Abstract
Background: 131I treatment (tx) of differentiated thyroid cancer (DTC) is associated with hematopoietic toxicity. It was hypothesized that metformin could have radioprotective effects on bone-marrow function. The objective was to determine whether metformin prevents 131I-induced changes in complete blood counts (CBC) in patients with DTC.
Methods: A retrospective analysis was performed of CBC values in DTC patients who were (40 patients: metformin group) or were not taking metformin (39 patients: control group) at the time of administration of 131I. Repeated measures analysis of variance was used for the analysis of the differences in the averages of CBC that were documented at baseline and at 1, 6, and 12 months post 131I tx.
Results: The groups were comparable in terms of age, sex, stage of DTC, 131I dose administered, and baseline CBC values. In the control group, the decrease in white blood cells (WBC) was 35.8% (p < 0.0001) at one month, 21.8% (p < 0.0001) at six months, and 19.4% (p < 0.0001) at 12 months. In the metformin group, the decrease in WBC was 17.1% (p < 0.0001) at one month, and 8.6% at six months (p = 0.01), while at 12 months WBC had returned to baseline values (p = 0.9). Differences between the two groups were highly statistically significant at all time points (p < 0.0001, p = 0.0027, and p < 0.0001, respectively). Lymphocytes were more sensitive to 131I, but metformin's radioprotective properties were more prominent in neutrophils. At 12 months, the decrease in platelets in the control group was 15.5% (p < 0.0001) versus 5.6% (p = 0.056) in the metformin group, while at one and six months the reductions in the two groups were comparable. No statistically significant differences were observed between the two groups in the change from baseline values for hemoglobin.
Conclusions: Metformin attenuated the 131I-induced decrease in CBC parameters, and its radioprotective properties were more prominent in WBC. Patients who were taking metformin during 131I tx also experienced a faster recovery in their blood counts, when compared to the control group. Further study is warranted in order to examine if the radioprotective properties of metformin observed in the current study for 131I tx can also apply to other forms of therapeutic chemo- and radiotherapy.
Introduction
Differentiated thyroid cancer (DTC) is the most common endocrine cancer, and its incidence is increasing at the fastest rate of any malignancy (1). DTC (Stage 2–4) is usually treated by total thyroidectomy followed by 131I therapy and life-long thyroid hormone therapy. Patients with DTC have an excellent prognosis, and 10-year survival rates range between 80% and 95% (2,3).
Though effective, radioactive iodine (RAI) treatment (tx) exhibits important side effects that can affect patients' quality of life. The side effects include, but are not limited to, sialoadenitis, xerostomia, dysgeusia, acute radiation pneumonitis, and pulmonary fibrosis (4). Moreover, the hematopoietic toxicity induced by RAI tx is another well-established side effect. Neutropenia, anemia, low platelet count, as well as leukemia and bone-marrow aplasia have been described (5). One study from the author's group examined the effect of dosimetry-guided RAI tx on bone marrow, and observed a significant decrease in all blood counts (6). The decrease was most prominent in the first month post RAI tx, and remained significant for as long as one year post therapy.
Metformin is a metabolic drug used to control diabetes mellitus type 2 and in women with polycystic ovary syndrome (PCOS). Metformin has been proposed to have antineoplastic properties against a variety of cancers. In thyroid cancer, a retrospective analysis of medical records of patients diagnosed with concomitant DTC and type 2 diabetes mellitus revealed increased progression-free survival in those who were on metformin tx (7). The comparison of thyroid cancer pathological data and tx efficacy between metformin-treated and non-treated groups showed that size of thyroid tumors was significantly lower in the metformin group compared with the non-metformin group, suggesting an inhibitory effect of the drug on tumor growth.
Over the last few years, several studies that examined the synergistic effect of metformin and radiation in various cancers have been published. In fact, the majority of these reports suggest that metformin sensitizes the cancer cells to the effects of radiation therapy. Fasih et al. proposed that the radiosensitization of pancreatic cells with metformin occurs through the activation of the adenosine monophospahate-activated protein kinase (AMPK) pathway (8). Similar findings have been replicated in non-small lung-cell lung cancer (9), breast cancer (10), and esophageal cancer (11).
