Key Teaching Points.
-
•
In atypical atrial flutter 3-dimensional electroanatomic mapping (3D EAM) is a key tool for proper analysis of the wavefront propagation and strategic ablation of the critical isthmus in the circuit.
-
•
A new feature of the 3D EAM system CARTO 3 version 6.0, high definition (HD) coloring (Biosense Webster, Diamond Bar, CA), allows high-quality display of the EAM and, in addition to the conventional early-meets-late, also highlights areas of potential conduction block. Thus, HD coloring provides clearer visualization of the arrhythmia mechanism than the conventional local activation time map.
-
•
In this case of atypical flutter, the HD coloring feature together with Multi-Electrode Mapping technology simplified the interpretation of the arrhythmia mechanism and facilitated effective ablation.
Introduction
Atypical atrial flutter is a regular arrhythmia characterized by a non–cavotricuspid isthmus–dependent reentry.1, 2, 3 Atypical atrial flutter can arise from complex reentry circuits involving areas of slow electrical conduction and scar tissue in both atria. Three-dimensional (3D) activation mapping is a key tool for analysis of the wavefront propagation. However, activation mapping techniques can be very time-consuming and in some cases the mapping can be difficult to analyze, leading to ineffective ablation procedures. A new feature of the 3D electroanatomic mapping (3D EAM) system CARTO 3 version 6.0, high definition (HD) coloring (Biosense Webster, Diamond Bar, CA), allows high-quality display of the EAM, and in addition to the conventional early-meets-late (EML) it also highlights areas of potential conduction block, called extended early-meets-late (EEML), thereby providing a basis for a better interpretation of the local activation time (LAT) and propagation map.
Here we report the first case completed with the HD coloring feature of an atypical atrial flutter in the right atrium and compare it with the conventional CARTO 3 software version.
Case report
A 67-year-old man with atypical atrial flutter was referred for ablation. Previous cardiac history includes surgery for a late diagnosed atrial septal defect with insertion of a pericardium patch and correction of partial anomalous pulmonary venous connection in 1990. In 2018 the patient presented with worsening shortness of breath on exertion and palpitations.
Interrogation of the pacemaker during symptoms revealed paroxysmal atrial flutter with 1:1 conduction and a ventricular rate of 200 beats per minute. Echocardiography showed normal left ventricular ejection fraction and no residual atrial septal defect or pulmonary hypertension.
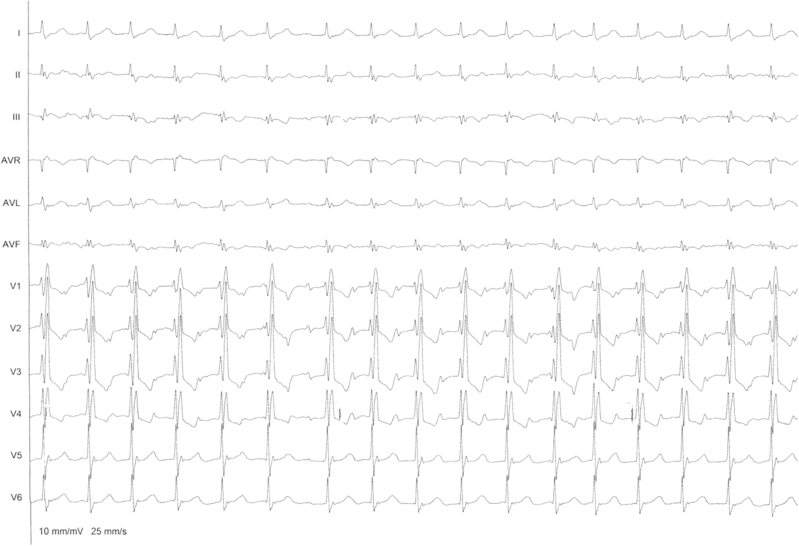
The patient arrived at the electrophysiology laboratory in atrial flutter with 2:1 conduction; the electrocardiogram is shown in Figure 1.
Figure 1.
The 12-lead electrocardiogram shows a 2:1 flutter with flutter wave morphology positive in V1, no visible F waves in lead I, and inverted P wave in leads II, III, and augmented vector foot (AVF) and a right bundle branch block pattern.
