Abstract
We identified SCN1A variants in two infants who died of Sudden Infant Death Syndrome (SIDS) from an exome sequencing study of 10 cases of SIDS with hippocampal abnormalities but no history of seizures. One harbored SCN1A G682V, and the other had two SCN1A variants in cis: L1296M and E1308D, a variant previously associated with epilepsy. Functional evaluation in a heterologous expression system demonstrated partial loss-of-function for both G682V and the compound variant L1296M/E1308D. Our cases represent a novel association between SCN1A and SIDS, extending the SCN1A spectrum from epilepsy to SIDS. Our findings provide insights into SIDS and support genetic evaluation focused on epilepsy genes in SIDS.
Keywords: Sudden unexpected death, sodium channel, epilepsy, dentate gyrus, hippocampus
Introduction
Sudden infant death syndrome (SIDS), the death of an infant less than 1 year of age that remains unexplained after complete autopsy and death scene investigation, is the leading cause of post-neonatal infant mortality in the United States. SIDS is hypothesized to result from the interaction of intrinsic vulnerabilities in the infant, a critical developmental period, and exogenous stressors in what has been called the ‘triple-risk’ model of SIDS.1 In one study, the majority of SIDS infants (57%) had at least two extrinsic risks and one intrinsic risk factor.2 Through research into underlying neuropathological causes of SIDS, we have reported hippocampal abnormalities in approximately 40% of infants dying of SIDS, chiefly bilamination of the granule cell layer in the dentate gyrus.3; 4 We report this same lesion in children over 1 year of age dying of Sudden Unexplained Death in Childhood (SUDC).3 Such lesions are classically associated with temporal lobe epilepsy.5
The association of epilepsy-related pathology with SIDS and SUDC,3; 4 recently called epilepsy in situ,6 leads to questions about epilepsy-related mechanisms in sudden death. Sudden Unexpected Death in Epilepsy (SUDEP) exemplifies the well-recognized association between epilepsy and sudden death. In addition, we and others have described an association between SUDC and personal or family history of febrile seizures (FS), suggesting possible shared genetic predispositions for these entities.3; 7 Notably, the SIDS infants and SUDC children with hippocampal abnormalities had not been diagnosed with epilepsy, though some had a history of FS.3 Collectively, these data support an association between sudden death and seizures, even in the absence of overt epilepsy.
There is active investigation into potential mechanisms involving epilepsy- and cardiac arrhythmia-related genes implicated in SUDEP, including SCN1A,8; 9in the predisposition of some children to sudden death. We performed whole exome sequencing (WES) to evaluate 10 cases of SIDS with the hypothesis that some cases are associated with epilepsy-associated genes. Here we report the discovery of SCN1A variants in two SIDS cases.
Methods
DNA from 10 SIDS cases was obtained through the Office of the Medical Examiner (OME), San Diego, CA in accordance with California law Chapter 955, Statutes of 1989 (SB1069), permitting the use of autopsy tissues and DNA from SIDS infants for research. Samples were de-identified; parental samples are not available. Antemortem history and autopsy findings were reviewed for evidence of known causes of death.
WES and analysis were performed using standard methods plus evaluation for the presence of variants in three SCN1A-specific databases: SCN1A Variant Database, SCN1A Infobase, and the Ghangzhou Medical University SCN1A Database. We focused further analysis on variants with population allele frequency <0.001 and OMIM disease associations, particularly sudden death, seizures, cardiac arrhythmia, and metabolic disease. We applied American College of Medical Genetics and Genomics (ACMG) guidelines for variant interpretation to each variant10. All variants considered likely pathogenic were confirmed by Sanger sequencing (primer sequences available on request). Functional evaluation of the variants was performed using manual patch-clamp recording. Mutagenesis of recombinant human NaV1.1 (encoded by SCN1A) was performed as previously described11; 12 to create G682V and the compound variant L1296M/E1308D. The open reading frames of all plasmid preparations were sequenced in their entirety prior to use in experiments. Heterologous co-expression of WT NaV1.1or the SIDS-associated variants with the human β1 and β2 subunits in tsA201 cells was performed as previously described.12 Whole-cell voltage clamp recording was performed at room temperature as previously described.12; 13
Results
From among the 10 cases ascertained with SIDS, 5 had hippocampal sections available; of these, 3/5 had dentate gyrus abnormalities, including the two reported here. The Supplementary Table contains available clinical data for all 10 cases.
