Main Text
A capsid variant of adeno-associated virus (AAV) serotype 9 called AAV-PHP.B is highly permeable to the blood-brain barrier (BBB) in C57BL/6J mice. A recent study, however, showed failure of BBB penetration by AAV-PHP.B in BALB/c mice and suggested that the BBB transmission of AAV-PHP.B might be limited to the C57BL/6J strain. Here, we further characterized the BBB permeability of AAV-PHP.B in different mouse strains. As reported, intravenously infused AAV-PHP.B did not transduce brains of BALB/c mice. However, different inbred strains showed comparable (C57BL/6N, SJL/J, and FVB/N) or even greater (DBA/2) CNS transduction than C57BL/6J. The F1 hybrids of “permeable” C57BL/6N or “highly permeable” DBA/2 and “impermeable” BALB/c were entirely impermeable to AAV-PHP.B, indicating that the impermeability was dominantly inherited. Intriguingly, outbred Institute of Cancer Research (ICR) mice were divided into two discrete “permeable” and “impermeable” groups. These results indicate that the BBB permeability of AAV-PHP.B is not restricted to the C57BL/6J strain, but rather that the impermeability is limited to particular inbred strains such as BALB/c and its filial generation. Comparing the BBB structure between BALB/c and other inbred strains may provide a key insight for the mechanism that defines the BBB transmission efficacy of AAV-PHP.B.
It is highly challenging to engineer a viral vector that penetrates the BBB following intravenous administration and efficiently transduces the mammalian CNS. In 2016, Deverman et al.1 reported such a vector, called PHP.B, which was a capsid variant of AAV serotype 9 (AAV9). PHP.B was isolated using the C57BL/6J mouse strain by screening capsid variants that crossed the BBB following intravenous infusion of the capsid library, with each variant having a different seven-amino acid insertion. The finding of PHP.B had a significant impact on scientists and clinicians in the field of gene therapy because it has great promise in delivering therapeutic genes to the human CNS without invasive procedures, simply by intravenously injecting PHP.B.
Using common marmosets, we recently tested whether PHP.B crossed the BBB and efficiently transduced the non-human primate CNS.2 It was proven, however, that intravenously injected PHP.B transduced the adult marmoset CNS with very low efficacy, which was almost comparable to the original AAV9 vectors. A different group reported similar results in which loss of efficient CNS transduction in a rhesus macaque following intravenous injection of PHP.B was demonstrated.3 In addition, this group provided a more notable finding: efficient CNS transduction following intravenous infusion of PHP.B was not found in a different, commonly used mouse strain, BALB/c. Based on this finding, they suggested that the remarkably enhanced CNS tropism of PHP.B was restricted to a mouse of C57BL/6J background, the model in which it was selected. Because this result was quite unexpected and surprising to us, we decided to verify their result and further characterize the CNS tropism of PHP.B using BALB/c and other inbred and outbred mouse strains.
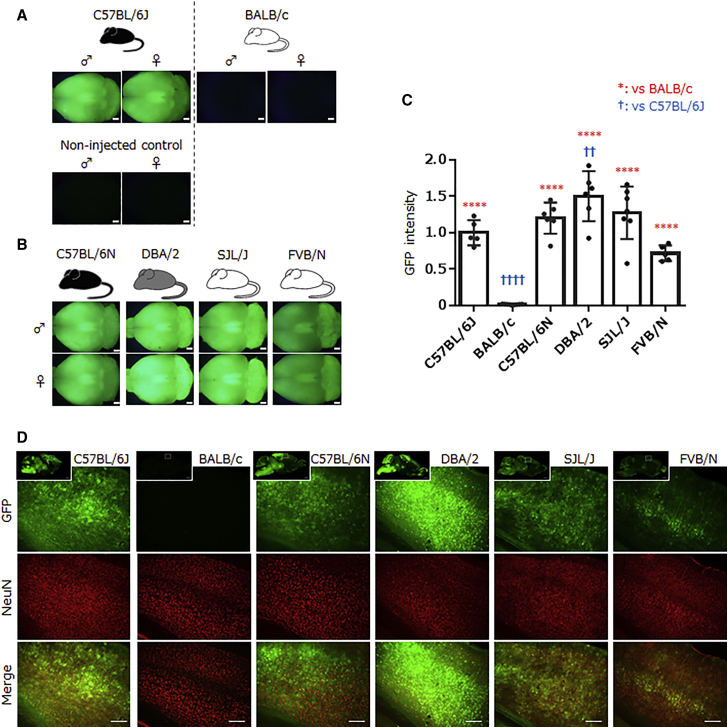
First, we compared the CNS tropism of PHP.B between C57BL/6J and BALB/c strains. PHP.B expressing EGFP under the control of a hybrid form of the chicken β-actin (CBh) promoter,4 which is composed cytomegalovirus early enhancer and chicken β-actin promoter, was infused into C57BL/6J (8 weeks of age) and BALB/c (7 weeks of age) mice through the orbital sinus. Two weeks after the injection, GFP expression in the brain was examined. As previously reported,1 robust GFP expression was observed throughout the CNS of the C57BL/6J mice (Figures 1A and S1A, left). In contrast, brains from BALB/c mice showed almost no GFP fluorescence, which was almost the same as non-injected control brains of C57BL/6J (Figures 1A, S1B, and S1C, right), in accordance with the previous report.3
Figure 1.