However, very little is known about the radioprotective effect of metformin on normal cells. Miller et al. recently performed in vitro and in vivo (in mice) experiments and demonstrated that metformin possesses radioprotective properties on mice splenic cells when administered 24 hours after radiation exposure (12). These data suggested that metformin could potentially be used as a radioprotective agent that can help to minimize the side effects from radiation therapy.
In view of these earlier observations, it was hypothesized that metformin could minimize the effects of RAI tx on patients' bone marrow, and a retrospective analysis was performed of the medical records of patients with DTC who were treated with RAI to examine this hypothesis.
Methods
Patients
For the metformin group, a retrospective analysis was performed on medical records of patients diagnosed with concomitant differentiated thyroid cancer (papillary, follicular, and mixed papillary-follicular histologic types) and diabetes mellitus type 2 that were treated with 131I at the MedStar Washington Hospital Center from January 2000 to December 2013. All patients met the following specific inclusion criteria: (i) differentiated thyroid cancer; (ii) near-total or total thyroidectomy; (iii) at least one 131I tx performed; (iv) a baseline pretreatment complete blood count (CBC); (v) at least one CBC available during follow-up; (vi) confirmed diagnosis of diabetes mellitus based on the American Diabetes Association criteria; and (vii) tx with the antidiabetic drug metformin during and for at least three months prior to their 131I tx.
Patients were excluded from the study if they: (i) were known to have any baseline hematologic disease; (ii) had an abnormal baseline CBC; (iii) had a systemic disease that could affect their bone marrow or peripheral blood cells (e.g., systemic lupus erythematosus, human immunodeficiency virus infection, rheumatoid arthritis); or (iv) received subsequent medication that could affect their hematologic state (e.g., tyrosine kinase inhibitors, cytotoxic chemotherapy).
For the control group, patients with DTC but no metformin tx were included. More specifically, in order to eliminate heterogeneity between the two groups, all patients found in the database who had DTC, diabetes mellitus type 2 and were not on metformin tx during and for at least three months prior to their 131I tx were included. Then, a random sample was selected from the database with patients who had DTC, were not diabetic, and were not on metformin tx. All patients in the control group met the first five aforementioned inclusion criteria and all the exclusion criteria.
CBC were used as a surrogate marker for the effect of RAI tx on patients' bone marrow. The blood counts of patients who were on metformin tx for their diabetes were compared to those of patients who were not treated with metformin. Blood parameters evaluated included hemoglobin, red blood-cell count (RBC), white blood-cell count (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and platelet count.
Due to the inherent variability of the timeframe from the date of RAI tx at which the CBC were drawn, the time points were categorized into the following reference ranges: 3–6 weeks for the one-month time frame, 4.5–7.5 months for the six-month time frame, and 10.5–13.5 months for the one-year time frame. This allowed the statistical analysis of mean and percent change for the established time intervals.
All follow-up CBC were drawn while the patients were on thyroid hormone replacement. The protocol for this study was approved by the local Institutional Review Board (IRB).
Statistical analysis
The change in CBC parameters over time for the two groups (control group and metformin group) was tested using repeated measures two-way analysis of variance (ANOVA) with interaction term between time and tx group. Least-square estimates (estimated means) along with the corresponding standard errors were calculated from this analysis. Comparison of baseline estimates to one-month, six-month, and 12-month estimates is provided, as well as how change over time differs between tx groups when tested from the same model. For the group receiving metformin, analysis was conducted using repeated-measures two-way ANOVA with time, 131I dose, and their interaction in the model. Descriptive statistics are presented using means and standard deviation, and percentages were calculated for categorical variables. The statistical significance level was set at p < 0.05 (two-sided). The analysis was conducted using SAS v9.3 (SAS Institute, Inc., Cary, NC).
Results
Baseline patient characteristics
The database included records from 1825 131I tx administered in 1685 patients with DTC and performed at the authors' institution between 2000 and 2013. The initial screening for diabetic patients with DTC who have been on metformin tx resulted in the identification of 465 patients. All these records were reviewed, and 40 patients met all the inclusion criteria. As a result, the study cohort (metformin group) consisted of 40 patients diagnosed with follicular cell-derived carcinomas who had undergone at least one 131I tx in the authors' institution and were on metformin tx for their diabetes mellitus type 2.