Case 1 was a Caucasian girl who died at 2 months, with cause of death recorded as SIDS. The infant had prenatal opioid exposure, a known risk factor for SIDS. She was born at 35 gestational weeks to an opioid-dependent mother who began methadone treatment at 5 gestational months. At birth, the infant required medication for neonatal opiate withdrawal syndrome for 19 days and subsequently metoclopramide and lansoprazole for gastroesophageal reflux disease (GERD). She was placed in foster care and had been healthy prior to death. Prior to death, she had been swaddled and placed supine to sleep, with the head of the bed elevated as recommended for GERD. She was found diaphoretic and unresponsive in the prone position. Toxicological assessment for drugs of abuse was negative. Neuropathological examination revealed no macroscopic abnormalities. Microscopic examination of the hippocampus revealed focal bilamination of the dentate gyrus (Fig. 1A-B).
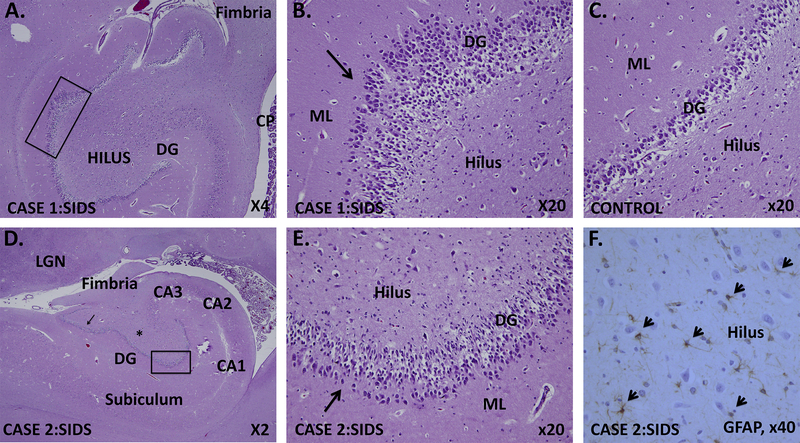
Figure 1.
Hippocampal developmental lesions in two infants with SIDS and variants in SCN1A.
A. Case 1: SIDS infant with SCN1A c.2045G>T, p.Gly682Val variant. Low power photograph of the hippocampus shows the abnormal dentate gyrus with a region of focal bilamination highlighted in the black rectangle. (Haematoxylin + Eosin stain, x4).
B. Case 1: Focal dentate bilamination with two layers of granule cells and intervening neuropil (arrow). Other abnormalities include mild hyperconvolution of the dentate gyrus, immature neuronal-like precursors in the subgranular zone, ectopic granule cells in the molecular layer and hilus, and mild hilar gliosis. (Haematoxylin + Eosin stain, x20).
C. Control dentate gyrus in an age-matched infant showing the normal single layer of dentate gyrus granule cells in row. (Haematoxylin + Eosin stain, x20).
D. Case 2: SIDS infant with c.3886T>A, Leu1296Met and c. 3924A>T, Glu1308Asp variants in cis: Low power photograph of the hippocampus shows dentate gyrus bilamination in two foci (black rectangle, arrow). The dentate gyrus is slightly hyperconvoluted. Other abnormalities include immature neuronal-like precursors in the subgranular zone, ectopic granule cells in the molecular layer and hilus, and mild hilar gliosis. (Haematoxylin + Eosin stain, x2).
E. Case 2: Focal dentate bilamination (and trilamination) (arrow) in the rectangle from Fig. 1.D. (Haematoxylin + Eosin stain, x20).
F. Hilar gliosis in Case 2, demonstrated with standard immunocytochemistry for glial fibrillary acidic protein (GFAP) to label reactive astrocytes (short arrow). GFAP, x40. Abbreviations: CP, choroid plexus; DG, dentate gyrus, ML, LGN, lateral geniculare nucleus; molecular layer.
