Abstract
We previously showed the safety of using cord blood (CB) expanded ex vivo in cocultures with allogeneic mesenchymal precursor cells (MPC) after myeloablative conditioning with faster recovery of neutrophils and platelets compared with historical controls.
Herein, we report the transplant outcomes of 27 patients with hematologic cancers who received one CB unit ex vivo expanded with MPCs in addition to an unmanipulated CB (MPC group) after reduced intensity conditioning (RIC). The results in this group were compared with 51 historical controls who received two unmanipulated CB units (control group). The analyses were stratified for two RIC treatment groups: 1) total body irradiation 200 cGy + cyclophosphamide + fludarabine) (TCF), and 2) Melphalan+ fludarabine (FM).
Co-culture of CB with MPCs led to an expansion of total nucleated cells by a median factor of 12 and of CD34+ cells by a median factor of 49. In patients that engraftment occurred, the median time to neutrophil engraftment was 12 days in the MPC group, as compared with 16 days in controls (p= 0.02). The faster neutrophil engraftment was observed in both RIC groups. The cumulative incidence of neutrophil engraftment on day 26 was 75% with expansion versus 50% without expansion in patients who received FM as the RIC regimen (p=0.03). Incidence of neutrophil engraftment was comparable in MPC and control groups if treated with TCF (82% versus 79%, p=0.4).
Transplantation of CB units expanded with MPCs is safe and effective with faster neutrophil engraftment even after RIC regimens.
Keywords: cord blood, expansion, reduced intensity conditioning, engraftment, neutrophil recovery
Article Summary
1. In this retrospective analysis, the infusion of one CB unit ex vivo expanded with MPCs in addition to an unmanipulated CB unit after RIC regimen increased the cell dose to be infused with improvement in time to neutrophil engraftment compared with infusion of two unmanipulated CB units.
2. Our results support the notion that strategies to increase cell dose infused in CB unit by manipulation may lead to a lower minimal TNC dose required for a safe CB transplantation even with RIC regimens and increase the number of acceptable CB units available in public CB banks.
INTRODUCTION
Cord blood transplantation (CBT) has historically been associated with prolonged time to engraftment, high risk of graft rejection, delayed immune reconstitution and resultant infections, primarily related to the naivety of CB cells and low total cellular content in the graft as compared with bone marrow (BM) or peripheral blood (PB) progenitor cells graft(1–8). In an attempt to improve the cell dose, several techniques have been devised to expand CB units ex vivo prior to infusion (9–13). Our group also previously reported encouraging outcomes of double unit CBT (DCBT) where one of the CB units was expanded ex vivo by co-culturing with mesenchymal precursor cells (MPC) and the second unit was infused unmanipulated.(14) Among patients who received the expanded CB unit, neutrophil engraftment occurred at a median of 15 days compared with 24 days in the historical controls. All patients in that study received myeloablative conditioning. However, high dose myeloablative regimens are poorly tolerated by medically infirm or older individuals. The use of reduced-intensity conditioning (RIC) with CBT has been an effective strategy for older and less fit patients with a potential curative intent (2, 15–20) with a low treatment related mortality.(2, 18, 21–23)
Herein, we retrospectively studied the outcomes of adult patients with hematological malignancies who received RIC followed by infusion of an MPC-expanded CB plus an unmanipulated unit compared to recipients of two unmanipulated CB units.
METHODS
Patient population
We evaluated all consecutive adult patients with hematological malignancies who received RIC followed by DCBT where one CB unit was infused unmanipulated and the second unit was expanded ex vivo by co-culturing it with MPCs prior to infusion (MPC group; n=27), as described previously (14) between January 2004 and January 2015. During the study period, the clinical trial with the use of one ex vivo expanded CB unit in addition to a non-expanded CB unit was a departmental priority for enrolment for all double CB patients eligible for RIC regimens. Major reasons for not enrolling into the clinical trial were not receiving insurance approval and/or patient and physician preferences. That group of adult patients who received the same RIC regimens as the study cohort, followed by the infusion of two unmanipulated CB units during the same time period was defined as the “control cohort” (n= 51). Patients with prior allogeneic hematopoietic stem cell transplantation were excluded from the analysis. All patients underwent transplantation between January 2004 and January 2015.
Ex vivo cord blood expansion
The criteria for CB unit selection and the technique for their expansion with MPCs were previously reported (14). Briefly, the CB unit with a lower total nucleated cell (TNC) dose was thawed two weeks prior to the planned CBT and co-cultured with MPCs. On the day of transplantation, the unmanipulated cord was thawed, washed and infused followed by the infusion of the MPC-expanded cord.
