Abstract
Venezuelan equine encephalitis virus (VEEV) is a known biological defense threat. A live-attenuated investigational vaccine, TC-83, is available, but it has a high non-response rate and can also cause severe reactogenicity. We generated two novel VEE vaccine candidates using self-amplifying mRNA (SAM). LAV-CNE is a live-attenuated VEE SAM vaccine formulated with synthetic cationic nanoemulsion (CNE) and carrying the RNA genome of TC-83. IAV-CNE is an irreversibly-attenuated VEE SAM vaccine formulated with CNE, delivering a TC-83 genome lacking the capsid gene. LAV-CNE launches a TC-83 infection cycle in vaccinated subjects but eliminates the need for live-attenuated vaccine production and potentially reduces manufacturing time and complexity. IAV-CNE produces a single cycle of RNA amplification and antigen expression without generating infectious viruses in subjects, thereby creating a potentially safer alternative to live-attenuated vaccine. Here, we demonstrated that mice vaccinated with LAV-CNE elicited immune responses similar to those of TC-83, providing 100% protection against aerosol VEEV challenge. IAV-CNE was also immunogenic, resulting in significant protection against VEEV challenge. These studies demonstrate the proof of concept for using the SAM platform to streamline the development of effective attenuated vaccines against VEEV and closely related alphavirus pathogens such as western and eastern equine encephalitis and Chikungunya viruses.
Keywords: SAM, self-amplifying RNA, VEEV vaccine, RNA vaccine, CNE
Yu and colleagues developed two self-amplifying mRNA (SAM) vaccines for Venezuelan equine encephalitis virus (VEEV). Both vaccines were immunogenic and protective, and one provided a complete protection against VEEV challenge in mice. These studies demonstrate the use of SAM in streamlining vaccine development for VEEV and other alphavirus pathogens.
Introduction
Venezuelan equine encephalitic virus (VEEV) is an important emerging zoonotic pathogen. Although human infection with VEEV typically causes a severe but self-limiting disease characterized by symptoms such as headache, fever, and malaise, some cases result in encephalitis. The overall human mortality rate (<1%) is low, but immune-compromised individuals and infants are particularly at risk. VEEV is also considered a biological threat agent that could be intentionally introduced by aerosol to cause widespread disease.1 Several VEE vaccines have been developed and used under the investigational new drug (IND) status in military personnel and laboratory workers at risk for VEEV infection. These vaccines include the live-attenuated viral vaccine TC-83 and a formalin-inactivated version of TC-83 known as C-84 that is used as a boost to improve the efficacy and duration of the immunity in individuals who have received the TC-83 vaccine.2 TC-83 has a long record of human clinical use as an FDA IND.3 Nonetheless, safety concerns remain, as TC-83 is reactogenic in approximately 20% of recipients,2 likely in part due to the reversion of point mutations that result in the production of virulent virus in vaccinees. In addition, approximately 18% of vaccinees do not seroconvert, indicating that TC-83 induces suboptimal immunogenicity in humans.2 An experimental vaccine V3526, which is also a live-attenuated viral vaccine, has been demonstrated to be less neurovirulent than TC-83 in mice and non-human primates.4 However, safety regarding neurovirulence of V3526 remains to be tested in humans,4, 5, 6 and the antibody response elicited by V3526 does not neutralize heterologous variety of VEEV strains.4 Due to the shortfalls associated with these live-attenuated viral vaccine candidates, new VEE vaccines that are more efficacious and less reactogenic would be highly desirable.
Nucleic-acid-based vaccines have the benefits of a generic delivery platform and in situ expression of the antigens.7, 8, 9, 10, 11, 12, 13, 14 Moreover, immunization with nucleic acid vaccines can mimic natural infections by live or attenuated organisms, inducing both humoral and cellular immunity.7, 15 Vaccine platforms derived from a replicating RNA viral genome are particularly attractive because replicating RNA itself potently stimulates the innate immune system by engaging pattern recognition receptors.16, 17, 18, 19 RNA amplification gives rise to many copies of transcripts that are used as templates to drive robust antigen expression. Furthermore, antigen expression from RNA vaccines peaks in hours and is followed by a rapid decay, resembling acute viral infection, which is effective for induction of robust antigen-specific immune responses.7 To take advantage of these unique attributes of a viral self-amplifying RNA-based vaccine, we have recently developed an innovative RNA vaccine platform based on non-viral delivery of self-amplifying mRNA (SAM).8 This vaccine platform is based on a synthetic RNA molecule derived from the positive-stranded alphavirus RNA genome. Previously, the alphavirus RNA genome was used for the viral replication particle (VRP)-based vaccine platform.20 However, the cell-culture-based process of producing VRP-based vaccines is complex and also has the potential risk of generating infectious virus due to spontaneous recombination with the helper sequence. To overcome these limitations, the synthetic RNA molecule is formulated with synthetic, non-viral delivery systems such as lipid nanoparticles (LNPs)8, 21 or cationic nanoemulsions (CNEs).22 CNE has the advantage that it can be prepared and stockpiled separately from the RNA for later use,23, 24 which would be particularly useful for the rapid response to a pandemic outbreak or emerging infectious threat such as VEEV that could also be used as a biological weapon. Previous work has demonstrated that CNE-formulated SAM vaccines elicit anticipated immune responses against various infectious targets such as influenza virus, HIV, cytomegalovirus, and malaria in preclinical animal studies.22, 25, 26, 27 For the experiments described in this article, we used the CNE delivery system, which is composed of the cationic lipid DOTAP (1,2-dioleoyl-sn-glycero-3-phosphocholine) and emulsified with the constituents of MF59, an emulsion adjuvant. MF59 has a long history as a vaccine adjuvant, has an established clinical safety profile, and is well tolerated in children, adults, and the elderly.28 The DOTAP imparts a cationic charge to the surface of the nanoemulsion to enable adsorption of the positively charged RNA, and this protects the RNA from degradation during delivery.22
We applied the SAM vaccine concept to develop a novel VEE vaccine by delivering genetically modified VEEV genomes with CNE. We designed two VEE vaccines based on the replication capacity of the delivered viral genome. A live-attenuated VEE (LAV) SAM vaccine, LAV-CNE, was developed to deliver a CNE-formulated, full-length genome of the TC-83 live-attenuated vaccine strain of VEEV to overcome the need for complex cell culture production of the TC-83 virus vaccine. An irreversibly attenuated VEE SAM vaccine, IAV-CNE, was developed to deliver a CNE-formulated TC-83 viral genome with the capsid gene deleted to eliminate any possible reversion to a virulent virus after vaccination. In the studies described here, we demonstrated that LAV-CNE vaccines elicited virus-specific neutralizing antibody (NAb) titers in mice and completely protected the animals against wild-type VEEV aerosol challenge after two immunizations. IAV-CNE vaccines elicited lower NAb titers than LAV-CNE vaccines in mice but still conferred significant protection against virus challenge. Our results provide proof-of-concept for using this synthetic vaccine platform to develop efficacious attenuated vaccines against VEEV and closely related alphavirus pathogens such as western and eastern equine encephalitis viruses (WEEV and EEEV) and Chikungunya virus.
Results
In Vitro Characterization of LAV and IAV RNAs
Sequences of LAV and IAV RNA were inserted into DNA constructs for use as the templates for the synthesis of LAV and IAV RNAs (Figure 1A). Mutations in the sub-genomic 5′ UTR and between the C-terminal region of E1 and the 3′ UTR as well as synonymous mutations in nsP1 were introduced to facilitate cloning procedures (see Materials and Methods). To confirm that these mutations do not alter viral replication, we compared the replication phenotypes of the LAV-derived virus (LAVV) with a reference stock of TC-83 virus. Transfection of LAV RNA resulted in infectious virus that had the same plaque morphology and replication kinetics as TC-83 (Figure 1B). Therefore, the mutations introduced into the LAV RNA to facilitate production of a template for RNA synthesis did not alter the biological characteristics of the launched virus as compared to TC-83.
Figure 1.
Generation of RNA molecules for LAV or IAV
(A) Schematic representation of the synthetic RNA genome of live-attenuated VEE vaccine (LAV) and irreversibly attenuated VEE vaccine (IAV). Both LAV and IAV encode the viral glycoprotein genes, but the capsid (CP) gene is deleted in IAV. (B) Viral growth of LAV-derived virus (LAVV). Infectious viral particles were produced from BHK cells transfected with LAV RNA, and the plaque morphology (left) and multistep growth kinetics (right) were compared to TC-83 virus. Standard deviations of titers are indicated in the graph. (C) Analysis of infectious particle production from LAV or IAV RNA. BHK cells were electroporated with LAV RNA, IAV RNA, or unrelated (control) RNA, and intracellular VEEV antigens were detected with α-VEEV hyperimmune ascites fluid by indirect immunofluorescence assay at 24 h post-transfection (left). The presence of infectious viral particles in cell culture supernatants was analyzed by plaque assay (right). (D) Immunoblot analysis of viral protein expression in BHK cells transfected with LAV or IAV RNA. Cells were collected 8 h post-transfection, and lysates of 1 × 106 cells were analyzed by immunoblotting with the α-VEEV hyperimmune ascites fluid. Indicated are VEEV non-structural proteins (nsPs), glycoproteins E1/E2, and capsid protein (CP). Lysates of cells mock transfected (control) or transfected with unrelated SAM-RNA (vector) were included as controls. (E) BHK cells were transfected with LAV or IAV RNA, cell culture supernatants were collected 24 h later, and RNA infectious units (RIUs) were determined by RNA infectivity assay. The specific infectivity was calculated as RIUs per μg of transfected RNA. SDs of the infectivity titers are indicated in the graph. Presented in this figure are the representative data.
To confirm that IAV RNA was non-infectious, in vitro-synthetized LAV RNA, IAV RNA, or an unrelated negative control SAM RNA was transfected into baby hamster kidney (BHK) cells in an attempt to recover virus. Expression of VEEV antigens was analyzed at 24 h post-transfection by immunofluorescence assay using α-VEEV hyperimmune mouse ascitic fluid. Mock-transfected cells or cells transfected with the control SAM RNA showed no VEEV antigen staining (Figure 1C). Conversely, ∼90% of the monolayer of cells transfected with LAV or IAV RNA was positive for VEEV antigen staining, indicating an efficient translation and RNA amplification of both VEEV RNAs. To evaluate production of infectious viral particles, supernatants of transfected cells were collected at 48 h post-transfection, and viral titers were determined by plaque assays (Figure 1C). Infectious virus with a titer of 1.4 × 108 plaque-forming units (PFU)/mL was detected in the supernatants of cells transfected with LAV RNA, but no infectious virus was detected in supernatants of cells transfected with IAV RNA at any dilution tested. This result confirmed that the IAV RNA was unable to generate infectious virus.