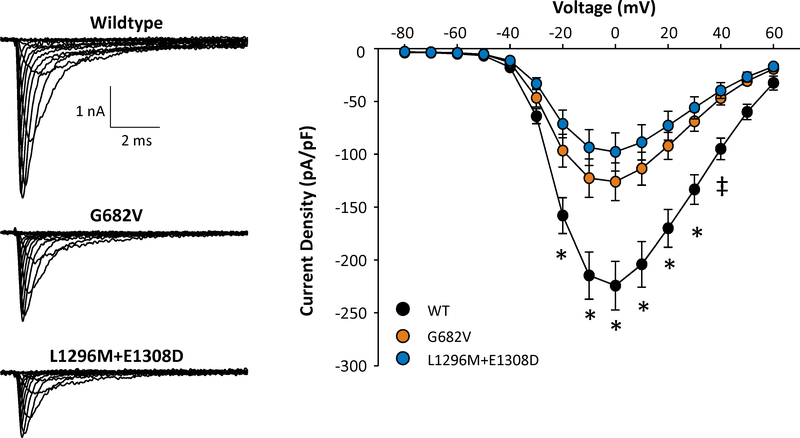
Exome analysis revealed SCN1A c.2045G>T, p.G682V (NM_001202435.1), confirmed by Sanger sequencing. Gly682 is a highly conserved amino acid in a cytoplasmic domain of SCN1A. SIFT score is 0 (deleterious), MutationTaster score is 1 (disease-causing), but Polyphen-2 score is 0.069 (benign). The variant is not seen in the ESP, ExAC, or the three referenced SCN1A databases, but reported nearby variants affecting the same domain have been associated with Dravet syndrome (D674G)14 and borderline severe myoclonic epilepsy of infancy, (T685LfsX5)15 (Figure 2.) Functional evaluation of this variant demonstrated significantly lower current density compared with WT channels, consistent with a partial loss-of-function effect (Figure 3); additional experiments showed no differences in the voltage-dependence of activation or inactivation, recovery from inactivation, or use-dependent channel rundown. No other variants were present that could plausibly explain the phenotype.
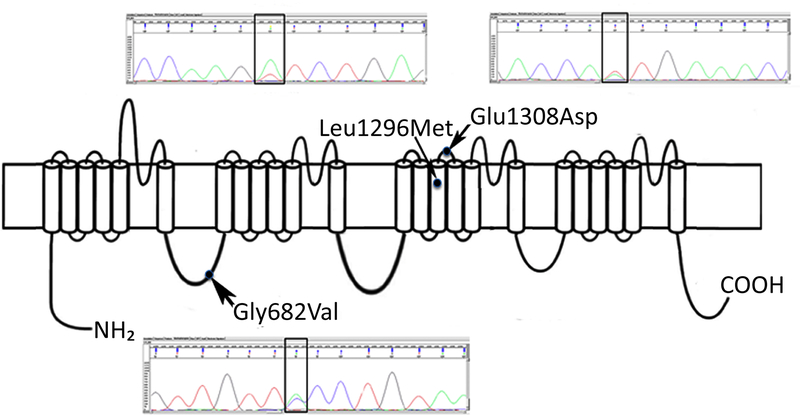
Figure 2.
Model of SCN1A protein and predicted pathogenic variants seen in two cases of SIDS. Case 1: c.2045G>T, p.Gly682Val. Case 2: c.3886T>A, Leu1296Met and c. 3924A>T, Glu1308Asp (present in cis configuration). Pathologic variants in close proximity to G682V, affecting the same transmembrane domain, have been associated with Dravet syndrome (D674G)14 and borderline severe myoclonic epilepsy of infancy, (T685LfsX5)15 and are depicted by dots on the model to show relative position. Previously reported pathologic variants in close proximity to Leu1296 are W1284X and F1289del; the patients were diagnosed with severe myoclonic epilepsy of infancy.16
Figure 3.
Functional evaluation of SCN1A variants.
A. Representative whole-cell sodium currents recorded from tsA201 cells expressing either WT NaV1.1 or SIDS associated variants. B. Current-voltage relationships of WT NaV1.1 and SIDS associated variants. All data are expressed as mean ± SEM for 14–15 measurements. Statistical differences were determined by ANOVA (*, p<0.05 for both variants compared to WT; ‡, p<0.05 between WT and L1296M / E1308D).
Case 2 was a Caucasian girl who died at age 7 weeks with cause of death reported as SIDS. The mother had received prenatal care beginning at 4.5 months gestation and was placed on bedrest at 6 months gestation due to potential placental abruption. The mother was positive for Group B Streptococcus (GBS); the infant’s GBS status was not reported. Exposures were limited to second-hand tobacco smoke. The infant fell asleep in her caregiver’s arms, was placed supine in an adult bed, and witnessed supine while sleeping. She was found prone and unresponsive. There were no macroscopic findings on neuropathological assessment. Examination of the hippocampi revealed focal areas of bilamination and a small amount of hilar gliosis (Fig. 1D-F).