A 6F 8-pole deflectable catheter and a 5F 4-pole catheter were placed into the coronary sinus (CS) and the right ventricle, respectively. A PENTARAY high-density mapping catheter (Biosense Webster) was advanced to the right atrium, later replaced by the ablation catheter, NaviStar RMT ThermoCool (Biosense Webster).
The tachycardia cycle length (TCL) was measured to 310 ms, with a concentric atrial activation (proximal-to-distal CS activation). 3D EAM was performed with the PENTARAY catheter and the CARTO 3 Multi-Electrode Mapping (MEM) technology. The MEM technology ensures multiple data sampling of the right atrium to provide high-resolution maps used for the HD coloring feature.
The window of interest was set to 90% of the TCL, with 50% to each side of the reference (CS 7–8), as seen in Figure 2B. The LAT map includes the entire CL, as seen in Figure 2B. An EAM of 1561 points of the right atrium, as seen in Figure 2A, was collected with the PENTARAY catheter in 5.4 minutes.
Figure 2.
Three-dimensional electroanatomic map of the right atrium performed with the PENTARAY catheter (Biosense Webster, Diamond Bar, CA) used with the CARTO 3 Multi-Electrode-Mapping technology (Biosense Webster). A: Local activation time (LAT) map. The earliest LAT point is -174 ms (red) and the latest LAT point is 120 ms (purple), thereby covering the entire tachycardia cycle length. B: Signals from the PENTARAY catheter that is placed in the region with conduction block and reentry, showing both late (eg, PR 15–16) and early (eg, PR 9–10) potentials. Local double potentials with a shift in near field and a far-field component and fractionation in between on PR 11–12 and PR 19–20 suggest that these poles are straddling the isthmus. The coronary sinus (CS) signals indicate a proximal-to-distal activation. Right lateral (RL) view.
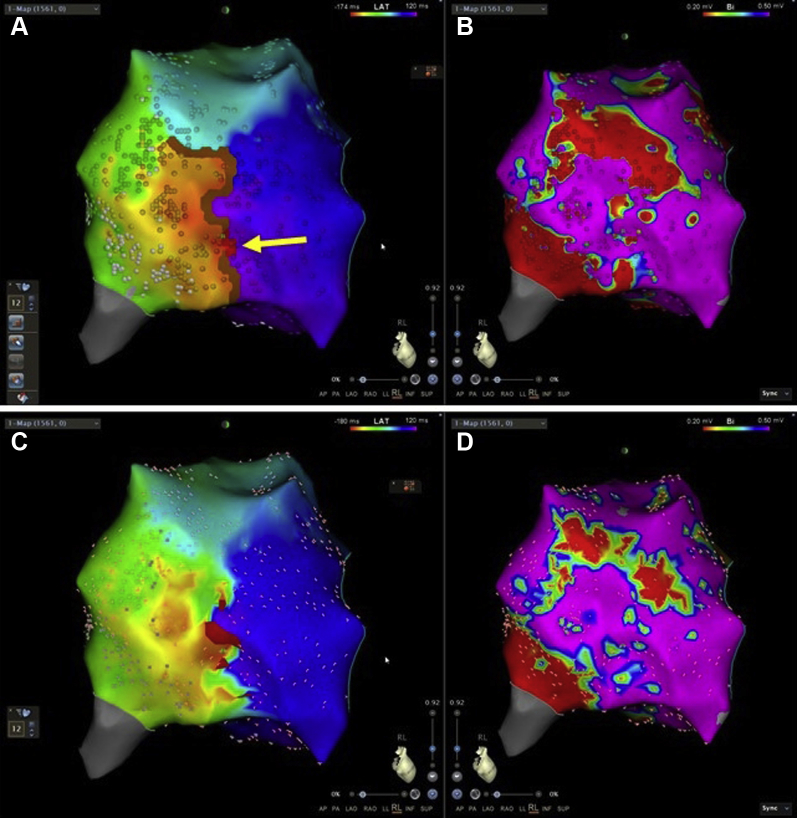
Interpretation of the LAT map shows a conduction block (brown line) situated in the lateral free wall inferiorly, and up toward the mid axis, as seen in Figure 3A. In the mid region of the conduction block, the EML reentry (red line and arrow) is situated, indicating that the wavefront is passing through this narrow path.
