7. [DOI] [PubMed] [Google Scholar]
- 13.Swank K.A., Wu E., Kortepeter C., McAninch J., Levin R.L. Adverse event detection using the FDA post-marketing drug safety surveillance system: Cardiotoxicity associated with loperamide abuse and misuse. J Am Pharm Assoc. 2017;57:S63–S67. doi: 10.1016/j.japh.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Kim R.B. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34:47–54. doi: 10.1081/dmr-120001389. [DOI] [PubMed] [Google Scholar]
- 15.Killinger J.M., Weintraub H.S., Fuller B.L. Human pharmacokinetics and comparative bioavailability of loperamide hydrochloride. J Clin Pharmacol. 1979;19:211–218. doi: 10.1002/j.1552-4604.1979.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu P.E., Juurlink D.N. Clinical review: loperamide toxicity. Ann Emerg Med. 2017;70:245–252. doi: 10.1016/j.annemergmed.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Zarghami M., Rezapour M. Loperamide dependency: a case report. Addict Health. 2017;9:59–63. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.