Exome analysis identified two SCN1A variants, c.3886T>A, p.L1296M and c. 3924A>T, p.E1308D, each confirmed by Sanger sequencing, and determined to be in cis configuration by direct inspection of the exome data in the Integrated Genomics Viewer (IGV). The L1296M variant affects the highly conserved L1296 amino acid in the SCN1A S3 helical loop of transmembrane domain III. SIFT score is 0 (deleterious), Mutation Taster score is 0.616 (polymorphism), and Polyphen-2 score is 0.897 (possibly damaging). The variant is not seen in the ESP, ExAC, or the referenced SCN1A databases but is in close proximity to a nonsense variant and an in-frame deletion associated with epilepsy16 (Figure 2).
The c. 3924A>T, p.E1308D variant affects a highly conserved amino acid in the extracellular domain of the transmembrane domain III of SCN1A (Figure 1). SIFT score is 0 (deleterious), MutationTaster score is 0.998 (disease-causing), and Polyphen-2 score is 0.042 (benign). The variant is present in ClinVar as a variant of uncertain significance (VUS), associated with Dravet syndrome. It is present in the ESP (ESP6500SIV2) in 0.09% of European Americans and in ExAC with allele frequency 0.075%. This variant has also been reported in association with familial febrile seizures17 and Dravet syndrome,15; 18 including in a child with a variant inherited from an asymptomatic parent,18 (referenced in SCN1A Infobase and Ghangzhou SCN1A database). Functional assessment of this compound variant (L1296M/E1308D) demonstrated lower whole-cell sodium current density compared to WT channels to a degree similar to G682V, consistent with a partial loss-of-function (Figure 3); additional experiments demonstrated no differences in the voltage-dependence of activation or inactivation, recovery from inactivation, or use-dependent channel rundown.
Exome data analysis also revealed a variant in AKAP9, NM_005751, ENST00000356239.3:c.1924G>A, p.Glu642Lys, predicted pathogenic. However, the gene is tolerant to missense variation (ExAC missense constraint metric z = −2.75), and the variant is not in proximity to the KCNQ1-binding domains of AKAP9 or the single published long QT syndrome-associated variant. Therefore, we conclude that the AKAP9 variant is not a contributor to SIDS in this case. No other disease-associated variants related to sudden death were present for this case.
Discussion
We describe a novel association between SCN1A and SIDS, evidence for a role for genetics in SIDS. From a cohort of 10 infants with SIDS, we identified two cases with heterozygous SCN1A variants. The variants we report, similar to those we have cited,8; 9; 14–20 are predicted to be pathogenic using in silico assessments. These predictions are further strengthened by association with previously reported cases with epilepsy, location of the variants in critical, disease-associated domains of the protein, and functional evidence that the variants present in both cases exhibit partial loss-of-function. SCN1A encodes NaV1.1, a voltage-gated sodium channel, expressed in human brain during fetal and early post-natal life.21 SCN1A variants are associated with the familial syndrome Genetic Epilepsy with Febrile Seizures Plus (GEFS+), with a wide phenotypic spectrum from unaffected or mildly affected with febrile seizures to severe epileptic encephalopathy. SCN1A is also associated with Dravet syndrome (severe myoclonic epilepsy of infancy), typically with de novo heterozygous truncating or missense mutations, with both types affecting the same translated protein domains. SCN1A is intolerant to missense variation (ExAc constraint metric z-score = 5.61), and its role in a clinically diverse group of epilepsies was highlighted in the largest genome-wide association study of epilepsy.22 Although we are unable to determine whether the variants in the two cases reported here are de novo or inherited because of lack of access to parental DNA, given the wide range of phenotypes associated with this gene and the demonstrated loss of function associated with these variants, the lack of parental data does not diminish the impact of our findings.