In the control group, first all patients who had DTC and diabetes mellitus type 2, were not on metformin tx, and met all the inclusion criteria were identified and included (n = 17). Then patients with DTC who were not diabetic (and consequently not on metformin tx) were included in order to reach approximately the same number of subjects as in the metformin group (n = 22). Finally, the control group consisted of 39 patients. The diabetic and the non-diabetic control groups were comparable, and demonstrated similar responses to 131I tx.
The pertinent characteristics of the two cohorts are presented in Table 1. No statistically significant difference was observed between the two groups in terms of age at diagnosis, age at 131I tx, and sex. The histopathologic breakdown was similar between groups, with classical papillary thyroid cancer being the most prevalent type of thyroid cancer. Similarly, no statistically significant difference was observed in the TNM staging (based on the American Joint Committee of Cancer classification, 6th edition) of patients between the two groups (p = 0.4). The two groups were comparable in terms of the dose of 131I received, the cumulative dose of 131I received, the number of tx that the patients had, and the percentage of patients who had received prior external beam radiation therapy.
Table 1.
Baseline Patient Characteristics
| Baseline characteristics | Control group, n = 39 (100%) | Metformin group, n = 40 (100%) | p-Value |
|---|---|---|---|
| Sex | |||
| Female | 15 (38%) | 12 (30%) | 0.43 |
| Male | 24 (62%) | 28 (70%) | |
| Age at diagnosis, M ± SD | 51.2 ± 14.9 | 54.8 ± 11.5 | 0.41 |
| Age at 131I tx, M ± SD | 53.2 ± 13.8 | 55.5 ± 11.6 | 0.23 |
| Histology | 0.98 | ||
| Papillary (PTC) | 33 (85%) | 32 (80%) | |
| Follicular (FTC) | 6 (15%) | 5 (12%) | |
| Mixed PTC-FTC | 0 (0%) | 3 (8%) | |
| TNM stage | 0.4 | ||
| 1 | 12 (32%) | 12 (30%) | |
| 2 | 5 (13%) | 4 (10%) | |
| 3 | 8 (21%) | 15 (38%) | |
| 4 | 13 (34%) | 9 (22%) | |
| Surgery, total thyroidectomy | 39 (100%) | 40 (100%) | |
| 131I tx | 39 (100%) | 40 (100%) | |
| Method of preparation for 131I tx | 0.93 | ||
| rhTSH | 26 (67%) | 27 (68%) | |
| THW | 13 (33%) | 13 (32%) | |
| Number of 131I tx (including the one documented for the study) | 0.55 | ||
| 1 | 32 (82%) | 33 (83%) | |
| 2 | 5 (13%) | 3 (7.5%) | |
| 3 | 2 (5%) | 3 (7.5%) | |
| 5 | 0 (0%) | 1 (2%) | |
| Activity of 131I (mCi) | 0.43 | ||
| M ± SD | 170.7 ± 68.6 | 157 ± 85 | |
| Range | 73.2–382 | 37.9–402 | |
| Cumulative activity of 131I (mCi) | 0.75 | ||
| M ± SD | 205.7 ± 133.6 | 220.3 ± 245.1 | |
| Median | 150 | 148.2 | |
| Range | 73.2–690 | 50–1417 | |
| EBRT | 3 (8%) | 1 (3%) | 0.32 |
SD, standard deviation; tx, treatment; mCi, millicuries; EBRT, external beam radiation therapy; rhTSH, recombinant human thyrotropin; THW, thyroid hormone withdrawal.