Conditioning regimens and graft-versus-host disease prophylaxis
RIC regimens consisted of 1) fludarabine 40 mg/m2 administered intravenously on days −6 to −2, cyclophosphamide 50 mg/kg intravenously on day −6 and 200cGy of total body irradiation on day −1 (TCF) or 2) fludarabine 40 mg/m2 administered intravenously on days −5 to −2 and melphalan 140 mg/m2 intravenously on day −2 (FM).
Graft-versus-host disease (GVHD) prophylaxis was provided with tacrolimus and mycophenolate mofetil. Tacrolimus 0.03 mg/kg intravenously continuous infusion daily was started on day −2, and the dose was adjusted to maintain serum trough levels between 5–15 ng/mL. The route was switched to oral dosing when tolerated and the taper was started around day +180 in the absence of GVHD. Mycophenolate mofetil 1 gram twice a day intravenously (or oral if tolerated) was started on day −3 and continued through day +100. Patients also received rabbit antithymocyte globulin (Genzyme) 1.25 mg/kg intravenously on days −4 and 1.75 mg/kg intravenously on day −3.
Supportive care
Filgrastrim 5 mcg/kg was administered subcutaneously daily starting day 0 until absolute neutrophil counts (ANC) was > 1000 ×106/L. Antimicrobial prophylaxis and blood products were administered according to the institutional guidelines.
Endpoints and Definitions
Our hypothesis was that the use of MPC-expanded CB unit along with one unmanipulated CB unit would lead to faster time to neutrophil engraftment as compared to patients who receive two unmanipulated CB units after RIC. The primary study endpoint was the median time to neutrophil engraftment, defined as the first of three consecutive days after DCBT with an ANC of > 0.5×109/L. Patients who had no neutrophil recovery by day 42 and had bone marrow cellularity <10% were treated as graft failure. Serial sampling of the BM and/or PB at days 30 and 100 after transplantation determined donor chimerism using eight highly polymorphic microsatellite markers (purchased from Integrated DNA Technologies, Inc., Coralville, IA, USA) in a multiplex polymerase chain reaction in recipient and donor units. It was determined that one unit was providing hematopoiesis solely if that unit contributed to more than 95% of the chimerism.
Other study endpoints included the median time to platelet engraftment, acute and chronic GVHD, non-relapse mortality (NRM), disease-free survival (DFS) and overall survival (OS). Time to platelet engraftment was defined as the first of seven consecutive days with a platelet count of >20×109/L without platelet transfusion support. Acute GVHD was assessed clinically as recommended (24) and the maximum overall grade was recorded. Chronic GVHD was recorded as limited or extensive (25). NRM was defined as death without evidence of disease recurrence. DFS was defined as the time from DCBT to either death or relapse. OS was defined as the time from DCBT to death from any cause.
Patients with different hematological malignancies were categorized by Disease Risk Index (DRI) defined by Armand et al (26).
Statistical Analysis
Patient and transplant characteristics in MPC and control groups were compared using the Wilcoxon rank-sum test for continuous variables and the chi-square test or Fisher-exact test for categorical variables. Time to neutrophil and platelet engraftment was estimated as the cumulative incidence function considering graft failure or early death as competing risks. The cumulative incidence of NRM was estimated considering disease progression or death due to disease as competing risks. The cumulative incidence of GVHD was estimated considering death or disease progression in the absence of GVHD as competing risks. Actuarial OS and DFS were calculated using the Kaplan-Meier method. Outcomes were compared using Cox’s proportional hazards regression analysis. Statistical significance was established at the 0.05 level. Statistical analysis was performed using STATA 11.0 (StataCorp. 2009. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP.)
RESULTS
Patient and disease characteristics of the comparison groups were similar except disease diagnoses as presented in Table 1. Of 27 patients in MPC group, 18 (67%) had AML or MDS as disease diagnosis compared with 20 of 51 patients in the control group (39%) (p=0.03). The median age at transplantation was similar with 59 (interquartile range (IQR), 49–67) in the MPC group and 57 (IQR, 48–63) in the control groups respectively (p=0.3). Disease Risk Index (DRI) (26) was high/very high in 9 (33%) and intermediate in 16 of 27 (59%) MPC patients compared with 8 (16%) and 38 (75%) in the control group respectively (p=0.1). More than half the patients in the study cohort; 15 patients in the MPC group (56%) and 31 (61%) in the control group; had advanced disease beyond first or second complete remission at transplantation.