To further analyze antigen expression from LAV RNA or IAV RNA, cytoplasmic fractions of RNA-transfected BHK cells were extracted at 8 h post-transfection and analyzed by immunoblot using an α-VEEV hyperimmune mouse ascitic fluid (see Materials and Methods) (Figure 1D). Two controls were included in the analysis: an unrelated RNA as a negative control (i.e., control) and an empty SAM RNA as a positive control for the expression of SAM non-structural proteins (nsPs; i.e., vector). As anticipated, similar levels of viral nsPs and glycoproteins E1/E2 were detected in cells transfected with IAV RNA or LAV RNA. However, viral capsid protein (CP) was detected in SAM-LAV-transfected cells, but not in SAM-IAV-transfected cells. This result confirms that CP expression is successfully abrogated in IAV RNA.
Molecular integrity and capping efficiency determine the effectiveness of the in vitro synthesized SAM RNA to launch RNA amplification and antigen expression in transfected cells and provide a critical quality checkpoint for generation of SAM RNA vaccines. To test this, a SAM RNA-specific infectivity assay was implemented to quantify this biological quality of the SAM RNA. The specific infectivity of LAV or IAV RNA was calculated as the number of RNA infectious units (RIUs) per μg of RNA transfected in BHK cells (see Materials and Methods). Using this test, the specific infectivity of LAV and IAV RNAs were found to be 9.5 × 105 ± 1.4 × 105 RIUs/μg and 1.6 × 106 ± 1.7 × 105 RIUs/μg, respectively (Figure 1E). This result demonstrated that both synthetic RNA molecules had similar infectivity and that the abrogation of CP had no detectable impact on the efficiency of launching IAV RNA translation and amplification in vitro.
In Vitro Characterization of VEEV E2 Antigen Expression
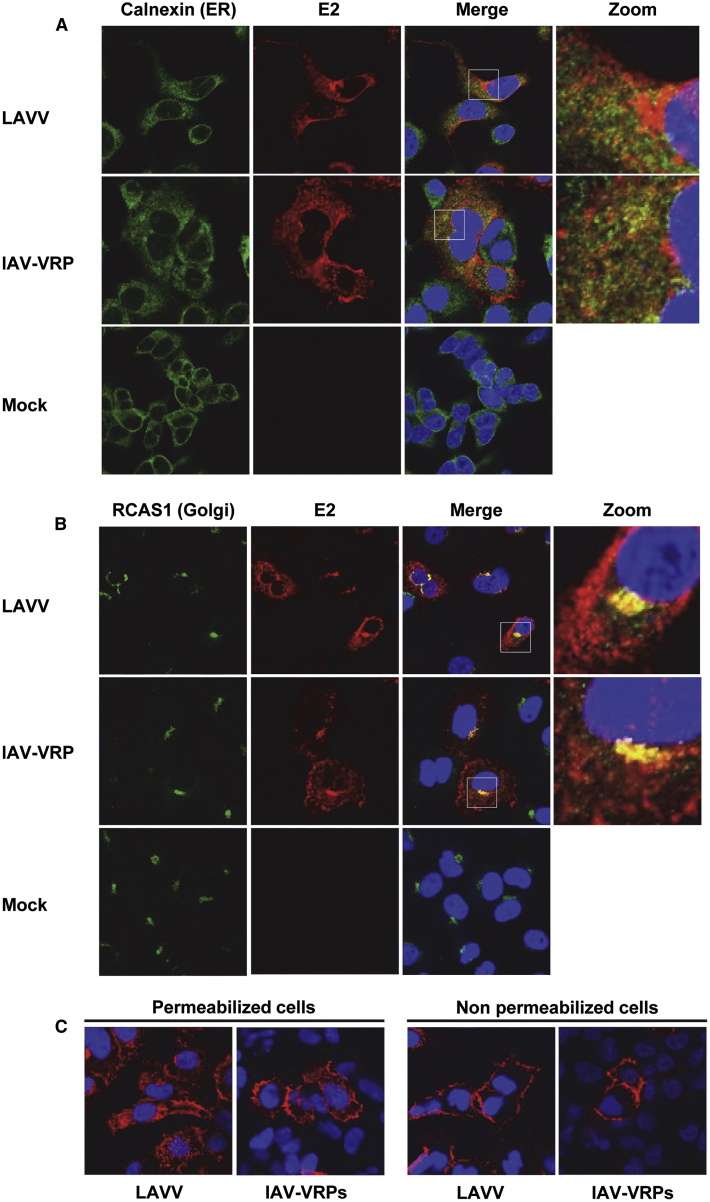
VEEV glycoprotein E2 is the main antigen targeted by α-VEEV NAbs. Consequently, we evaluated the processing of its precursor pE2-6K-E1 by comparing E2 subcellular distribution in A549 cells infected with VRPs derived from IAV RNA (i.e., IAV-VRPs) or virus derived from LAV RNA (i.e., LAVV). In this study, high-multiplicity IAV-VRP or LAVV infection was used instead of SAM RNA transfection, in order to facilitate production of a synchronized culture of cells containing similar numbers of transduced cells. At 24 h post-infection, cells were fixed, permeabilized, stained with an α-E2 monoclonal antibody, and analyzed by confocal immunofluorescence microscopy (Figure 2). Cells were also co-stained with antibodies to the endoplasmic reticulum (ER) marker Calnexin or the Golgi marker RCAS1. In cells infected with LAVV, E2 was found throughout in the cytoplasm and perinuclear area, but not directly co-localized with the ER (Figure 2A). Instead, E2 was found co-localized with the Golgi, consistent with a previous report on Sindbis virus, another alphavirus (Figure 2B).29 Importantly, a similar subcellular distribution of E2 was detected in cells infected with IAV-VRPs (Figures 2A and 2B). As viral glycoproteins are presented on the cell surface following VEEV infection, E2 localization was also examined in non-permeabilized cells infected with IAV-VRPs or LAVV (Figure 2C). A strong E2 staining was detected on the peripheral surface of cells infected with IAV-VRPs or LAVV. In summary, no difference was observed for E2 subcellular localization between cells infected with LAVV and IAV-VRPs, indicating that IAV RNA is capable of launching proper expression, processing, and presentation of pE2-6K-E1 glycoproteins in the absence of the VEEV capsid.
Figure 2.
Intracellular Localization of the Major VEEV Antigen E2 in A549 Cells Infected with LAV-Derived Virus or IAV-VRPs
A549 cells were infected with LAV-derived virus (LAVV) or IAV-VRPs at an MOI of 5, fixed, and permeabilized at 24 h post-infection and co-stained with a mouse monoclonal antibody for the viral glycoprotein E2 (red) and DAPI for cell nuclei (blue). Cells were also co-stained for the endoplasmic reticulum marker Calnexin (A) or Golgi apparatus marker RCAS1 (B), or cells were not permeabilized prior to staining (C). Subcellular localizations of VEEV glycoprotein E2 and subcellular markers were determined by confocal microscopy. Presented are the representatives of multiple fields.
LAV and IAV SAM Vaccines Elicit Robust Immune Responses in Mice
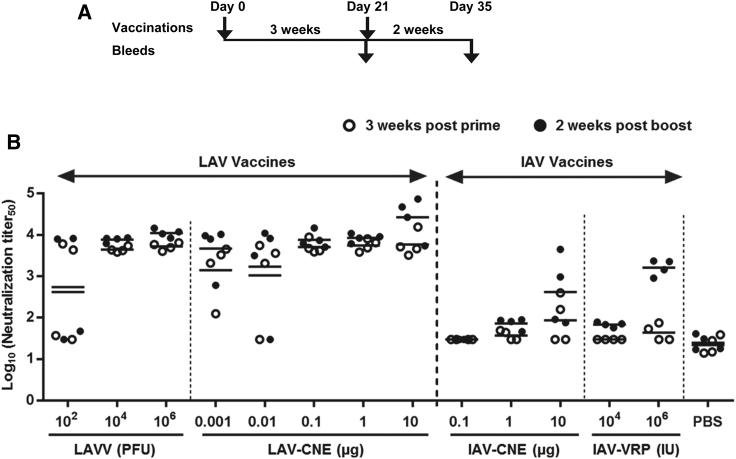
Next, the immunogenicity of LAV and IAV vaccines was evaluated in mice with both SAM vaccines delivered with CNE. For each vaccination group, mice received a priming immunization at day 0 followed by a booster immunization at day 21 (Figure 3A). Serum samples were collected 3 weeks post-prime and 2 weeks post-boost, and the NAb titers were measured by neutralization of TC-83 VRP infection, an assay that could be performed at BSL-2 (Figure 3B). The samples of post-boost vaccination were also analyzed by plaque reduction neutralization test (PRNT), an assay that could be performed only at BSL-3 to correlate VRP-based neutralization titers among groups (Figure S1).
Figure 3.
LAV and IAV RNA Vaccines Elicit Neutralizing Antibody Responses in Mice
(A) Schedule of vaccination and blood collection. Groups of female BALB/c mice (N = 4) were vaccinated by intramuscular injection with LAV or IAV vaccine delivered by CNE (LAV-CNE or IAV-CNE), and IAV vaccine delivered by VRPs (IAV-VRP), PBS control, or by intraperitoneal injection with LAV-derived virus (LAVV). Animals were vaccinated twice at day 0 (prime) and day 21 (boost). Retrorbital bleeds were performed 3 weeks post-prime and 2 weeks post-boost. (B) Neutralization titers of sera determined by TC-83 VRP neutralization assay. The titers were expressed as the reciprocal of the serum dilution yielding 50% reduction of infected cellular foci in the log scale (Log10 neutralization titer50). Shown are NAb titers from individual animals (depicted as dots) and geometric mean titers (GMTs; depicted as solid bars).