Figure 3.
Electroanatomic mapping of the right atrium performed with the PENTARAY catheter (Biosense Webster, Diamond Bar, CA). Top row with the CARTO 3 high definition (HD) coloring feature (Biosense Webster). A: Local activation time (LAT) map showing conduction block (brown line) and reentry path (red line and arrow). B: The bipolar voltage map (lower threshold: 0.2 mV, upper threshold: 0.5 mV) shows that regions with scar tissue correlate with the areas of conduction block in the LAT map. Moreover, the region of healthy tissue between scars correlates with the reentry in the LAT map in panel A. The propagating wavefront is passing through the narrow path between the 2 regions of scar tissue and thereby facilitating a reentry. Bottom row shows same map as top, but without HD coloring. C: LAT map showing reentry (red line – early meets late at 80%). D: Bipolar voltage map (lower threshold: 0.2 mV, upper threshold: 0.5 mV).
The HD coloring feature allows 2 ranges of EML: an upper range that identifies where the EML is in the reentry circuit (red line and arrow), and an EEML with a lower range (brown line) that identifies possible conduction block. EEML calculates the LAT difference between adjacent points, and if this difference is higher than the percentage selected (ie, an upper threshold of 75% and a lower threshold of 25%) of the cycle length mapped, a brown line will be drawn between those adjacent points (brown line in Figure 3A). HD coloring provides clearer visualization of the arrhythmia mechanism than the conventional LAT map shown in Figure 3C and D. This is accomplished by better projection of the points onto the surface. With HD coloring the points are projected on voxels of a 0.8 mm cube, while in the standard coloring the map is divided in triangles (mesh), to which fewer points can be projected. resulting in more color interpolation. As a result, by using HD coloring, more points are projected and there is less color interpolation, leading to the automatic collected line of block representing EEML. In the supplementary material, Video 1 illustrates the 3D EAM of the right atrium without the HD coloring feature, and Video 2 illustrates the 3D EAM of the right atrium with the HD coloring feature.
Ablation was performed with the Niobe ES system (Stereotaxis, St. Louis, MO) using the NaviStar RMT ThermoCool catheter with a irrigated flow of 10 mL/min and power output of 35 watts. Based on the electroanatomic maps, the ablation strategy was to complete the conduction block, and ablation was therefore performed in the lateral area between the 2 regions of scar tissue, as seen with the arrow in Figure 3. The ablation terminated the flutter during first application, and a complete block was achieved with total radiofrequency time of 3.1 minutes. The total procedure time was 70 minutes.
Conclusion
In this case of atypical flutter, focus on EML and conduction block using CARTO 3 version 6, with HD coloring feature, together with MEM technology provides a higher-resolution display of electroanatomic maps, simplifies the interpretation of arrhythmia mechanism, and facilitates effective ablation.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2019.01.003.
Appendix. Supplementary data
Shows the three-dimensional electro-anatomical mapping of the right atrium performed with the PENTARAY Catheter without the HD coloring feature.
Shows the three-dimensional electro-anatomical mapping of the right atrium performed with the PENTARAY Catheter and the CARTO 3 HD coloring feature. The Local-activation-time map shows the conduction block (brown line) and re-entry path (red line).
References
- 1.Page R.L., Joglar J.A., Caldwell M.A. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67:e27–e115. doi: 10.1016/j.jacc.2015.08.856. [DOI] [PubMed] [Google Scholar]
- 2.Cosío F.G. Atrial flutter, typical and atypical: a review. Arrhythm Electrophysiol Rev. 2017;6:55–62. doi: 10.15420/aer.2017.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu R.C., Berger R., Calkins H. Catheter ablation of atrial flutter and macroreentrant atrial tachycardia. Curr Opin Cardiol. 2002;17:58–64. doi: 10.1097/00001573-200201000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shows the three-dimensional electro-anatomical mapping of the right atrium performed with the PENTARAY Catheter without the HD coloring feature.
Shows the three-dimensional electro-anatomical mapping of the right atrium performed with the PENTARAY Catheter and the CARTO 3 HD coloring feature. The Local-activation-time map shows the conduction block (brown line) and re-entry path (red line).