Comparison of control and metformin groups
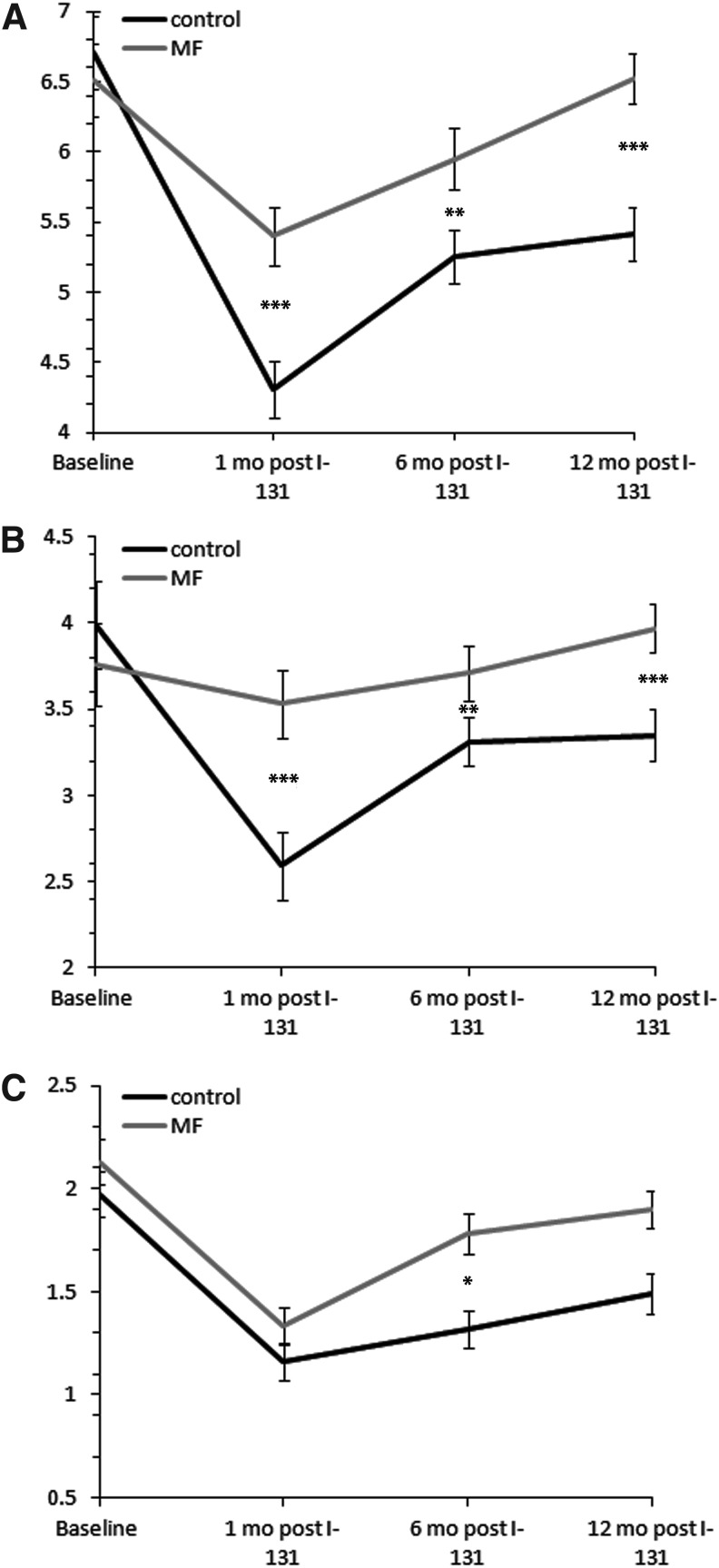
WBC, ANC, ALC, PLT, Hgb, and RBC were documented at baseline (<1 month before 131I tx) and at one, six, and twelve months post 131I tx. In all parameters, the pattern of reaction to 131I tx was consistent to the one presented previously by the authors' group (6): the CBC values reached a nadir at one month post 131I, and then they gradually returned toward baseline values over time. The mean decreases with standard errors, the percent differences from the baseline values, as well as p-values for the comparison between the two groups for all CBC parameters are presented in Figure 1 and Tables 2 and 3.
FIG. 1.
Comparison between the control group and the metformin group for the change in WBC (A), ANC (B), and ALC (C) post 131I treatment (tx) over time. WBC, ANC, and ALC reached a nadir at one month post 131I, and then they slowly returned toward baseline values in both groups. Patients on MF had a smaller initial drop in WBC and ANC, and a more rapid recovery to baseline values. A statistically significant difference was noted in ALC between the two groups at six months post 131I tx. MF, metformin; WBC, white blood-cell count; ANC; absolute neutrophil count; ALC, absolute lymphocyte count. A statistically significant difference for the change from baseline values at the specific time points between the two groups is signified at the level of *p < 0.05, **p < 0.01, and ***p < 0.0001. Error bars represent the standard error. WBC, ANC, and ALC counts in the y-axis are in 109/L.