The delivery of LAV RNA is designed to launch complete viral cycles similar to the attenuated virus vaccine TC-83, producing infectious viral particles that spread to other cells in vivo. Therefore, we tested the immunogenicity of the LAV vaccine as CNE-formulated SAM vaccine (LAV-CNE) at multiple RNA doses (0.001, 0.01, 0.1, 1, or 10 μg) or as the traditional viral particles produced from LAVV at a dose of 1 × 102, 1 × 104, or 1 × 106 PFU. On the other hand, the delivery of IAV RNA is designed to launch a single-cycle of RNA amplification and unable to produce infectious viral particles. Thus, we tested higher doses of IAV vaccines delivered either as CNE-formulated SAM vaccine (IAV-CNE) at 0.1, 1, or 10 μg of RNA or as VRPs (IAV-VRP) at 1 × 104 or 1 × 106 IU. Here, VRPs were included as a reference for viral delivery, as these single-cycle infectious particles represent a delivery system that has recently been demonstrated to elicit potent immune responses and protection against VEEV, EEEV, and WEEV in mice and non-human primates30 and against CMV antigens in human clinical trials.20
A dose of 1 × 102 PFU of the LAVV vaccine generated a mean neutralization titer of 5.6 × 102 as measured by TC-83 VRP neutralization assay after the second immunization (Figure 3B). However, individual titers in this group of animals were binary, consistent with the expectation that this low dose of live virus vaccine was unable to initiate replication in some of the inoculated mice. Virus vaccine at a dose of 1 × 104 PFU or above resulted in consistent neutralization titers of approximately 1 × 104. The LAV-CNE vaccine was immunogenic at doses as low as 1 ng, and mice inoculated with LAV-CNE at doses of 0.1 μg or above generated NAbs titers comparable to those elicited by 1 × 104 PFU or more of LAVV vaccine (Figure 3B). The LAV-CNE vaccine at doses below 0.1 μg resulted in inconsistent responses, similar to those detected with the lowest dose tested with LAV-derived live virus vaccine (1 × 102 PFU). In the animals that responded to the first dose of LAV-CNE at doses ranging from 0.001 to 1 μg, the second immunization did not appear to significantly enhance the immune response (comparing the titers at 3 weeks post-prime versus 2 weeks post-boost) (Figure 3B). However, the second immunization in mice vaccinated with 10 μg of the LAV-CNE vaccine demonstrated boosting the neutralization titers approximately 5-fold over the titers detected following the initial immunization.
Animals vaccinated with IAV-CNE or IAV-VRP induced lower levels of neutralization titers compared to those inoculated with the LAV-CNE vaccine or LAV-derived live virus vaccine (Figure 3B). While NAb responses induced by the LAV-CNE vaccine reached titers similar to 104 PFU of the LAV-derived live virus vaccine at the 1-μg dose, those induced by the IAV-CNE vaccine remained dose-dependent at doses of 1–10 μg. Importantly, a boosting effect was observed after the second immunization. At the 10-μg dose of the IAV-CNE vaccine, high neutralization titers were elicited in two of the four animals following the second inoculation. However, the mean neutralization titers induced by 10 μg of the IAV-CNE vaccine after the second immunization was ∼100-fold lower than those of the LAV-CNE vaccine at the same dose and time points. The neutralization titers elicited by the IAV-CNE vaccine after the second immunization also appeared to be more variable than those elicited by 1 × 106 IU of IAV-VRPs. Although the study was not powered to demonstrate statistical difference, the geometric mean titer (GMT) of IAV-CNE was about 4-fold lower than those of IAV-VRPs, possibly due to a more efficient vaccine delivery by VRPs.
In summary, results from this initial mouse immunogenicity study demonstrated that immunization with the LAV-CNE vaccine at a dose as low as 0.1 μg elicits neutralization titers after one immunization comparable to peak titers by the LAVV vaccine. Vaccination with the IAV-CNE or IAV-VRP vaccine also induced NAb responses, but the titers were approximately 10- to 50-fold lower than those achieved with the LAV-CNE vaccine or LAVV vaccine.
Impact of Antigen Codon Modification on the Immunogenicity of VEE SAM Vaccines in Mice
Based on the immunogenicity profiles observed with the LAV-CNE and IAV-CNE SAM vaccines, further improvement of these vaccines was attempted. For the LAV-CNE vaccines, we hypothesized that its high immunogenicity was the result of its capacity to produce infectious particles upon delivery into vaccinated animals. Therefore, we wanted to further attenuate the virus genome to make the LAV less infectious but still produce sufficient levels of viral particles in vaccinated animals for the robust induction of immune responses. To test this, we generated an additional LAV, termed LAVm, which carried mutations to replace all the positively charged amino acid residues at the N-terminal region of the CP with the non-charged residues as previously described for VEEV.31 This previously described mutant VEEV virus has been demonstrated to retain the ability to produce progeny for spread, but at levels 5–6 orders of magnitude lower than wild-type virus, yet still produce high levels of virus-like particles.
For IAV-CNE vaccines, we hypothesized that their reduced immunogenicity relative to LAV-CNE vaccines was due to the inability to produce infectious particles in vivo and spread. This would lead to a lower total availability of antigen compared to LAV-CNE. We attempted to improve the potency of the IAV-CNE vaccine by generating an alternative RNA molecule, namely IAVco, where the viral glycoprotein gene was codon optimized for increased expression in mammals. Coding sequence optimization has been widely used to increase heterologous gene expression in mammalian cells. For example, DNA vaccines expressing codon-optimized VEEV glycoprotein genes have been shown to have improved immunogenicity and protective immunity in mice and non-human primates.32, 33
In RNA-transfected BHK cells, these RNA molecules showed no detectable differences in glycoprotein expression or RNA-specific infectivity compared to parental LAV or IAV RNAs (data not shown). To test these modified RNA vaccines in vivo, groups of 10 mice were vaccinated with the RNA vaccines delivered by either CNE or VRPs at days 0 and 21. Sera were collected at day 35, and total anti-VEEV immunoglobulin G (IgG) titers or anti-VEEV NAb titers were determined by ELISA or by PRNT, respectively (Figure 4). LAV-CNE and LAVm-CNE vaccines were administered at a dose of 0.1 or 1 μg and 1 and 10 μg, respectively. Additionally, the LAVm RNA was delivered as VRPs (LAVm-VRP) at a dose of 1 × 106 IU. For IAV and IAVco vaccines, RNA was either formulated with CNE or as VRP and was delivered at a dose of 10 μg or 1 × 106 IU, respectively. 1 × 104 PFU of the viral vaccine TC-83 was administrated as a live-attenuated vaccine control.
Figure 4.
The Impact of Genetic Modification of LAV and IAV Vaccines on Their Immunogenicity in Mice
(A) Schedule of vaccination and immunology analysis. Groups of female BALB/c mice (N = 10) were vaccinated intramuscularly with live attenuated virus vaccine TC-83, RNA vaccines delivered by CNE, RNA vaccines delivered by VRPs, or PBS control as indicated. Animals were vaccinated at day 0 and day 21, and submandibular bleeds were performed at day 35 for immune analysis. (B) Total α-VEEV IgG antibody titers of serum samples measured by ELISA and (C) neutralizing titers determined by plaque reduction neutralization test (PRNTs) using VEEV subtype IAB (strain Trinidad donkey) and expressed as the reciprocal of the serum dilution yielding 80% reduction of plaques forming units (PRNT80). Shown are titers from individual animals (depicted as dots) and GMTs (depicted as solid bars). Statistical analysis was performed by one-way ANOVA using Graph Pad Prism 7.0 software. p values were corrected by Bonferroni methods (n/s, not statistically significant; ****p < 0.0001).
As anticipated, total IgG titers of mice vaccinated with the LAV-CNE vaccine peaked at the 0.1-μg dose and were at a level similar to those of the TC-83 vaccine group (Figure 4B). The highly-attenuated LAVm-CNE vaccine elicited IgG antibodies in a dose-dependent manner at doses of 1–10 μg. At the high dose of 10 μg vaccine, animals developed IgG titers comparable to those in the TC-83 group. Similarly, mice vaccinated with the LAVm-VRP vaccine also generated IgG titers comparable to those in the TC-83 group. For anti-VEEV NAbs, the PRNT titers elicited in mice vaccinated with LAV-CNE also peaked at the 0.1 μg dose. The titers were not statistically different from those obtained for the TC-83-vaccinated group but trended higher and were less variable (Figure 4C). PRNT titers elicited by 10 μg of LAVm-CNE were 50-fold lower than those of TC-83. In contrast, the LAVm-VRP vaccine elicited 100-fold higher PRNT titers than LAVm-CNE vaccines and comparable to those of TC-83.
The IAV vaccines (IAV and IAVco) delivered by CNE or by VRPs elicited IgG antibody titers comparable to those of the TC-83 vaccine (Figure 4B). An apparent improvement on IgG titers was observed in animals vaccinated with the IAVco-CNE relative to the IAV-CNE; however, this difference was not statistically significant and also diminished when the RNAs were delivered as VRPs. Similar to that observed in Figure 3, the PRNT titers elicited by both IAV-CNE vaccines were lower than those of the IAV-VRP vaccines (Figure 4C). There was no significant difference in PRNT titers elicited by the IAVco-VRPs or by IAV-VRPs (Figure 4C). The NAb titers of IAV-CNE vaccines in this study were lower than those presented in Figure 3, likely due to the different neutralization assays used in these studies (i.e., PRNT assay versus TC-83 VRP neutralization assay).