A unique feature of the two cases we report with SIDS and SCN1A variants is hippocampal dentate gyrus bilamination, a variant of granule cell dispersion classically associated with temporal lobe epilepsy.5 This feature has been described previously in association with SIDS and SUDC3; 4 but not with SCN1A prior to this report. The limited literature on neuropathological abnormalities in patients with SCN1A-related epilepsy includes hippocampal sclerosis, focal cortical dysplasia, periventricular heterotopia, micronodular dysplasia of the medial temporal lobe, and granule cell dispersion of the dentate gyrus.23 Dentate bilamination, as seen in our two cases without overt epilepsy before death, may represent a primary developmental lesion and may represent an epileptogenic nidus for the generation of seizures, in these cases subclinical. Alternatively, the dentate bilamination may be secondary to seizures, again subclinical, that arose in the hippocampus due to SCN1A dysfunction. The extent to which SCN1A and other epilepsy-related genes are associated with the developmental hippocampal abnormalities that we have observed in 40–50% of cases with SIDS and SUDC,3; 4 remains to be determined.
SCN1A-related mechanisms have been postulated to underlie a risk of SUDEP in patients with Dravet syndrome,8 with evidence from rodent models providing supporting evidence.24 In recent reports, al., SCN1A was not included in the panel of genes interrogated.9; 20 A report of SCN1A Leu61Pro in association with SUDC25 underscores the potential for SIDS and SUDC to share SCN1A-related mechanisms. Additional SCN1A variants associated with SUDEP8 support the extension of the spectrum of SCN1A into the realm of SUDEP. All three conditions, regardless of age, concern unexpected death in the sleep state, and seizure-related mechanisms have been invoked in their pathogenesis.3; 4; 6 Together, our cases and the previously published cases of SUDC and SUDEP present a potential link between an epileptogenic lesion in the hippocampus, variants in a gene associated with epilepsy, and sudden, unexplained, sleep-related death. In keeping with the ‘triple-risk’ model of SIDS1, we conclude that the SCN1A variants in the cases reported represent an intrinsic vulnerability that, in combination with other endogenous and exogenous factors, may have contributed to the risk of SIDS. Further genetic studies should be included as part of a ‘molecular autopsy’ for as many cases of SIDS as possible, and investigation into the mechanisms that may lead to sudden death in individuals with variants in SCN1A and other epilepsy-associated genes is warranted.
Conclusions
The association between SCN1A and SIDS, coupled with recent reports of cases with SUDC25 and SUDEP,8 extends the spectrum of SCN1A from febrile seizures and epilepsy to sudden death. Notably, infants and children classified as SIDS and SUDC with the SCN1A variants and dentate gyral lesions did not have a reported history of epilepsy. The novel association of SCN1A with SIDS supports further intense efforts to understand epilepsy-related mechanisms into sudden death across the age spectrum in individuals with and without an overt history of seizures or epilepsy.
Supplementary Material
Acknowledgements
We thank the many families who have lost a child to SIDS and have participated in SIDS research. We also thank the coroners, medical examiners, and pathologists who helped in ensuring the evaluation of the forensic autopsy materials. We thank Dr. David Paterson for his early work on the SIDS cohort and Jean-Marc DeKeyser for assistance with engineering sodium channel variant plasmids. We appreciate the administrative assistance of Dr. Roya Dastjerdi, Ms Kelly Journey, and Ms Kalen Fletcher. We thank Dr. Calum MacRae and Alan Taylor, MS, CGC for thoughtful discussion, and Dr. Dawna D. Armstrong for critical reading of the manuscript. This study was supported by the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center funded by National Institutes of Health P30 HD18655, NIH grant HL131914 (ALG), Robert’s Program on Sudden Unexpected Death in Pediatrics, Citizens United for Research in Epilepsy/the Isaiah Stone Foundation Award, and the Departments of Pathology, Pediatrics, and Neurology at Boston Children’s Hospital.
Footnotes
Ethical Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures
Author C. Brownstein is a paid consultant for WuXi NextCode. Author A. Poduri is an Associate Editor for Epilepsia, serves on the Editorial Board for Annals of Neurology, and served on the Scientific Advisory Board of the Dravet Syndrome Foundation. Author M. Bainbridge is founder of Codified Genomics, a DNA sequencing analytics company. Author A. George serves on a scientific advisory board for Otsuka Pharmaceutics, Inc., and is an unpaid scientific advisor for Praxis Precision Medicines, Inc. None of the remaining authors have any conflicts of interest to disclose.