Efficient CNS Transduction by Systemic Infusion of PHP.B in Different Inbred Strains except for BALB/c
Adult mice of various inbred strains received intravenous injection of PHP.B expressing GFP under the control of the CBh promoter. Brains were observed 2 weeks post-injection. (A) The native GFP fluorescence images of whole brains from AAV-PHP.B-injected and non-injected C57BL/6J and injected BALB/c mice. In contrast to robust GFP expression in brains of C57BL/6J mice, those of BALB/c emitted no GFP fluorescence. Scale bars: 1 mm. (B) The consistent transduction of the brains from other inbred strains. Native GFP fluorescent images of the whole brains from C57BL/6N, DBA/2, SJL/J, and FVB/N mice. Upper and lower images were obtained from male and female mice, respectively. The hair color of each mouse strain is displayed as an illustration above each brain image. Scale bars: 1 mm. (C) A summarized graph showing the GFP fluorescent intensity from whole brains of each mouse strain. Black plots represent fluorescence intensity from individual mouse brains relative to the mean value (1.0) from C57BL/6J mouse brains. Error bars indicate SEM. ****p < 0.0001 (versus BALB/c), ††p < 0.01, and ††††p < 0.0001 (versus C57BL/6J) by ANOVA with Dunnett’s post hoc test. (D) The efficient transduction of cortical neurons and glia in PHP.B-treated mice, except for BALB/c mice. Sagittal sections of the whole brains from the strains indicated were immunostained for GFP (insets) and NeuN. The top, center, and bottom images are high magnification of the cerebral cortex immunostained for GFP and NeuN and the superposition, respectively. Scale bars: 1 mm (insets) and 200 μm.
We next aimed to clarify whether the enhanced CNS tropism of PHP.B was restricted to C57BL/6J mice. To explore this, we injected PHP.B intravenously into four different inbred strains: C57BL/6N (7 to 8 weeks of age), DBA/2 (7 weeks of age), SJL/J (4 weeks of age), and FVB/N (15 or 18 weeks of age) (see Table 1 for details). We examined the brains at 2 weeks after the injection. Surprisingly, strong GFP expression was observed in all four mouse strains, which was comparable to or even stronger than that in C57BL/6J mice (Figures 1B and S1D–S1G). Statistical analyses showed that GFP fluorescence intensity of whole brains was comparable between C57BL/6J mice and C57BL/6N (p = 0.4960), SJL/J (p = 0.1879), or FVB/N (p = 0.2305) mice, while it was significantly greater in DBA2 mice than in C57BL/6J mice (p = 0.0055) (Figure 1C). To verify that transduction was not limited to vascular endothelial cells, we produced sagittal sections of the PHP.B-transduced brains, followed by double immunostaining for GFP and NeuN. High magnification images of the cerebral cortex showed GFP expression in cortical cells, including numerous NeuN-positive neurons, in all strains that showed robust transduction of the whole brain (Figure 1D), indicating efficient permeation of the PHP.B through the BBB in various strains except for BALB/c. These results indicate that the increased CNS tropism of PHP.B in the C57BL/6J strain was shared with other inbred strains and that the limited CNS tropism was likely restricted to the BALB/c strain.
Table 1.