Table 2.
Comparison of the Change in Platelets Over Time Post 131I in the Control Group and the Metformin Group
| Control group, M (SE) | MF group, M (SE) | MF vs. control | |
|---|---|---|---|
| Baseline, 109/L | 257.61 (11.55) | 278.12 (11.38) | 20.52 (16.22), p = 0.21** |
| Change at 1 month post tx | −60.13 (7.15) | −45.39 (7.44) | p = 0.16** |
| % | −23.3% | −16.3% | |
| p-value | <0.0001* | <0.0001* | |
| Change at 6 months post tx | −35.43 (5.86) | −33.97 (6.52) | p = 0.87** |
| % | −13.7% | −12.2% | |
| p-value | <0.0001* | <0.0001* | |
| Change at 12 months post tx | −39.79 (8.31) | −15.58 (8.04) | p = 0.04** |
| % | −15.5% | −5.6% | |
| p-value | <0.0001* | 0.056* |
Statistically significant differences (p < 0.05) are noted in bold.
Represents the comparison performed between the value at a specific time point and the baseline value.
Represents the comparison performed between the control and the metformin group at each specific time point.
MF, metformin; SE, standard error.
Table 3.
Comparison of the Change in Hemoglobin Over Time Post 131I in the Control Group and the Metformin Group
| Control group, M (SE) | MF group, M (SE) | MF vs. control | |
|---|---|---|---|
| Baseline, g/dL | 13.65 (0.24) | 12.93 (0.23) | −0.72 (0.33), p = 0.03** |
| Change at 1 month post tx | −0.88 (0.18) | −0.43 (0.18) | p = 0.08** |
| % | −6.5% | −3.3% | |
| p-value | <0.0001* | 0.02* | |
| Change at 6 months post tx | −0.54 (0.17) | −0.24 (0.20) | p = 0.26** |
| % | −4% | −1.9% | |
| p-value | 0.002* | 0.22* | |
| Change at 12 months post tx | −0.18 (0.17) | −0.12 (0.16) | p = 0.82** |
| % | −1.3% | −0.9% | |
| p-value | 0.3* | 0.45* |
Statistically significant differences (p < 0.05) are noted in bold.
Represents the comparison performed between the value at a specific time point and the baseline value.
Represents the comparison performed between the control and the metformin group at each specific time point.
WBC
Baseline values for WBC were similar in both groups (p = 0.6). Data on WBC are presented in Figure 1A. The decrease of WBC from baseline values in the control group was 35.8%, 21.8%, and 19.4% at one, six, and 12 months post 131I, respectively, with all differences being highly statistically significant (p < 0.0001). On the contrary, in the metformin group, changes from baseline were a decrease of 17.1% and 8.6% at one and six months post 131I (p < 0.0001 and p = 0.01, respectively), and a slight increase of 0.2% (p = 0.93) at 12 months. When the changes in WBC were compared between the two groups, highly statistically significant differences were found at all time points (p < 0.0001 at one month, p = 0.0027 at six months, and p < 0.0001 at 12 months). These data suggest that metformin attenuated the decrease in WBC post 131I tx, and that patients concomitantly treated with metformin returned to their baseline values much sooner.
Because the observed effect of metformin was so prominent on WBC, the two major constituents of the WBC (ANC and ALC) were analyzed in order to examine whether the two blood-cell lineages were equally sensitive to metformin's effects. The effect on ANC was even more prominent than on WBC (Fig. 1B). At one month, the decrease was 35% (p < 0.0001) in the control compared with 6.1% (p = 0.25) in the metformin group. At six months, the decrease was 17% (p < 0.0001) in the control compared with 1.3% (p = 0.74) in the metformin group. At 12 months, ANC decreased by 16% (p < 0.0001) in the control group, while in the metformin group the change was an increase of 5.6% (p = 0.13). The differences in the changes between the two groups were highly significant (p < 0.0001 for 1 and 12 months, and p = 0.004 for 6 months).