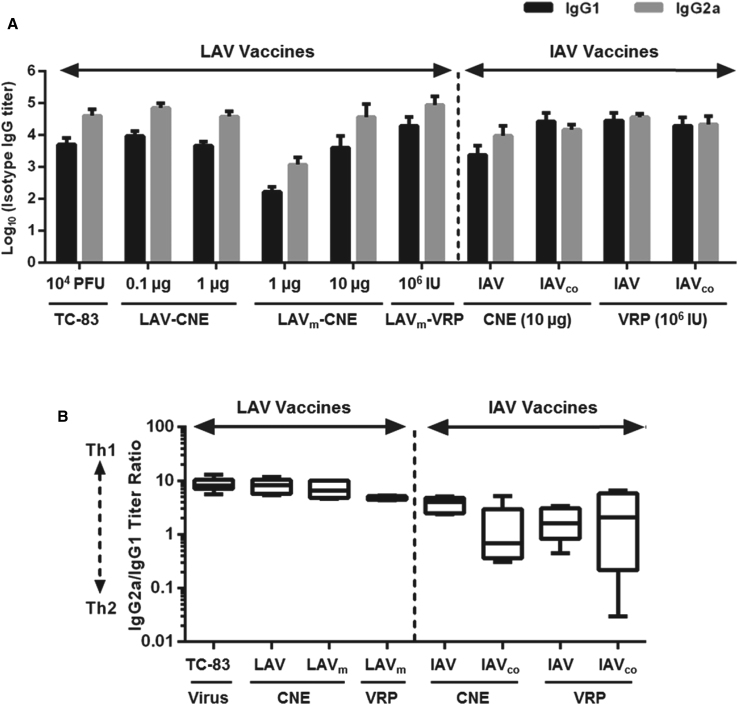
Although NAbs are critical for protection against natural alphavirus infections,3, 34, 35 other components of the host immune response such as cell-mediated immunity can also play an important role. For example, passive immunization by administration of NAbs does not protect mice from an intranasal challenge.3, 36, 37 This suggests that a broad immune response involving both humoral and cell-mediated immunity may be required for full protection against aerosol VEEV infection. In our study, we assessed vaccine-elicited Th1 and Th2 responses by analyzing titers of IgG antibody isotypes in the same serum samples used for total IgG and NAb analysis (Figure 5). IgG1 and IgG2a were used as surrogate markers for Th2- and Th1-skewed responses, respectively. IgG isotype ELISA analysis demonstrated that all live-attenuated vaccines, including TC-83, LAV-CNE, LAVm-CNE, and LAVm-VRPs, elicited greater IgG2a titers than IgG1 titers (Figure 5A). The ratios of the IgG2a titers/IgG1 titers elicited by the vaccines were also plotted to assess the relative weight of Th1 and Th2 responses (Figure 5B). Consistent with previous reports, mice vaccinated with TC-83 and other live attenuated vaccines had approximately 10-fold more IgG2a than IgG1, suggesting a Th1-skewed immunity.33, 38 Similarly, IAV-CNE also showed high IgG2a/IgG1 ratios of 4 (Figure 5B). However, IAVco-CNE and all VRP-delivered IAV vaccines (IAV and IAVco) showed similar levels of IgG2a and IgG1 titers, suggesting a more balanced Th1 and Th2 immune response, which could result in a more effective VEE vaccine (Figure 5B).33, 39
Figure 5.
A Subset of LAV and IAV Vaccines Elicited a Th1/Th2 Balanced Immune Response
Sera from vaccinated animals as described in Figure 4 were analyzed for IgG1 and IgG2a α-VEEV antibody titers by isotype ELISA assay. Shown are (A) the titers of isotype IgG1 and IgG2a of each group and (B) the ratio of IgG2a/IgG1 titers of the group immunized with TC-83 (104 PFU), LAV-CNE (1 μg), LAVm-CNE (1 μg), LAVm-VRP (106 IU), various IAV-CNE (10 μg), or various IAV-VRP (106 IU) as indicated. Black or gray bar presents IgG1 or IgG2a, respectively. SDs of IgG titers are indicated in the graph.
LAV and IAV SAM Vaccines Confer Protection against Aerosol VEEV Infection in Mice
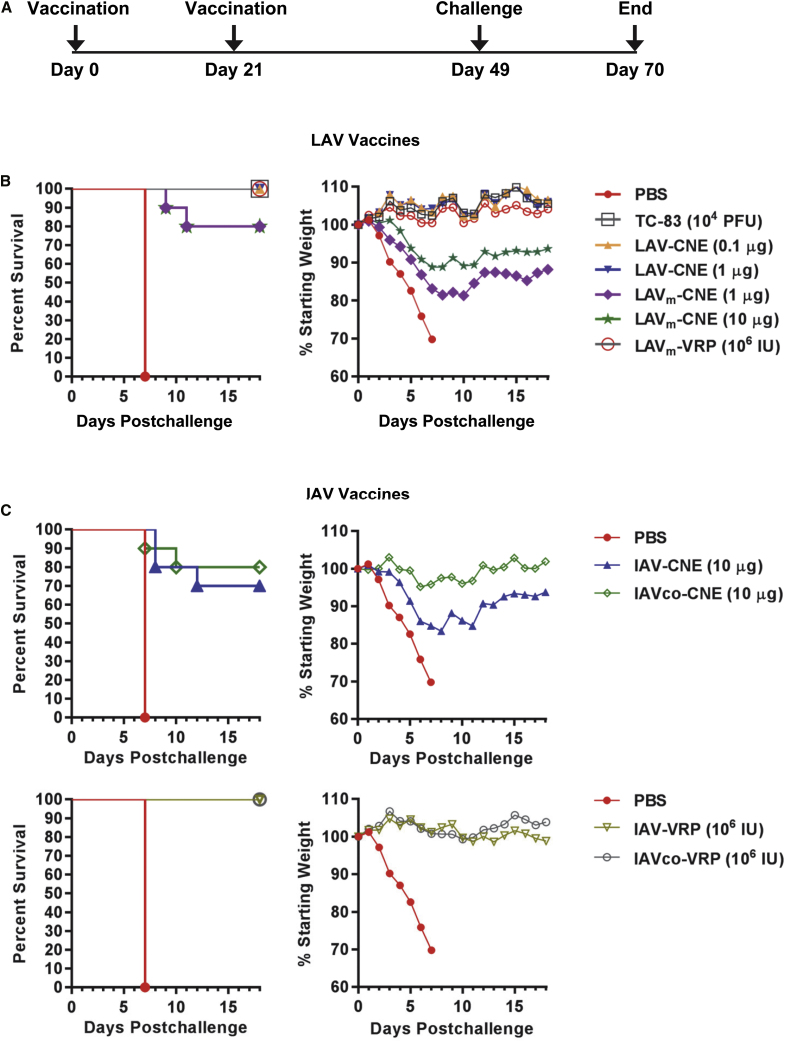
Finally, protective efficacy of the VEE RNA vaccines was tested in a homologous mouse challenge model. In the study presented in Figure 4A, groups of 10 mice that were vaccinated and analyzed for immunogenicity were also challenged 28 days after the second vaccination with 1 × 104 PFU (equivalent to ≥ 10,000 50% lethal doses [LD50]) of VEEV subtype IAB strain Trinidad donkey by the aerosol route (Figure 6A). All of the mice in the control group vaccinated with PBS displayed clinical signs of disease and weight loss and were euthanized due to morbidity at day 7 post-challenge (Figure 6). In contrast, all of the mice vaccinated with live-attenuated vaccines TC-83 (1 × 104 PFU), LAV-CNE (0.1 or 1 μg), or LAVm-VRPs (1 × 106 IU) survived the challenge (Figure 6B). For TC-83, LAV-CNE, and LAVm-VRPs, no signs of illness were observed in these groups, and average body weights were maintained during the course of the challenge (Figure 6B). For the LAVm-CNE vaccine, seven mice vaccinated at the 1-μg dose and three mice vaccinated at the 10-μg dose showed signs of sickness starting at day 6 but recovered thereafter. Two animals in each group never recovered, succumbing at day 9 and 11 (Figure 6B). A reduction in the average body weight of 20% or 10% was also detected in the group vaccinated with 1 μg or 10 μg of the LAVm-CNE, respectively (Figure 6B).
Figure 6.
LAV and IAV RNA Vaccines Confer Protection against Aerosol VEEV Infection in Mice
(A) Schedule of vaccination and challenge. BALB/c mice were vaccinated as described in Figure 4, challenged with 1 × 104 PFU (equivalent to ≥ 10,000 LD50) of VEEV IAB strain Trinidad donkey by the aerosol route at day 49 and were then monitored for mortality and morbidity daily for an additional 21 days. (B) Percentage of survival (left) and changes in body weights (right) after immunization with various LAV RNA vaccines as indicated. (C) Percentage of survival (left) and changes in body weights (right) after immunization with IAV RNA vaccines as indicated.
The IAV and IAVco vaccines delivered as VRPs (1 × 106 IU) provided complete protection against VEEV aerosol challenge (Figure 6C). Only one animal in the IAVco-VRP group displayed signs of sickness starting at day 9 but recovered by day 11 post-challenge. Moreover, no loss of body weight was observed in IAV-VRP or IAVco-VRP vaccinated mice. The IAV and IAVco vaccines formulated with CNE and delivered as SAM (10 μg) elicited protection in 70%–80% of vaccinated animals (Figure 6C). Seventy percent of the mice vaccinated with the IAV-CNE survived the challenge. Two animals in this group displayed signs of sickness at day 6 and succumbed at day 8 post-challenge. A third animal became sick at day 9 and succumbed at day 12 post-challenge. Eighty percent of the animals vaccinated with the IAVco-CNE survived the challenge. One animal succumbed at day 7, and a second animal succumbed at day 10. The trend in improved survival in the IAVco-CNE vaccinated group relative to the IAV-CNE vaccinated group was consistent with the weight retention of the animals in these two groups post-vaccination. Mice vaccinated with IAV-CNE showed a rapid and significant loss of body weight after challenge (Figure 6C). However, mice vaccinated with IAVco-CNE maintained initial body weights until day 5 post-challenge had a gradual and mild loss of body weight (<5%) between day 5 and day 10 and recovered thereafter (Figure 6C). These results are consistent with the hypothesis that coding-sequence modifications could have a positive impact on vaccine potency and protective efficacy delivered as a SAM vaccine.
Taken together, these results provide evidence to support the use of a synthetically delivered RNA approach to develop effective, virus-based vaccines against positive-strand RNA viral pathogens such as VEEV.
Discussion
Venezuelan equine encephalitis virus is an important emerging pathogen in equines and humans and is also considered a biological threat agent. Despite its importance, no commercial human vaccines against the diseases caused by VEEV are currently available. In the studies described here, we applied a synthetic SAM vaccine delivery platform to investigate novel vaccine candidates for preventing disease caused by VEEV. Previously, we have shown that the SAM platform can elicit potent and broad immune responses against various targeted antigens. The ability of SAM vaccines to specifically induce T cell responses has also been demonstrated with different antigens. The SAM vaccine expressing influenza virus hemagglutinin (HA) antigen has been shown to induce CD4+ and CD8+ T cell responses to mediate heterologous protection against a different influenza strain in mice.27 CD4+ and CD8+ T cell responses were also detected in rhesus macaques vaccinated with SAM vaccines expressing human cytomegalovirus (HCMV) proteins gB + pp65-IE1,22 HIV envelope glycoprotein,26 and respiratory syncytial virus (RSV) F antigen.8 More recently, a malaria SAM vaccine candidate expressing the plasmodium protein PMIF, which modulates the host inflammatory response to malaria, has been shown to confer T-cell-mediated protection in a lethal mouse model.25 Importantly, an extensive body of the work has also demonstrated that the SAM platform can elicit potent antibody responses against an array of pathogens, such as RSV, HCMV, influenza virus, HIV/simian immunodeficiency virus (SIV), group A streptococcus (GAS), and group B streptococcus (GBS).40 Previous preclinical animal studies have shown that SAM vaccines can drive equivalent or higher antigen expression, immune responses, or protection at much lower doses than DNA vaccines8 or conventional mRNA vaccines.41 In this study, we tested the potential of this platform to overcome manufacturing limitations for live virus vaccines and provide the opportunity for further engineering to enhance potency and safety.