Profiles and Numbers of Mice, Viral Titers, and Duration between the Viral Injection to Sacrifice
| Strain | Age (Weeks) | Number of Mice |
Average of BW (g) |
Dose of Viral Vector (× 1011 vg) | Average Viral Titer (× 1012 vg/kg) |
Sacrifice (dai) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Male | Female | |||||
| Inbred | C57BL/6J | 7 or 8 | 8 | 9 | 17 | 22.1 | 20.1 | 1.0 | 4.5 | 5.0 | 14 |
| Inbred | BALB/c | 7 | 9 | 3 | 12 | 23.0 | 19.7 | 1.0 | 4.4 | 5.1 | 14 |
| Inbred | C57BL/6N | 7 | 3 | 3 | 6 | 22.5 | 18.9 | 1.0 | 4.5 | 5.3 | 14 |
| Inbred | DBA/2 | 7 | 3 | 3 | 6 | 22.7 | 18.7 | 1.0 | 4.4 | 5.4 | 14 |
| Inbred | SJL/J | 4 | 5 | 2 | 7 | 18.6 | 16.1 | 1.0 | 5.4 | 6.2 | 14 |
| Inbred | FVB/N | 15 or 18 | 4 | 1 | 5 | 31.0 | 22.0 | 1.0 | 3.2 | 4.5 | 14 |
| Hybrid | CB6F1 | 7 | 6 | 3 | 9 | 31.6 | 26.1 | 1.0 | 3.6 | 4.4 | 14 |
| Hybrid | CD2F1 | 8 | 3 | 3 | 6 | 25.7 | 20.5 | 1.0 | 3.9 | 4.9 | 14 or 15 |
| Closed colony | ICR | 7 or 8 | 17 | 8 | 25 | 27.4 | 22.6 | 1.0 | 3.2 | 3.8 | 14 or 17 |
BW, body weight; dai, days after injection; vg, viral genome.
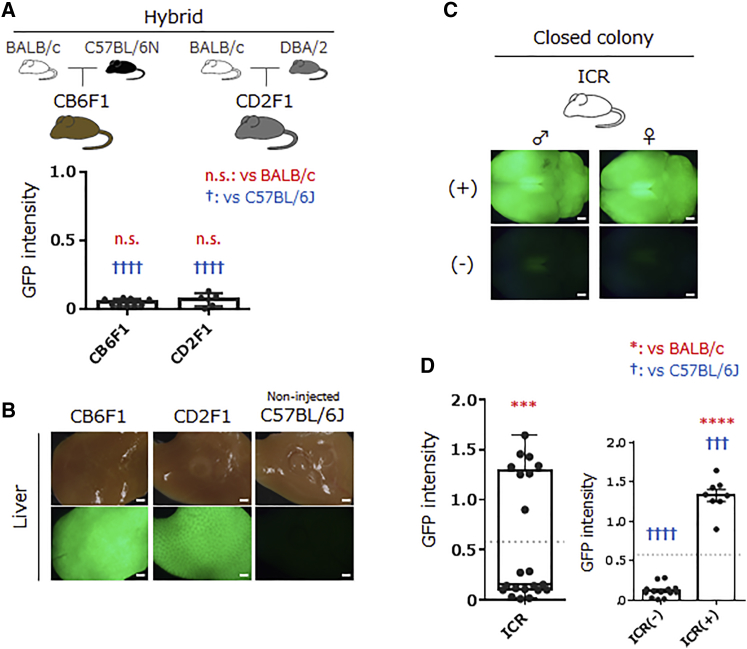
To gain insight into the strain-dependent BBB permeability of PHP.B, we tested filial descendants that were obtained by crossing “permeable” mouse strains with the “impermeable” BALB/c mouse. First, we used 7-week-old CB6F1 mice, F1 mice from “impermeable” BALB/c, and “permeable” C57BL/6N. Intravenous administration of PHP.B totally failed to transduce the brains of all nine CB6F1 mice tested (Figures 2A and S1H). Statistical analysis of GFP fluorescence intensity confirmed the significant difference between C57BL/6J and CB6F1 (p < 0.0001) and no difference between CB6F1 and BALB/c (p = 0.6673) (Figure 2A). Similarly, PHP.B did not cross the BBB in 8-week-old CD2F1 mice, F1 mice from “impermeable” BALB/c, and “highly permeable” DBA2 (p < 0.0001, versus C57BL/6J and p = 0.4016, versus BALB/c) (Figures 2A and S1I). In contrast, the livers of both CB6F1 and CD2F1 mice expressed robust GFP (Figure 2B). These results suggest that the BBB-impermeable feature of PHP.B in the BALB/c background is dominantly inherited and that the limited CNS tropism is likely restricted to BALB/c mice and their filial descendants.
Figure 2.