As far as ALC are concerned (Fig. 1C), the decrease in the control group was 41.1% (p < 0.0001) at one month, 33% (p < 0.0001) at six months, and 24.4% (p < 0.0001) at 12 months. In the metformin group, the decrease was 37.6% (p < 0.0001) at one months, 16.4% (p = 0.0006) at six months, and 10.8% (p = 0.013) at 12 months. At one month, the decrease was similar between the two groups (p = 0.95). The difference between the two groups was statistically significant only at six months (p = 0.029), and approached statistical significance at 12 months (p = 0.069). Despite the fact that ALC seemed to be more sensitive to 131I, based upon a more significant drop at all time points, the radioprotective effect of metformin was more prominent on ANC.
Platelets
Data on platelets are presented in Table 2. The difference observed between the decreases in the two groups was not significant at either one (p = 0.16) or at six months (p = 0.87). However, a statistically significant difference was identified at 12 months post 131I (p = 0.04). At that time point, the decrease was 15.5% (p < 0.0001) in the control compared with 5.6% (p = 0.056) in the metformin group.
Hemoglobin and RBC
The baseline values of hemoglobin were statistically different between the two groups (p = 0.03). No statistically significant difference was observed between the decreases from baseline at any time point (p-values 0.08, 0.26, and 0.82, respectively). The analytical data appear in Table 3. Data from RBC (not shown) were very similar to data on hemoglobin.
Correlation between metformin dose and radioprotective effects
Then, whether the radioprotective effect of metformin was dose-dependent was examined. Fifteen patients of the study cohort were treated with a metformin dose up to 1000 mg/day, 21 patients with a dose ranging between 1001 and 2000 mg/day, and four patients with a dose exceeding 2000 mg/day. Repeated-measures two-way ANOVA was used to adjust for within-patient correlation over time for each CBC parameter. No statistically significant correlation was found between the dose of metformin and its radioprotective properties over time. More specifically, the corresponding p-values for this correlation were 0.83 for WBC, 0.88 for ANC, 0.49 for ALC, 0.72 for platelets, and 0.69 for hemoglobin.
Discussion
Hematopoietic toxicity is a well-established side effect of 131I tx (13,14). Several methods have been implemented in an effort to limit the effects of 131I to the bone marrow and peripheral blood cells. Dosimetry is used to determine the maximum safe dose of RAI that can be delivered without causing permanent damage to the bone marrow, which corresponds to <200 rads absorption from the bone marrow. However, the use of dosimetry does not preclude hematopoietic toxicity of varying degrees. Padovani et al. recently reported that CBC are frequently abnormal in patients who have received dosimetry-guided 131I tx (15). As a result, it is apparent that new or modified strategies are necessary in order to prevent and/or correct the hematopoietic toxicity caused by 131I.
To the authors' knowledge, this study is the first to demonstrate that metformin has radioprotective properties in humans. More specifically, the results of this study demonstrated that the significant hematopoietic toxicity caused by 131I tx was attenuated by the concomitant use of metformin. Moreover, patients who were taking metformin during 131I tx experienced a faster recovery in their blood counts compared with the control group. The radioprotective effect of metformin was most prominent in total WBC and ANC, and was not dose dependent. Metformin did not seem to be radioprotective for RBC. However, a potential effect could be masked by the non-sensitivity of RBC to 131I, as this is the blood-cell lineage that was least affected by RAI tx.
The mechanism by which the metabolic drug metformin exerts its radioprotective effect is not fully elucidated. Metformin is recognized as a mitochondrial complex I inhibitor (16), and an activator of AMPK (17). It is well-established that ionizing radiation destroys both mature blood cells and hematopoietic progenitor/stem cells in the bone-marrow compartment (18). Experimental studies demonstrated that ionizing radiation induces senescence and apoptosis of the hematopoietic stem cells (HSC) resulting in exhaustion of the stem-cell pool (19). It has been shown that reprogramming of cellular metabolism from oxidative phosphorylation to glycolysis resulted in a remarkable preservation of long-term HSC through hyperactivation of AMPK (20). Inhibition of mitochondrial metabolism by metformin also resulted in activation of AMPK and helped maintain stem cells in culture (20). It is possible that the protective effects of metformin on bone-marrow function in thyroid cancer patients treated with 131I could be related to an effect of metformin on AMPK signaling in HSC. This hypothesis needs to be confirmed by further experimental studies.