We demonstrated that the delivery of 0.1 μg of the synthetic RNA genome of TC-83 by CNE (LAV-CNE) was able to elicit NAb titers comparable to those by TC-83 live attenuated virus vaccine and confer complete protection against an aerosol challenge with wild-type VEEV strain IAB. Interestingly, a boost immunization provided little benefit except at the highest dose tested (10 μg). It is possible that an interval of greater than 3 weeks between prime and boost could allow for a more complete contraction of the initial immune response, which could result in a stronger boosting effect. However, the interval chosen for the study was typical of that used in SAM vaccine studies in mice. We have previously shown that expression from a SAM vaccine carrying RSV F antigen peaked at day 7, followed by a > 100-fold decay at day 14, and became undetectable at day 21.16 While we have not performed the expression study with VEEV antigens in the present work, we believe that SAM expression and antibody response could follow a similar pattern. Moreover, if there were a failure for the initial immune response to contract in the presented study, we would also expect a stronger boost effect at lower doses, where antigen levels were lower and immune responses were more likely to have started to contract, instead of the high dose of 10 μg. It is also possible that ongoing replication of the launched virus from the SAM prime could activate systemic innate immune responses attenuating the launch of the SAM boost. However, it is more likely that the effectiveness of the LAV at this low dose of RNA could be attributed to the in vivo generation of infectious LAV virus, which eliminates the need for boost.
The CNE delivery of a highly attenuated VEEV genome (LAVm-CNE) resulted in partial protection (80% survival), whereas viral delivery of the same genome as virus-replicon particles (LAVm-VRP) conferred 100% survival. This difference in the protective efficacy between non-viral (i.e., CNE) and viral delivery methods (i.e., VRPs) was also observed with irreversibly attenuated vaccines, where the IAV RNA delivered by CNE gave 70%–80% protection but the same molecules delivered by VRP gave 90%–100% protection, which correlated with the levels of NAbs. It is possible that this may reflect a stronger innate recognition of CNE-delivered RNA by cellular pattern recognition pathways over that of VRP-delivered RNA, which limits the potency of SAM vaccine. Alternatively, we speculate that the lower potency of a highly attenuated or inactivated VEE RNA vaccine could be overcome by further improvement of the synthetic non-viral delivery system, for example, by optimizing CNE specifically for VEEV SAM vaccine or utilizing alternative delivery strategies such as LNPs.8, 10, 42, 43 On a related note, the attenuated LAVm-CNE vaccine was significantly less immunogenic than the parental LAV-CNE vaccine, whereas LAVm-VRPs was similar to LAV-VRPs. It is possible that the VRP presents glycoproteins authentically to allow for more efficient uptake of the particles as compared to CNE so that both LAVm-VRPs and LAV-VRPs could efficiently launch to induce robust immune responses. When delivered by CNE, LAV RNA could reconstitute live attenuated virus in vivo to spread and amplify the response, whereas LAVm-CNE could only undergo RNA amplification but was unable to spread. It is also possible that glycoproteins presented in both VLPs contribute to the elicitation of the protective immune response, therefore diminishing the immunogenic difference between LAVm-VLPs and LAVV.
Interestingly, studies that compared the efficacy of our original CNE-formulated IAV-CNE vaccine and a derivative that contained codon-optimized glycoprotein coding sequences (IAVco-CNE) revealed a trend toward decreased disease severity for the vaccine containing the codon modification. IAVco-CNE did not enhance the NAb titers or protective efficacy against the challenge, but the total IgG titers that it elicited trended higher as compared to those of IAV-CNE (Figure 4B). Mice vaccinated with IAVco-CNE had less change in body weight after challenge than mice vaccinated with IAV-CNE. The live-attenuated vaccines, TC-83, LAV-CNE, and LAVm-CNE, as well as the other irreversibly attenuated vaccine IAV-CNE, developed higher levels of IgG2a antibodies relative to IgG1, consistent with a Th1-skewed immune response that has been previously described for TC-83 live virus vaccine.33, 38 Recent studies indicate that the effective protection against VEEV may also be enhanced by a vaccine-elicited T cell response, so a balanced immune response might enhance the protective efficacy of a vaccine.33, 36, 37, 44 Interestingly, IAVco-CNE induced similar IgG2a titers to IAV-CNE but significantly more IgG1 titers than other VEE SAM vaccines (Figure 5A), suggesting a more balanced Th1/Th2 response (Figure 5B). A similarly balanced Th1/Th2 response has been reported for a DNA vaccine that expresses codon-optimized VEEV envelope glycoprotein genes, elicits improved protective immunity in mice and NHPs, and has recently been evaluated in a phase 1 clinical trial.33, 39 While the NAbs directed against the envelope glycoproteins are the most widely accepted correlate of protection,35, 45, 46, 47, 48 they are not always significantly associated with protection against VEEV challenge by the aerosol route.4, 49 Non-NAbs can also mediate protection against encephalitis caused by alphaviruses.50, 51 Roles of mucosal antibody responses and antibody-dependent cellular cytotoxicity in protection against aerosol VEEV challenge in mice have also been documented.44, 52, 53 While cytotoxic T cell activity was not observed in previous studies with TC-83, recent studies have revealed an importance for certain populations of T cells in protection against lethal encephalitis caused by VEEV in mice.36, 37, 54, 55 For SAM vaccines, a more thorough characterization of the elicited immune responses in future studies would further elucidate the contributing role of these responses in the protection observed against aerosol VEEV challenge.
The concept of launching a live-attenuated virus vaccine from a nucleic acid construct has been demonstrated previously,56, 57 but the present work is the first to effectively deliver a live-attenuated vaccine using a non-viral, synthetic formulation. The delivery of the infectious RNA genome of a live-attenuated tick-borne encephalitis (TBE) virus, a flavivirus, can elicit strong protection in mice even at low RNA doses.56 More recently, the delivery of a DNA plasmid encoding the full TC-83 RNA genome under the CMV promoter has been shown to elicit protection against a subcutaneous challenge with VEEV IAB.57 However, in these studies, the viral RNA genome was delivered by devices (e.g., gene gun or electroporation), which are invasive and likely impractical for large-scale vaccination in humans. In this work, we have demonstrated that a fully synthetic vaccine consisting of RNA encoding LAV formulated with CNE and administrated by intramuscular injection was potent and protective against homologous aerosol VEEV infection in mice. This provides proof of principle for an effective VEE RNA vaccine with potential to enable simplified manufacturing, rapid scale-up capability, and compatibility with standard vaccine administration methods in humans.
The VEE SAM vaccines presented in this work provide opportunities to address two key limitations of VEE vaccine development, namely manufacturability and risk of pathogenic reversion. While the LAV RNA vaccine is derived from TC-83 and reversion to pathogenic virus remains a possibility during in vitro transcription, it circumvents the need for serial passage of virus vaccine in cell culture, which has the potential to select for growth of pathogenic revertants.57 Importantly, the synthetic aspect of the SAM approach will eliminate the need for growth and purification of live-attenuated virus, thus greatly streamlining the manufacturing process. Highly attenuated vaccines (e.g., LAVm-CNE) and irreversibly attenuated vaccines (e.g., IAV SAM variants) represent a potentially safer alternative to TC-83 due to a partial or total loss of viral replication. The use of a synthetic delivery system would overcome the challenges associated with the production of VRP-based vaccines, which are difficult to produce at large scale and which are susceptible to the production of replication-competent virus due to recombination with helper constructs required for their manufacture. IAV vaccines delivered by CNE in their current format are less efficacious compared to LAV-CNE vaccines. However, immunogenicity and efficacy of an IAV SAM vaccine could potentially be enhanced by utilizing alternative delivery strategies, such as LNP. Finally, this work also provides evidence to support the use of the SAM platform to deliver RNA vaccines for closely related alphavirus pathogens such as WEEV, EEEV, and Chikungunya viruses.
Materials and Methods
Cells and Viruses
BHK cells were cultured in DMEM supplemented with 5% fetal bovine serum (FBS). Human lung cell line A549 was cultured with DMEM supplemented with 10% FBS. DMEM media was also supplemented with 10 mM sodium pyruvate and 100 μg/mL penicillin-streptomycin. Both cell lines were maintained at 37°C and 5% CO2. Live-attenuated VEEV virus TC-83 used in Figures 1 or Figures 4, 5, and 6 was a generous gift from Dr. Robert Tesh (University of Texas Medical Branch, USA) or generated at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID), respectively. LAVVs were obtained by electroporating 1 × 106 BHK cells with 4.2 μg LAV RNA, collecting cell-culture supernatants 24 h post-electroporation, pelleting cell-free virus by centrifugation at 8,000 × g and storage at −80°C.
Analysis of Virus Titers, Plaque Morphology, and Growth Kinetics
Virus titers were determined by standard plaque assay. In brief, BHK cells plated in 24-well plates were inoculated with 10-fold serial dilutions of viral samples in DMEM with 1% FBS at 37°C, 5% CO2 for 2 h. Wells were rinsed with PBS, overlaid with DMEM supplemented with 1.2% methylcellulose and 1% FBS, and incubated at 37°C, 5% CO2 for 3 days. Cells were then fixed by adding 1 mL of 10% formalin per well and stained with 0.2% crystal violet in 4% paraformaldehyde and 20% ethanol. Plaques were counted for titer calculation, and morphology was analyzed by image acquisition (ChemiDoc Imaging System, Bio-Rad). For multistep virus growth analysis, BHK cells plated in 6-well dishes were inoculated with TC-83 or SAM-LAVVs at an MOI of 0.01 per cell at 37°C, 5% CO2 for 1 h, rinsed twice with PBS, and replenished with DMEM with 1% FBS. Infected cells were cultured at 37°C, 5% CO2, cell culture supernatants were collected at 0, 3, 6, 9, 12, 24, and 48 h, and virus titers were determined by plaque assay.