References
- 1.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate 1994;65:194–197. [DOI] [PubMed] [Google Scholar]
- 2.Trachtenberg FL, Haas EA, Kinney HC, et al. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics 2012;129:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinney HC, Poduri AH, Cryan JB, et al. Hippocampal Formation Maldevelopment and Sudden Unexpected Death across the Pediatric Age Spectrum. J Neuropathol Exp Neurol 2016;75:981–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinney HC, Cryan JB, Haynes RL, et al. Dentate gyrus abnormalities in sudden unexplained death in infants: morphological marker of underlying brain vulnerability. Acta neuropathologica 2015;129:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res 1990;535:195–204. [DOI] [PubMed] [Google Scholar]
- 6.Noebels J Hippocampal abnormalities and sudden childhood death. Forensic science, medicine, and pathology 2016;12:198–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesdorffer DC, Crandall LA, Friedman D, et al. Sudden unexplained death in childhood: A comparison of cases with and without a febrile seizure history. Epilepsia 2015;56:1294–1300. [DOI] [PubMed] [Google Scholar]
- 8.Bagnall RD, Crompton DE, Petrovski S, et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol 2016;79:522–534. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer J, Lecca MR, Russo G, et al. Post-mortem whole-exome analysis in a large sudden infant death syndrome cohort with a focus on cardiovascular and metabolic genetic diseases. European journal of human genetics : EJHG 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahlig KM, Rhodes TH, Pusch M, et al. Divergent sodium channel defects in familial hemiplegic migraine. Proceedings of the National Academy of Sciences of the United States of America 2008;105:9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson CH, Kahlig KM, George AL Jr. SCN1A splice variants exhibit divergent sensitivity to commonly used antiepileptic drugs. Epilepsia 2011;52:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson CH, Hawkins NA, Kearney JA, et al. CaMKII modulates sodium current in neurons from epileptic Scn2a mutant mice. Proceedings of the National Academy of Sciences of the United States of America 2017;114:1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harkin LA, McMahon JM, Iona X, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain : a journal of neurology 2007;130:843–852. [DOI] [PubMed] [Google Scholar]
- 15.Zuberi SM, Brunklaus A, Birch R, et al. Genotype-phenotype associations in SCN1A-related epilepsies. Neurology 2011;76:594–600. [DOI] [PubMed] [Google Scholar]
- 16.Wallace RH, Hodgson BL, Grinton BE, et al. Sodium channel alpha1-subunit mutations in severe myoclonic epilepsy of infancy and infantile spasms. Neurology 2003;61:765–769. [DOI] [PubMed] [Google Scholar]
- 17.Orrico A, Galli L, Grosso S, et al. Mutational analysis of the SCN1A, SCN1B and GABRG2 genes in 150 Italian patients with idiopathic childhood epilepsies. Clinical genetics 2009;75:579–581. [DOI] [PubMed] [Google Scholar]
- 18.Nicita F, Spalice A, Papetti L, et al. Genotype-phenotype correlations in a group of 15 SCN1A-mutated Italian patients with GEFS+ spectrum (seizures plus, classical and borderline severe myoclonic epilepsy of infancy). J Child Neurol 2010;25:1369–1376. [DOI] [PubMed] [Google Scholar]
- 19.Wallace RH, Scheffer IE, Barnett S, et al. Neuronal sodium-channel alpha1-subunit mutations in generalized epilepsy with febrile seizures plus. American journal of human genetics 2001;68:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Methner DN, Scherer SE, Welch K, et al. Postmortem genetic screening for the identification, verification, and reporting of genetic variants contributing to the sudden death of the young. Genome research 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Takashima S, Segawa Y, et al. The developmental changes of Na(v)1.1 and Na(v)1.2 expression in the human hippocampus and temporal lobe. Brain research 2011;1389:61–70. [DOI] [PubMed] [Google Scholar]
- 22.Genetic determinants of common epilepsies: a meta-analysis of genome-wide association studies. The Lancet. Neurology 2014;13:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catarino CB, Liu JY, Liagkouras I, et al. Dravet syndrome as epileptic encephalopathy: evidence from long-term course and neuropathology. Brain : a journal of neurology 2011;134:2982–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 2015;7:282ra246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halvorsen M, Petrovski S, Shellhaas R, et al. Mosaic mutations in early-onset genetic diseases. Genetics in medicine : official journal of the American College of Medical Genetics 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.