The above-described hypothesis does not preclude the existence of alternative mechanisms that could explain the radioprotective effects of metformin. 131I exerts its therapeutic effects by emitting beta particles of radiation, which cause DNA damage and production of reactive oxygen species (ROS) (21,22). There are reports in the literature that metformin in vitro prevents short-term oxidant-induced DNA damage in lymphocytes by decreasing apoptotic DNA fragmentation and DNA strand breaks (23). Consistent with these reports, Xu et al. reported that administration of metformin to mice significantly attenuated irradiation-induced increases in ROS production and DNA damage and upregulation of NADPH oxidase 4 expression in bone-marrow HSC, thus inhibiting chronic oxidative stress and senescence in HSC (24). Together, these reports could potentially explain the attenuation of the drop in CBC that was observed in patients treated with metformin.
One of the key findings of this study is that the radioprotective effect of metformin was most prominent in WBC, especially ANC. It is believed that this phenomenon is due to the inherent sensitivity of WBC to 131I. Several groups have demonstrated that WBC are more susceptible to 131I than other blood cells are (6,13). As a result, it is reasonable to expect that the greatest radioprotective effect is observed in the most radiosensitive cell lineage. The fact that the radioprotective effects of metformin were not dose dependent is discordant with what has been reported in the literature about the drug's anticancer and antidiabetic effects (25).
The current study is novel and could be of great clinical significance, as these results could potentially be extrapolated to other forms of therapeutic radiation used in diverse clinical settings. Despite its strengths, this study has certain limitations. First, due to the retrospective nature of this study, there is the potential for bias that cannot be accounted for. The control group of this study is more heterogeneous than the metformin group, as it contains both diabetic and non-diabetic patients, while the metformin group by definition contains only diabetic patients. To account for this difference, the control group included as many diabetic patients (who met the inclusion criteria) as were found in the records, and then non-diabetic patients were included in order to reach approximately the number of patients in the metformin group. The two groups were comparable in all parameters, as demonstrated in Table 1, but the potential for bias exists. Moreover, CBC determinations were not always performed at exactly the same time points, but it is believed that the categorization did not influence the results of the study. Finally, because of the small number of patients, this pilot study is underpowered in order to examine whether metformin prevents serious hematopoietic toxicity (i.e., leukemia, myelodysplasia) and further study is warranted.
In summary, it has been demonstrated that metformin attenuated hematopoietic toxicity caused by 131I tx in patients with differentiated thyroid cancer. The data highlight that metformin has the potential to be used as a novel radioprotective agent for the bone marrow. However, larger-scale prospective studies are needed in order to elucidate the mechanism that is responsible for the radioprotective properties of metformin, to verify these results in other forms of therapeutic radiation, and to examine whether metformin's radioprotective properties extend to other tissues besides the hematopoietic system.
Acknowledgments
The authors would like to thank Dr. Kristbjorn Gudmundsson (Pediatrics, USUHS, Bethesda, MD) for providing valuable insight, and discussion. The results of this study were presented in form of an oral presentation in the Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI 2015—Baltimore, MD—6/2015).