DNA Constructs
The nucleotide sequences of LAV RNA, IAV RNA, and their variants were derived from the VEEV TC-83 sequence (GenBank: L01443) and inserted after the T7 promoter in the SAM-derived DNA vector as previously described.8 To facilitate cloning, the following mutations were introduced in the TC-83 sequence (positions indicated according to TC-83): A-to-C at NT# (nucleotide acid number) 7560, G-to-C at NT# 7561 (both within Kazak sequence), and insertion of GA at NT# 7575 (to create a SalI restriction site) in the sub-genomic 5′ UTR; G-to-A at NT# 11300 and insertion of TAGTAAGCGGCCGC at NT# 114123 (to create two tandem E1 stop codons as well as a Not I restriction site) in the region between C-terminal region of E1 and 3′ UTR; and C-to-A at NT#1622 (to create a synonymous mutation removing a SalI restriction site) in nsP1. Codon-optimized VEEV glycoprotein genes were generated by subjecting the wild-type 26S structural gene sequences minus the CP coding region (E3-E2-6K-E1) of VEEV IAB strain Trinidad donkey (GenBank: L01442) to the Genewiz proprietary bioinformatic algorithm for optimized expression in Homo sapiens followed by synthesis of the codon-optimized genes. The synthesized genes were then used to create the IAVCO RNA. Plasmids were grown in E. coli and purified using QIAGEN Endo Free Maxi Preps Kits.
RNA Synthesis
In vitro RNA transcription was performed as previously described.8 In brief, DNA plasmids encoding the RNA vaccines were linearized by restriction digestion with BspQI at the precise 3′ end of SAM sequences and purified by phenol-chloroform extraction. Linearized DNA templates were in vitro transcribed using T7 RNA polymerase (MEGAscript kit T7, Ambion AM1333) and purified by LiCl precipitation. RNAs were capped using a vaccinia capping kit (ScriptCap m7G, Epicenter Biotechnologies SCCE 0610), purified again by LiCl precipitation, and dissolved in nuclease-free water (AM9937, Ambion). The quality and integrity of the RNA was analyzed by 1% agarose gel electrophoresis. RNA was stored at −80°C before use.
CNE/RNA Formulation
CNE/RNA formulations were prepared as previously described.22 In brief, squalene, DOTAP, and sorbitan trioleate were combined and heated to 37°C. The resulting oil phase was then combined with an aqueous phase consisting of polysorbate 80 in 10 mM citrate buffer at pH 6.5. The final weight-by-weight percentages of squalene, DOTAP, sorbitan trioleate, and polysorbate 80 were 4.3%, 0.4%, 0.5%, and 0.5%, respectively. This mixture was homogenized using a T25 homogenizer with a 13.4-mm diameter rotor (IKA) at 24,000 rpm to produce a primary emulsion. This was then passed through an M-110P Microfluidizer (Microfluidics) with an ice bath cooling coil at a homogenization pressure of 137 Mpa approximately eight times. The formulation was stored at 4°C before use. The 100-nm CNE had a positive surface charge, which was used to adsorb the RNA to the surface of the oil droplet through an electrostatic interaction with the negatively charged phosphate backbone. RNA was diluted to 200 μg/mL and was added to an equal volume of CNE, mixed, and allowed to equilibrate on ice for 30 min to 2 h. Endotoxin levels were measured by the gel clot limulus amebocyte lysate (LAL) assay per the manufacturer’s instructions (Cape Cod Associates).
Production of VRPs
VRPs were produced by co-transfecting BHK cells with IAV-derived RNAs and a helper DNA construct encoding the capsid gene under the CMV promoter as previously described.58 At 24 h post-transfection, cell culture supernatants were collected and VRPs were purified by tangential flow filtration (Spectrum, P-C1-500E-100-01N) in the exchange buffer (10 mM sodium phosphate, 0.5 M NaCl, pH 7.3). Purified VRPs were titrated by standard methods as preciously described58 and stored at −80°C until use. VRPs were diluted to desired doses in PBS prior to inoculation of animals.
Production of Mouse α-VEEV Ascites Fluid
Female CD-1 mice were immunized with TC-83 virus and boosted twice with TC-83 mixed with incomplete Freund’s adjuvant. At 7 days post-final-immunization, mice were injected with mouse-adapted Src180 cells. The tumor cells were tapped three times before ascites fluid collection.
Immunoblotting
One million of BHK cells and 0.1 μg of RNA were mixed in 300 μL of Opti-MEM and electroporated using 0.2-cm cuvettes (GenePulser X-cell, Bio-Rad Laboratories, 1 pulse of 120 V for 25 ms). Cells were resuspended in 2 mL DMEM supplemented with 1% FBS, 10 mM sodium pyruvate, 100 μg/mL penicillin-streptomycin, plated on a 60-mm dish, and cultured at 37°C, 5% CO2. Under this condition, about 60% of cells were positive with transfected RNA at 8 h post-electroporation. Cells were then collected and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS, pH 7.4). Soluble fractions were resolved by precast polyacrylamide gel-lithium dodecyl sulfate (NuPAGE-LDS) gel electrophoresis and transferred to a nitrocellulose membrane (Blot Transfer Stack, Thermo Fisher). The membrane was hybridized with α-VEEV immune ascites fluid, incubated with horseradish peroxidase (HRP)-linked goat α-mouse IgG antibody (Thermo Fisher 31430), and VEEV proteins were detected with enhanced chemiluminescence (ECL) western blotting detection reagents (GE Healthcare RPN2106).
RNA-Specific Infectivity Assay
One million of BHK cells were electroporated with 0.1 μg of LAV- or IAV-derived RNAs as described above. Eight hours post-transfection, cells were split 1:10 and co-cultured with fresh BHK cells. Cells transfected with LAV RNA were plated with a semi-solid overlay consisting of 2.4% carboxymethyl cellulose in DMEM. Cells transfected with IAV RNA were incubated in normal DMEM. In both cases, DMEM was supplemented with 1% FBS, 10 mM sodium pyruvate, and penicillin and streptomycin. At 24 h post-electroporation, cells were fixed with methanol/acetone (50:50) and stained with the α-VEEV hyperimmune ascites fluid. Infection foci of LAV particles or positive cells of IAV-replicating RNA were countered as RNA infectious units (RIUs). The specific infectivity was expressed as RIUs per μg of transfected RNA.
Immunofluorescence Assay
For immunofluorescence analysis of transfected cells, BHK cells were electroporated as described above. At 24 h post-electroporation, cells were fixed with methanol/acetone (50:50) and immunostained with the α-VEEV hyperimmune ascites fluid followed by α-mouse Alexa 488 (Molecular Probes A11029). For immunofluorescence analysis of cells infected with VRPs, A549 cells were plated in Nunc Lab-Tek Chambers (Thermo 177445) and incubated for 4 h before infection with VRP-LAV or VRP-IAV at an MOI of 5. At 24 h post-infection, cells were fixed with 4% paraformaldehyde in PBS with or without subsequent permeabilization with 0.1% Triton X-100 in PBS and stained with the following antibodies: α-VEEV glycoprotein E2 monoclonal mouse antibody (Millipore MAB8755), α-calnexin rabbit polyclonal antibody (Cell Signaling #2433), α-RCAS1 rabbit polyclonal antibody (Cell Signaling #6960), α-mouse goat Alexa 488 (Molecular Probes A11029), and α-rabbit goat Alexa 555 (Molecular Probes A21429). Nuclei were counter-stained with antifade reagent with DAPI (Molecular Probes P36935). Images were captured with a Carl Zeiss LSM700 confocal microscope.
VEEV Neutralization Assays
Neutralization titers of sera from vaccinated animals were determined either by TC-83 VRP neutralization assay (in Figure 3) or by PRNT (in Figure 4). For the TC-83 VRP neutralization assay, sera from vaccinated animals were heat inactivated at 56°C for 30 min, 3-fold serial dilution of the serum samples in 5% DMEM were mixed with an equal volume of TC-83 VRP stock with an infectivity of 500–700 infected cells per counting field and incubated at 37°C for 90 min. TC-83 VRPs were derived from TC-83 replicon expressing GFP, trans-encapsidated using TC-83 glycoproteins, and were generated as described for VRP production. Mixed serum and VRP samples were added to BHK cells grown in 96-well half-area cell-culture plates (Corning). At 20 h post-infection, GFP-positive cells were scored using an Immunospot S5 UV Analyzer (Cellular Technology). 50% neutralization titers, defined as the reciprocal of the serum dilution yielding 50% reduction in the infected cell count (relative to infected cell count in diluent plus virus control wells), were calculated by linear regression interpolation between the two dilutions with wells yielding average infected cell counts above and below the 50% value.
PRNT assay was used to determine the neutralizing titers against VEEV subtype IAB (strain Trinidad donkey) as described previously.59 In brief, 2-fold serial dilutions of sera starting at 1:10 were mixed with equal volumes of medium containing ∼200 PFU of virus and incubated for 24 h at 4°C. The virus and antibody mixtures were then used to infect confluent monolayers of Vero cells in 6-well plates for 1 h at 37°C, after which an overlay consisting of 0.6% agar (Genemate, catalog #E-3121-125) in complete Eagle’s Basal Medium with Earle’s salts (EBME) without phenol red (Invitrogen, catalog #A15950DK) was added. The plates were stained 24 h later by the addition of an overlay containing 5% neutral red (Gibco, catalog #02-0066DG) and 0.6% agar in complete EBME without phenol red, and the plaques were counted 24 h after staining. The NAb titers were then calculated as a reciprocal of the highest dilution, resulting in an 80% reduction of the plaque number as compared to virus-only control wells.
ELISA
Total IgG α-VEEV antibody titers for serum samples were determined by indirect enzyme-linked immunosorbent assay (ELISA) using sucrose-purified, irradiated whole VEEV IAB strain Trinidad donkey antigen as described previously.59 In brief, 2-fold serial dilutions of sera starting at 1:100 were incubated with 250 ng per well of antigen in 96-well plates. HRP-conjugated anti-mouse IgG antibodies (Sigma-Aldrich, catalog #A3673) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic) acid (ABTS) peroxidase substrate (KPL, catalog #50-62-01) were used for detection. For antibody isotype ELISA, HRP-conjugated anti-mouse IgG1 and anti-mouse IgG2a secondary antibodies (Bethyl Laboratories, catalog #A90-105P and A90-107P, respectively) were used. The optical density at 405 nm was determined using a SpectraMax M2e microplate reader (Molecular Devices), and the endpoint titers were calculated in Softmax Pro v5 (Molecular Devices) using a 4-parameter logistic curve fit and a cutoff value equal to the mean optical density of the negative control samples plus three SDs.