Table 1:
Patient characteristics
| MPC Expanded group (N=27) |
Control group (N=51) |
p value | |
|---|---|---|---|
| Age, in years | |||
| median (interquartile range) | 59 (49, 67) | 57 (48, 63) | 0.3 |
| Gender, n (%) | |||
| Male | 14 (52%) | 24 (47%) | 0.7 |
| Diagnosis, n (%) | |||
| AML/MDS | 18 (67%) | 20 (39%) | |
| ALL | 3 (11%) | 10 (20%) | |
| Non-Hodgkin lymphoma | 4 (15%) | 14 (27%) | |
| Hodgkin’s lymphoma | 0 (0%) | 3 (6%) | |
| CLL | 1 (4%) | 3 (6%) | |
| CML/MPD | 1 (4%) | 0 (0%) | |
| Other | 0 (0%) | 1 (2%) | |
| Disease Status, n (%) | |||
| CR1 | 7 (26%) | 12 (24%) | |
| CR2 | 5 (19%) | 8 (16%) | |
| Other | 15 (56%) | 31 (61%) | 0.7 |
| Disease Risk Index, n (%) | |||
| V. High | 2 (7%) | 1 (2%) | |
| High | 7 (26%) | 7 (14%) | 0.1 |
| Intermediate | 16 (59%) | 38 (75%) | |
| Low | 2 (7%) | 3 (6%) | |
| Undetermined | 0 (0%) | 2 (4%) | |
| Comorbidity index, n (%) | |||
| 0–1 | 13 (48%) | 24 (47%) | |
| 2–4 | 11 (41%) | 22 (43%) | |
| >4 | 3 (11%) | 5 (10%) | 0.97 |
| Median (range) number of prior chemotherapies | 2 (1–5) | 2 (1–7) | 0.4 |
| Median (range) time to transplantation, in months | 15 (3.5–141) | 19 (4–162) | 0.6 |
| Conditioning regimen, n (%) | |||
| Flu/Mel | 16 (59%) | 22 (43%) | |
| Flu/Cy/TBI | 11 (41%) | 29 (57%) | 0.2 |
| HLA of dominant unit | 0.6 | ||
| 3–4/8 | 5 (19%) | 8 (16%) | |
| 5–6/8 | 16 (59%) | 29 (57%) | |
| 7–8/8 | 2 (7%) | 6 (12%) | |
| Other* | 4 (15%) | 8 (16%) | |
| HLA of non-dominant unit | |||
| 3–4/8 | 2 (7%) | 8 (16%) | 0.2 |
| 5–6/8 | 21 (78%) | 29 (57%) | |
| 7–8/8 | 0 (0%) | 6 (12%) | |
| Other* | 4 (15%) | 8 (16%) | |
|
TNC (x 107/Kg); median (range), pre expansion, (MPC- expanded CB unit only) |
0.55 (0.12–1.3) | - | |
|
TNC (x 107/Kg); median (range), post expansion, (MPC- expanded CB unit only) |
5.7 (1.35–11.8) | - | |
| TNC (x 107/Kg); median (range), total infused | 7.9 (3.5–16.1) | 4.25 (2.8–432.0) | <0.001 |
|
CD34 (x 105/Kg); median (range), pre expansion, (MPC- expanded CB unit only) |
0.3 (0.1–1.2) | - | |
|
CD34 (x 105/Kg); median (range), post expansion, (MPC- expanded CB unit only) |
16 (0.4–53) | - | |
| CD34+ (x105/Kg); median (range), total infused | 19.7 (2.0–57.4) | 4.3 (1.5–173.1) | <0.001 |
| Median time (range) to follow-up, months | 39 (12–86) | 22 (3–88) |
Patients with missing high resolution HLA typing (n=2), early death (n=2) or graft failure (n=7), chimerism not available (n=1).
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; MPD, myeloproliferative disorder; CR1, first complete remission; CR2, second complete remission; Flu, fludarabine; Mel, melphalan; Cy, cyclophosphamide; TBI, total body irradiation; HLA, Human Leucocyte Antigen; TNC, total nucleated cells; CB, umbilical cord blood.
The RIC regimen used for transplantation was TCF in 11 MPC (41%) and 29 (57%) control group patients. The rest of the patients received FM as the conditioning regimen. The distribution of human leukocyte antigen (HLA) matching at 4 loci (-A, B, C and DRB1) using high resolution testing was similar between comparison groups. The dominant unit was matched to the recipient at 5–6/8 allele-level in 16 (59.3%) patients and in 28 (54.9%) patients of the MPC and control groups respectively (p=0.6).