Author Disclosure Statement
The authors have nothing relevant to disclose.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. 1998. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger MJ. 1998. Papillary and follicular thyroid carcinoma. N Engl J Med 338:297–306 [DOI] [PubMed] [Google Scholar]
- 3.Sherman SI. 2003. Thyroid carcinoma. Lancet 361:501–511 [DOI] [PubMed] [Google Scholar]
- 4.Van Nostrand D. 2009. The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid 19:1381–1391 [DOI] [PubMed] [Google Scholar]
- 5.Van Nostrand D, Freitas J. 2006. Side effects of I-131 for ablation and treatment of well-differentiated thyroid carcinoma. In: Wartofsky L, Van Nostrand D. (eds) Thyroid Cancer: A Comprehensive Guide to Clinical Management. Humana Press, Totowa, NJ, pp 459–484 [Google Scholar]
- 6.Bikas A, Burman K, Desale S, Mete M, Wartofsky L, Van Nostrand D 2015 Effects of dosimetry-guided 131I therapy for metastatic differentiated thyroid cancer on complete blood counts. Presented at the 97th Annual Meeting, The Endocrine Society, San Diego, CA [Google Scholar]
- 7.Klubo-Gwiezdzinska J, Costello J, Jr, Patel A, Bauer A, Jensen K, Mete M, Burman KD, Wartofsky L, Vasko V. 2013. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab 98:3269–3279 [DOI] [PubMed] [Google Scholar]
- 8.Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. 2014. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res 182:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G, Tsakiridis T. 2013. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer 108:2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song CW, Lee H, Dings RP, Williams B, Powers J, Santos TD, Choi BH, Park HJ. 2012. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep 2:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng T, Li L, Ling S, Fan N, Fang M, Zhang H, Fang X, Lan W, Hou Z, Meng Q, Jin D, Xu F, Li Y. 2015. Metformin enhances radiation response of ECa109 cells through activation of ATM and AMPK. Biomed Pharmacother 69:260–266 [DOI] [PubMed] [Google Scholar]
- 12.Miller RC, Murley JS, Grdina DJ. 2014. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat Res 181:464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molinaro E, Leboeuf R, Shue B, Martorella AJ, Fleisher M, Larson S, Tuttle RM. 2009. Mild decreases in white blood cell and platelet counts are present one year after radioactive iodine remnant ablation. Thyroid 19:1035–1041 [DOI] [PubMed] [Google Scholar]
- 14.Fard-Esfahani A, Emami-Ardekani A, Fallahi B, Fard-Esfahani P, Beiki D, Hassanzadeh-Rad A, Eftekhari M. 2014. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl Med Commun 35:808–817 [DOI] [PubMed] [Google Scholar]
- 15.Padovani RP, Tuttle RM, Grewal R, Larson SM, Boucai L. 2014. Complete blood counts are frequently abnormal 1 year after dosimetry-guided radioactive iodine therapy for metastatic thyroid cancer. Endocr Pract 20:213–220 [DOI] [PubMed] [Google Scholar]
- 16.Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, Chandel NS. 2014. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 3:e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng S, Cao J, He Q, Xiong L, Chang E, Radovick S, Wondisford FE, He L. 2015. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J Biol Chem 290:3793–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ. 1995. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 31:1319–1339 [DOI] [PubMed] [Google Scholar]
- 19.Shao L, Wang Y, Chang J, Luo Y, Meng A, Zhou D. 2013. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl Cancer Res 2:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Zheng H, Yu WM, Cooper TM, Bunting KD, Qu CK. 2015. Maintenance of mouse hematopoietic stem cells ex vivo by reprogramming cellular metabolism. Blood 125:1562–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doai M, Watanabe N, Takahashi T, Taniguchi M, Tonami H, Iwabuchi K, Kayano D, Fukuoka M, Kinuya S. 2013. Sensitive immunodetection of radiotoxicity after iodine-131 therapy for thyroid cancer using γ-H2AX foci of DNA damage in lymphocytes. Ann Nucl Med 27:233–238 [DOI] [PubMed] [Google Scholar]
- 22.Wyszomirska A. 2012. Iodine-131 for therapy of thyroid diseases. Physical and biological basis. Nucl Med Rev Cent East Eur 15:120–123 [PubMed] [Google Scholar]
- 23.Kanigür-Sultuybek G, Ozdas SB, Curgunlu A, Tezcan V, Onaran I. 2007. Does metformin prevent short-term oxidant-induced DNA damage? In vitro study on lymphocytes from aged subjects. J Basic Clin Physiol Pharmacol 18:129–140 [DOI] [PubMed] [Google Scholar]
- 24.Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y, Zhang H, Lu L, Li C, Huang S, Xing Y, Zhou D, Meng A. 2015. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med 87:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. 2010. Metformin: taking away the candy for cancer? Eur J Cancer 46:2369–2380 [DOI] [PubMed] [Google Scholar]