Animals, Vaccinations, and Blood Collections
Female BALB/c mice (6–8 weeks old, Charles River Laboratories) were anesthetized with intramuscular (IM) injection of a diluted acepromazine-ketamine-xylazine mixture or with isoflurane gas and were then injected into one tibialis anterior muscle with vaccines diluted to the appropriate concentration as described in the text and shown in the figures. At various times after vaccination, as described in the text and shown in the figures, blood samples were collected from anesthetized mice by retro-orbital or submandibular vein bleed, and serum was recovered by centrifugation.
Aerosol Challenge of Mice
Mice were placed into a class III biological safety cabinet located inside a biosafety level 3 containment suite and exposed in a whole-body aerosol chamber to a VEEV aerosol created by a collison nebulizer for 10 min as previously described.60 Sucrose-purified VEEV IAB strain Trinidad donkey was diluted to an appropriate starting concentration in Hank’s Balanced Salt Solution (Gibco, catalog #14175-095) containing 1% FBS (Thermo Scientific, catalog #SH30071.03) for use in aerosol generation. Samples collected from the all-glass impinger attached to the aerosol chamber were analyzed by plaque assay on Vero cells using standard methods as previously described to determine the inhaled dose of VEEV.61 The mice were monitored at least twice daily for clinical signs of disease and survival for 21 days post-challenge, and any animals found to meet early-endpoint criteria were euthanized. After the post-challenge observation period was completed, the protection data was used to generate Kaplan-Meier survival curves.
Ethics Statement
All animal research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the “Guide for the Care and Use of Laboratory Animals,” Institute for Laboratory Animal Research, Division of Earth and Life Studies, National Research Council, National Academies Press, Washington, DC, 2011. The USAMRIID and GSK facilities where this animal research was conducted are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Author Contributions
M.M.S., L.C.D., C.W.B., P.W.M., A.J.G., J.B.U., and D.Y. were involved in the conception and design of the study. M.M.S., L.C.D., and C.M.S. acquired the data. M.M.S., L.C.D., C.W.B., P.W.M., A.J.G., and D.Y. analyzed and interpreted the results. All authors were involved in drafting the manuscript or revising it critically for important intellectual content. All authors had full access to the data and approved the manuscript before it was submitted by the corresponding author.
Conflicts of Interest
M.M.S., C.W.B., P.W.M., A.J.G., J.B.U., and D.Y. were employees of Novartis Vaccines at the time of the study. M.M.S., C.W.B., A.J.G., J.B.U., and D.Y. are or were employees of the GSK group of companies. J.B.U. and D.Y. are shareholders of GSK. M.M.S., C.W.B., P.W.M., A.J.G., J.B.U., and D.Y. are listed as inventors on patents owned by the GSK group of companies. L.C.D., C.M.S., and C.S.S. declare no competing interests.
Acknowledgments
We thank the RNA vaccine platform team at GSK Vaccines for their support of this work. We thank Giuseppe Palladino (currently at Seqirus) for performing the TC-83 VRP neutralization assay; Thomas Carsillo (currently at Novartis Institute for BioMedical Research) for the generation of the α-VEEV hyperimmune ascites fluid; Luis Brito and Kimberly Hassett (both currently at Moderna Therapeutics) for CNE formulations; the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) Veterinary Medicine Division for performing mouse anesthetizations and blood collections; the USAMRIID Center for Aerobiological Sciences for performing the aerosol exposures; Robert Tesh (University of Texas Medical Branch and World Reference Center for Emerging Viruses and Arboviruses, USA) for the generous gift of the live-attenuated VEE virus TC-83; and Giulietta Maruggi, Robert van den Berg, and Ulrike Krause for critical reading of the manuscript. This work was supported in part by grant CB3947 to USAMRIID from the Joint Science and Technology Office for Chemical and Biological Defense of the Defense Threat and Reduction Agency. This research was performed under U.S. Army Medical Research and Material Command Cooperative Research and Development Agreement W81XWH-11-0294 A7 between USAMRIID and Novartis Vaccines/GlaxoSmithKline Biologicals SA. In March 2015, the Novartis non-influenza vaccines business was acquired by the GSK group of companies. The opinions, interpretations, conclusions, and recommendations contained herein are those of the authors and are not necessarily endorsed by the U.S. Army.
Footnotes
Supplemental Information includes one figure and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.12.013.
Contributor Information
Marcelo M. Samsa, Email: marcelo.m.samsa@gsk.com.
Dong Yu, Email: dong.x.yu@gsk.com.
Supplemental Information
References
- 1.Hawley R.J., Eitzen E.M., Jr. Biological weapons—a primer for microbiologists. Annu. Rev. Microbiol. 2001;55:235–253. doi: 10.1146/annurev.micro.55.1.235. [DOI] [PubMed] [Google Scholar]
- 2.Pittman P.R., Makuch R.S., Mangiafico J.A., Cannon T.L., Gibbs P.H., Peters C.J. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14:337–343. doi: 10.1016/0264-410x(95)00168-z. [DOI] [PubMed] [Google Scholar]
- 3.Paessler S., Weaver S.C. Vaccines for Venezuelan equine encephalitis. Vaccine. 2009;27(Suppl 4):D80–D85. doi: 10.1016/j.vaccine.2009.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed D.S., Lind C.M., Lackemeyer M.G., Sullivan L.J., Pratt W.D., Parker M.D. Genetically engineered, live, attenuated vaccines protect nonhuman primates against aerosol challenge with a virulent IE strain of Venezuelan equine encephalitis virus. Vaccine. 2005;23:3139–3147. doi: 10.1016/j.vaccine.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig G.V., Turell M.J., Vogel P., Kondig J.P., Kell W.K., Smith J.F., Pratt W.D. Comparative neurovirulence of attenuated and non-attenuated strains of Venezuelan equine encephalitis virus in mice. Am. J. Trop. Med. Hyg. 2001;64:49–55. doi: 10.4269/ajtmh.2001.64.49. [DOI] [PubMed] [Google Scholar]
- 6.Fine D.L., Roberts B.A., Terpening S.J., Mott J., Vasconcelos D., House R.V. Neurovirulence evaluation of Venezuelan equine encephalitis (VEE) vaccine candidate V3526 in nonhuman primates. Vaccine. 2008;26:3497–3506. doi: 10.1016/j.vaccine.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Ulmer J.B., Mason P.W., Geall A., Mandl C.W. RNA-based vaccines. Vaccine. 2012;30:4414–4418. doi: 10.1016/j.vaccine.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 8.Geall A.J., Verma A., Otten G.R., Shaw C.A., Hekele A., Banerjee K., Cu Y., Beard C.W., Brito L.A., Krucker T. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA. 2012;109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz J., Lazzaro S., Habbeddine M., Schmidt K.E., Baumhof P., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Heidenreich R., Fotin-Mleczek M. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines. 2017;2:29. doi: 10.1038/s41541-017-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly J.J., Ulmer J.B., Shiver J.W., Liu M.A. DNA vaccines. Annu. Rev. Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 12.Barouch D.H. Rational design of gene-based vaccines. J. Pathol. 2006;208:283–289. doi: 10.1002/path.1874. [DOI] [PubMed] [Google Scholar]
- 13.Gurunathan S., Klinman D.M., Seder R.A. DNA vaccines: immunology, application, and optimization*. Annu. Rev. Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 14.Robinson H.L., Pertmer T.M. DNA vaccines for viral infections: basic studies and applications. Adv. Virus Res. 2000;55:1–74. doi: 10.1016/s0065-3527(00)55001-5. [DOI] [PubMed] [Google Scholar]
- 15.Ulmer J.B., Donnelly J.J., Parker S.E., Rhodes G.H., Felgner P.L., Dwarki V.J., Gromkowski S.H., Deck R.R., DeWitt C.M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 16.Pepini T., Pulichino A.M., Carsillo T., Carlson A.L., Sari-Sarraf F., Ramsauer K., Debasitis J.C., Maruggi G., Otten G.R., Geall A.J. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. J. Immunol. 2017;198:4012–4024. doi: 10.4049/jimmunol.1601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo D. Toward a crystal-clear view of the viral RNA sensing and response by RIG-I-like receptors. RNA Biol. 2014;11:25–32. doi: 10.4161/rna.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atasheva S., Akhrymuk M., Frolova E.I., Frolov I. New PARP gene with an anti-alphavirus function. J. Virol. 2012;86:8147–8160. doi: 10.1128/JVI.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneyama M., Fujita T. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 2010;20:4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein D.I., Reap E.A., Katen K., Watson A., Smith K., Norberg P., Olmsted R.A., Hoeper A., Morris J., Negri S. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009;28:484–493. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 21.Ljungberg K., Liljeström P. Self-replicating alphavirus RNA vaccines. Expert Rev. Vaccines. 2015;14:177–194. doi: 10.1586/14760584.2015.965690. [DOI] [PubMed] [Google Scholar]
- 22.Brito L.A., Chan M., Shaw C.A., Hekele A., Carsillo T., Schaefer M., Archer J., Seubert A., Otten G.R., Beard C.W. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014;22:2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geall A.J., Settembre E.C., Ulmer J.B. Using self-amplifying mRNA vaccines to facilitate a rapid response to pandemic influenza. European Pharmaceutical Review. 2014;19:20–23. [Google Scholar]