Dominant Inheritance of BBB Impermeability of PHP.B in the BALB/c Filial Generation and Separation of Two Discrete Groups of Outbred ICR Mice in Terms of CNS Transduction by Intravenously Infused PHP.B
(A) A summarized graph showing the GFP fluorescence intensity from whole brains of hybrid strains. PHP.B was systemically injected into CB6F1 and CD2F1 mice that were obtained by crossing BBB-impermeable BALB/c mice with permeable C57BL/6N (CB6F1) or highly permeable DBA2 (CD2F1) mice. The hair color of each mouse strain is displayed as an illustration. Black plots in the graph represent fluorescence intensity from individual mouse brains relative to the mean value (1.0) from C57BL/6J mouse brains. Not significant (n.s.) (versus BALB/c) and ††††p < 0.0001 (versus C57BL/6J) by ANOVA with Dunnett’s post hoc test. (B) CB6F1 and CD2F1 mice that received intravenous PHP.B injection showed robust GFP expression in the livers (left and center). The right image shows the liver from a non-injected control C57BL/6J mouse. (C) Representative native GFP fluorescence images of whole brains from 7- or 8-week-old ICR mice 2 weeks after intravenous injection of PHP.B expressing GFP under the CBh promoter. The ICR mice had white hair, as illustrated. Among 21 mice tested, about one third of the mice showed bright GFP fluorescence, while the other two thirds emitted almost no fluorescence (see Figures S1J and S1K). (D) A summarized graph showing the GFP fluorescence intensity from whole brains of the ICR mice, which was normalized to the mean value (1.0) of C57BL/6J (Figure 1). Black plots represent fluorescence intensity from individual mouse brains. All 21 ICR mice were plotted in the left graph, while the right graph shows the 21 mice separated into two groups based on whether fluorescence intensity was above or below the mean value (0.580) of GFP fluorescence intensity from ICR mouse brains: a group that showed intense GFP fluorescence [8 mice named ICR(+)] and another group with no or only little GFP fluorescence [13 mice named ICR(–)]. ***p < 0.001 and ****p < 0.0001 (versus BALB/c), and ††††p < 0.0001 and †††p = 0.0004 (versus C57BL/6J) by ANOVA with Dunnett’s post hoc test. (A and D) Error bars indicate SEM. (B and C) Scale bars: 1 mm.
Next, we asked what would happen if we use an outbred strain, which was bred by random mating within the stock, and thus contains more genetic diversity than inbred mice. To answer this question, we used outbred ICR mice. PHP.B was similarly infused into 21 ICR mice (7 to 8 weeks of age), and the brains were examined 2 weeks after the viral injection. Intriguingly, about one third of the mice showed bright GFP fluorescence, while the rest of the mice exhibited almost no fluorescence (Figures 2C, S1J, and S1K), indicating that the ICR mice could be divided into two groups: one efficiently transduced by intravenously administered PHP.B [named ICR(+)], and another one having little BBB permeability of PHP.B [named ICR(–)] (Figure 2D). Statistical analysis by one-way ANOVA with Dunnett’s post hoc test revealed that GFP fluorescence intensity from ICR(+) mice was significantly stronger than that from age-matched C57BL/6J mice (p = 0.0004) and that there was no significant difference in the GFP fluorescence intensity between ICR(–) mice and BALB/c mice (p = 0.1728) (Figure 2D).
The mechanism underlying PHP.B entry from peripheral circulation to the brain in divergent mouse strains remains unknown. There are two possibilities that would account for PHP.B penetration of the BBB. One is transcytosis of the viruses through the brain microvascular endothelial cells (BMECs), as has been proven using BMEC cultures and AAV9 vectors.5 Another possibility is disruption of the integrity of the BBB, which is composed of tight and adherens junctions. Indeed, many neurotropic viruses enter the brain by the latter approach.6 The capsid variant of PHP.B may gain a capacity to bind to a receptor on the BMECs, which triggers signaling for cytoskeletal rearrangements and possibly leads to disruption of the tight junction.6 If this is the case, AAV9 particles co-administered with PHP.B would cross the disrupted BBB more efficiently with PHP.B. We verified this possibility by intravenously infusing AAV9 vectors with PHP.B.