- 24.Hekele A., Bertholet S., Archer J., Gibson D.G., Palladino G., Brito L.A., Otten G.R., Brazzoli M., Buccato S., Bonci A. Rapidly produced SAM(®) vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microbes Infect. 2013;2:e52. doi: 10.1038/emi.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeza Garcia A., Siu E., Sun T., Exler V., Brito L., Hekele A., Otten G., Augustijn K., Janse C.J., Ulmer J.B. Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat. Commun. 2018;9:2714. doi: 10.1038/s41467-018-05041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogers W.M., Oostermeijer H., Mooij P., Koopman G., Verschoor E.J., Davis D., Ulmer J.B., Brito L.A., Cu Y., Banerjee K. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J. Infect. Dis. 2015;211:947–955. doi: 10.1093/infdis/jiu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brazzoli M., Magini D., Bonci A., Buccato S., Giovani C., Kratzer R., Zurli V., Mangiavacchi S., Casini D., Brito L.M. Induction of Broad-Based Immunity and Protective Efficacy by Self-amplifying mRNA Vaccines Encoding Influenza Virus Hemagglutinin. J. Virol. 2015;90:332–344. doi: 10.1128/JVI.01786-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Hagan D.T., Ott G.S., Nest G.V., Rappuoli R., Giudice G.D. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert Rev. Vaccines. 2013;12:13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 29.Jose J., Snyder J.E., Kuhn R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009;4:837–856. doi: 10.2217/fmb.09.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed D.S., Glass P.J., Bakken R.R., Barth J.F., Lind C.M., da Silva L., Hart M.K., Rayner J., Alterson K., Custer M. Combined alphavirus replicon particle vaccine induces durable and cross-protective immune responses against equine encephalitis viruses. J. Virol. 2014;88:12077–12086. doi: 10.1128/JVI.01406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atasheva S., Kim D.Y., Akhrymuk M., Morgan D.G., Frolova E.I., Frolov I. Pseudoinfectious Venezuelan equine encephalitis virus: a new means of alphavirus attenuation. J. Virol. 2013;87:2023–2035. doi: 10.1128/JVI.02881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams A.J., O’Brien L.M., Phillpotts R.J., Perkins S.D. Improved efficacy of a gene optimised adenovirus-based vaccine for venezuelan equine encephalitis virus. Virol. J. 2009;6:118. doi: 10.1186/1743-422X-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupuy L.C., Richards M.J., Ellefsen B., Chau L., Luxembourg A., Hannaman D., Livingston B.D., Schmaljohn C.S. A DNA vaccine for venezuelan equine encephalitis virus delivered by intramuscular electroporation elicits high levels of neutralizing antibodies in multiple animal models and provides protective immunity to mice and nonhuman primates. Clin. Vaccine Immunol. 2011;18:707–716. doi: 10.1128/CVI.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillpotts R.J., O’brien L., Appleton R.E., Carr S., Bennett A. Intranasal immunisation with defective adenovirus serotype 5 expressing the Venezuelan equine encephalitis virus E2 glycoprotein protects against airborne challenge with virulent virus. Vaccine. 2005;23:1615–1623. doi: 10.1016/j.vaccine.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 35.Phillpotts R.J., Jones L.D., Howard S.C. Monoclonal antibody protects mice against infection and disease when given either before or up to 24 h after airborne challenge with virulent Venezuelan equine encephalitis virus. Vaccine. 2002;20:1497–1504. doi: 10.1016/s0264-410x(01)00505-9. [DOI] [PubMed] [Google Scholar]
- 36.Paessler S., Yun N.E., Judy B.M., Dziuba N., Zacks M.A., Grund A.H., Frolov I., Campbell G.A., Weaver S.C., Estes D.M. Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology. 2007;367:307–323. doi: 10.1016/j.virol.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun N.E., Peng B.H., Bertke A.S., Borisevich V., Smith J.K., Smith J.N., Poussard A.L., Salazar M., Judy B.M., Zacks M.A. CD4+ T cells provide protection against acute lethal encephalitis caused by Venezuelan equine encephalitis virus. Vaccine. 2009;27:4064–4073. doi: 10.1016/j.vaccine.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riemenschneider J., Garrison A., Geisbert J., Jahrling P., Hevey M., Negley D., Schmaljohn A., Lee J., Hart M.K., Vanderzanden L. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21:4071–4080. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 39.Hannaman D., Dupuy L.C., Ellefsen B., Schmaljohn C.S. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine. 2016;34:3607–3612. doi: 10.1016/j.vaccine.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 40.Maruggi G., Chiarot E., Giovani C., Buccato S., Bonacci S., Frigimelica E., Margarit I., Geall A., Bensi G., Maione D. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35:361–368. doi: 10.1016/j.vaccine.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 41.Vogel A.B., Lambert L., Kinnear E., Busse D., Erbar S., Reuter K.C., Wicke L., Perkovic M., Beissert T., Haas H. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018;26:446–455. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedic M., Senn J.J., Lynn A., Laska M., Smith M., Platz S.J., Bolen J., Hoge S., Bulychev A., Jacquinet E. Safety Evaluation of Lipid Nanoparticle-Formulated Modified mRNA in the Sprague-Dawley Rat and Cynomolgus Monkey. Vet. Pathol. 2018;55:341–354. doi: 10.1177/0300985817738095. [DOI] [PubMed] [Google Scholar]
- 43.Iavarone C., O’hagan D.T., Yu D., Delahaye N.F., Ulmer J.B. Mechanism of action of mRNA-based vaccines. Expert Rev. Vaccines. 2017;16:871–881. doi: 10.1080/14760584.2017.1355245. [DOI] [PubMed] [Google Scholar]
- 44.Elvin S.J., Bennett A.M., Phillpotts R.J. Role for mucosal immune responses and cell-mediated immune functions in protection from airborne challenge with Venezuelan equine encephalitis virus. J. Med. Virol. 2002;67:384–393. doi: 10.1002/jmv.10086. [DOI] [PubMed] [Google Scholar]
- 45.Mathews J.H., Roehrig J.T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J. Immunol. 1982;129:2763–2767. [PubMed] [Google Scholar]
- 46.Roehrig J.T., Mathews J.H. The neutralization site on the E2 glycoprotein of Venezuelan equine encephalomyelitis (TC-83) virus is composed of multiple conformationally stable epitopes. Virology. 1985;142:347–356. doi: 10.1016/0042-6822(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 47.Hunt A.R., Roehrig J.T. Localization of a protective epitope on a Venezuelan equine encephalomyelitis (VEE) virus peptide that protects mice from both epizootic and enzootic VEE virus challenge and is immunogenic in horses. Vaccine. 1995;13:281–288. doi: 10.1016/0264-410x(95)93315-z. [DOI] [PubMed] [Google Scholar]
- 48.Hunt A.R., Frederickson S., Hinkel C., Bowdish K.S., Roehrig J.T. A humanized murine monoclonal antibody protects mice either before or after challenge with virulent Venezuelan equine encephalomyelitis virus. J. Gen. Virol. 2006;87:2467–2476. doi: 10.1099/vir.0.81925-0. [DOI] [PubMed] [Google Scholar]
- 49.Bennett A.M., Elvin S.J., Wright A.J., Jones S.M., Phillpotts R.J. An immunological profile of Balb/c mice protected from airborne challenge following vaccination with a live attenuated Venezuelan equine encephalitis virus vaccine. Vaccine. 2000;19:337–347. doi: 10.1016/s0264-410x(00)00123-7. [DOI] [PubMed] [Google Scholar]
- 50.Schmaljohn A.L., Johnson E.D., Dalrymple J.M., Cole G.A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982;297:70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- 51.Parker M.D., Buckley M.J., Melanson V.R., Glass P.J., Norwood D., Hart M.K. Antibody to the E3 glycoprotein protects mice against lethal venezuelan equine encephalitis virus infection. J. Virol. 2010;84:12683–12690. doi: 10.1128/JVI.01345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathews J.H., Roehrig J.T., Trent D.W. Role of complement and the Fc portion of immunoglobulin G in immunity to Venezuelan equine encephalomyelitis virus infection with glycoprotein-specific monoclonal antibodies. J. Virol. 1985;55:594–600. doi: 10.1128/jvi.55.3.594-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brooke C.B., Schäfer A., Matsushima G.K., White L.J., Johnston R.E. Early activation of the host complement system is required to restrict central nervous system invasion and limit neuropathology during Venezuelan equine encephalitis virus infection. J. Gen. Virol. 2012;93:797–806. doi: 10.1099/vir.0.038281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones L.D., Bennett A.M., Moss S.R., Gould E.A., Phillpotts R.J. Cytotoxic T-cell activity is not detectable in Venezuelan equine encephalitis virus-infected mice. Virus Res. 2003;91:255–259. doi: 10.1016/s0168-1702(02)00275-7. [DOI] [PubMed] [Google Scholar]
- 55.Brooke C.B., Deming D.J., Whitmore A.C., White L.J., Johnston R.E. T cells facilitate recovery from Venezuelan equine encephalitis virus-induced encephalomyelitis in the absence of antibody. J. Virol. 2010;84:4556–4568. doi: 10.1128/JVI.02545-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandl C.W., Aberle J.H., Aberle S.W., Holzmann H., Allison S.L., Heinz F.X. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat. Med. 1998;4:1438–1440. doi: 10.1038/4031. [DOI] [PubMed] [Google Scholar]
- 57.Tretyakova I., Lukashevich I.S., Glass P., Wang E., Weaver S., Pushko P. Novel vaccine against Venezuelan equine encephalitis combines advantages of DNA immunization and a live attenuated vaccine. Vaccine. 2013;31:1019–1025. doi: 10.1016/j.vaccine.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loomis R.J., Lilja A.E., Monroe J., Balabanis K.A., Brito L.A., Palladino G., Franti M., Mandl C.W., Barnett S.W., Mason P.W. Vectored co-delivery of human cytomegalovirus gH and gL proteins elicits potent complement-independent neutralizing antibodies. Vaccine. 2013;31:919–926. doi: 10.1016/j.vaccine.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Hodgson L.A., Ludwig G.V., Smith J.F. Expression, processing, and immunogenicity of the structural proteins of Venezuelan equine encephalitis virus from recombinant baculovirus vectors. Vaccine. 1999;17:1151–1160. doi: 10.1016/s0264-410x(98)00335-1. [DOI] [PubMed] [Google Scholar]
- 60.Hart M.K., Pratt W., Panelo F., Tammariello R., Dertzbaugh M. Venezuelan equine encephalitis virus vaccines induce mucosal IgA responses and protection from airborne infection in BALB/c, but not C3H/HeN mice. Vaccine. 1997;15:363–369. doi: 10.1016/s0264-410x(96)00204-6. [DOI] [PubMed] [Google Scholar]
- 61.Pratt W.D., Gibbs P., Pitt M.L., Schmaljohn A.L. Use of telemetry to assess vaccine-induced protection against parenteral and aerosol infections of Venezuelan equine encephalitis virus in non-human primates. Vaccine. 1998;16:1056–1064. doi: 10.1016/s0264-410x(97)00192-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.