First, C57BL/6J mice received intravenous injection of conventional AAV9 vectors alone, which were designed to express mCherry under the control of the CBh promoter. Although the efficiency is much less than that of PHP.B, AAV9 vectors have some capacity of crossing the BBB and transducing brain cells in C57BL/6 mice. However, because of the low vector dose, we observed only slight CNS transduction along with efficient transduction of peripheral organs (Figures S2C and S2G). We then confirmed that intravenous injection of PHP.B with a similar viral titer to that of the AAV9 vectors resulted in very efficient transduction of both the peripheral organs and the brain (Figures S2B and S2F). Subsequently, we intravenously injected C57BL/6J mice with the two-virus-mixture. The results revealed that the brain expressed GFP but not mCherry (Figure S2D), in contrast to efficient expression of both mCherry and GFP in the peripheral organs, including the liver (Figure S2H). These results suggest that co-administration of PHP.B did not enhance BBB permeability of AAV9 vectors and that PHP.B likely permeates the BBB by transcytosis through the BMECs.
Our results revealed that inbred mouse strains could be divided into two discrete groups in terms of BBB penetration by PHP.B. One group shows high BBB permeability (most inbred strains) and the other exhibits very low BBB permeability (BALB/c). The hybrid mice (CB6F1 and CD2F1) that were obtained by crossing BBB-permeable C57BL/6N or DBA2 mice with BBB-impermeable BALB/c mice dominantly inherited the BBB-impermeable nature from BALB/c mice. In addition, outbred ICR mice were divided into two groups of marked and poor BBB permeability. These results imply that marked BBB permeability of PHP.B could possibly be regulated by a single gene. The presence or absence of a molecule encoded by the gene may regulate the binding affinity of PHP.B to putative membrane receptors on BMECs. In this context, comparing molecules expressed in the BMECs from “impermeable” BALB/c mice with those from “permeable” strains, such as C57BL/6J mice, would be a promising approach to identify the molecule that determines PHP.B permeability through the BBB. Identification of such a molecule would hopefully lead to identification of AAV capsid variants that allow efficient CNS transduction in other animal species.
Intravenous administration of AAV vectors coated with a capsid that efficiently crosses the human BBB is a promising therapeutic intervention that allows for noninvasive transfer of a therapeutic gene to the whole CNS. However, since human populations have genetic variations similar to or larger than outbred mouse strains,7 a capsid variant that efficiently crosses the BBB in some individuals may show no BBB permeability in others. Unraveling a molecular mechanism that mediates efficient crossing of the BBB by AAV vectors may enable us to design BBB-permeable AAV capsids that are specific to each individual.
Author Contributions
Y.M., A.K., and H.H. conceived, designed, and conducted experiments; A.K. constructed AAV vectors; Y.M., M.T., S.H., T.M., and R.M. injected AAV-PHP.B into the orbital sinus of mice, sacrificed them, and captured pictures of whole brains; Y.M. summarized and analyzed experimental data; and Y.M. and H.H. drafted the manuscript. All authors shared information and commented on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Asako Ohnishi and Junko Sugiyama for production and titration of AAV-PHP.B and raising FVB/N mice, respectively. Dr. Ryosuke Kaneko (Gunma University Bioresource Center) kindly provided SJL/J mice. This research is supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and Development (AMED) under the grant no. JP18dm0207057.
Footnotes
Supplemental Information can be found with this article online at https://doi.org/10.1016/j.ymthe.2019.02.016.
Supplemental Information
References
- 1.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A., Wu W.L., Yang B., Huber N., Pasca S.P., Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuzaki Y., Konno A., Mochizuki R., Shinohara Y., Nitta K., Okada Y., Hirai H. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci. Lett. 2018;665:182–188. doi: 10.1016/j.neulet.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 3.Hordeaux J., Wang Q., Katz N., Buza E.L., Bell P., Wilson J.M. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Mol. Ther. 2018;26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray S.J., Foti S.B., Schwartz J.W., Bachaboina L., Taylor-Blake B., Coleman J., Ehlers M.D., Zylka M.J., McCown T.J., Samulski R.J. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther. 2011;22:1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merkel S.F., Andrews A.M., Lutton E.M., Mu D., Hudry E., Hyman B.T., Maguire C.A., Ramirez S.H. Trafficking of adeno-associated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. J. Neurochem. 2017;140:216–230. doi: 10.1111/jnc.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels B.P., Klein R.S. Viral sensing at the blood-brain barrier: new roles for innate immunity at the CNS vasculature. Clin. Pharmacol. Ther. 2015;97:372–379. doi: 10.1002/cpt.75. [DOI] [PubMed] [Google Scholar]
- 7.Aldinger K.A., Sokoloff G., Rosenberg D.M., Palmer A.A., Millen K.J. Genetic variation and population substructure in outbred CD-1 mice: implications for genome-wide association studies. PLoS ONE. 2009;4:e4729. doi: 10.1371/journal.pone.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.