The median follow-up was 39 months (range 12–86) in the MPC group and 22 months (range 3–88) in the controls.
Co-culture of CB units with mesenchymal precursor cells led to an increase in the total nucleated cell dose and CD34+ cell dose infused
Co-culture of CB units with MPCs led to an expansion of TNC by a median factor of 12 (range, 3–46.55) and that of CD34+ cells by a median factor of 49 (range, 3.5–98.8) as shown in Figure 1. After the expansion, the median TNC dose increased to 5.7 × 107/kg from a pre-expansion median dose of 0.55 × 107/kg. Similarly, expanded units had higher CD34+ cell dose with a median of 16 × 105/kg compared with pre-expansion dose of 0.3 × 105/kg.
Figure 1:
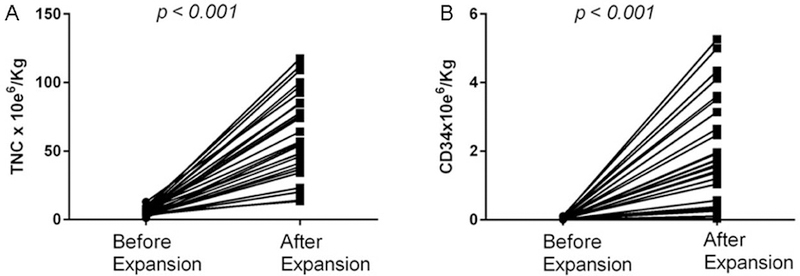
Co-culture of CB units with MPCs led to an expansion of TNC (A) by a median factor of 12 (range, 3–46.55) and that of CD34+ cells (B) by a median factor of 49 (range, 3.5–98.8).
This increase in the number of TNC and CD34+ cells doses after CB expansion was associated with an increase in the total stem cell dose infused for the MPC group compared with the control group. Patients in the expanded group received a median TNC dose of 7.9 × 107/kg and CD34+ cell dose of 19.7 × 105/kg compared with a median TNC dose of 4.25 × 107/kg and CD34+ cell dose of 4.3 × 105/kg in the control group, respectively (p<0.001 for both TNC and CD34+).
Infusion of the CB units expanded ex vivo with co-culture of mesenchymal precursor cells was associated with improved time to neutrophil recovery
Primary graft failure was observed in 3 of 27 patients in the MPC group and 4 of 51 in the control group; p=0.4 (supplemental table 1). Among patients who engrafted, the median time to neutrophil recovery was significantly faster in the MPC group with a median of 12 days (range 1–28) compared with the control group who engrafted within a median of 16 days (range 5–48) (p= 0.02) after the transplant. The improvement in time to neutrophil recovery observed in the MPC group was independent of the conditioning regimen used. The median time to neutrophil recovery was 6 days versus 11 days for MPC and control groups respectively if they were treated with TCF (p=0.04). The magnitude of improvement in time to neutrophil recovery was larger if patients were treated with FM. The median time to neutrophil recovery was 14 vs. 22 days for MPC and control groups (p=0.001).
The cumulative incidence of neutrophil engraftment was 78% in the MPC group compared with 67% in the control group (Hazard ratio (HR) 1.5, 95% confidence interval (CI), 0.9–2.6, p=0.1), as presented in figure 2a. However, there was a significant improvement in the incidence of neutrophil engraftment from 50% up to 75% in patients treated with FM regimen and received an expanded CB unit (p=0.03), as shown in figure 2b. This benefit was not observed in patients treated with TCF who had a higher incidence of neutrophil engraftment as 79% even with the infusion of two unmanipulated CB units (figure 2c).
Figure 2:
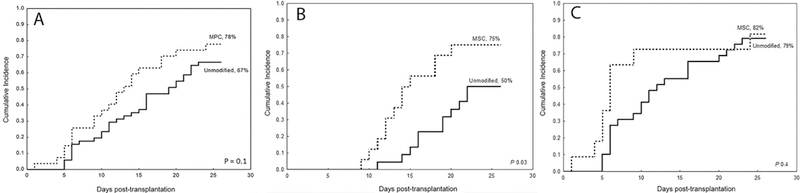
The cumulative incidence of neutrophil engraftment was 78% in the MPC group (dotted line) compared with 67% in the control group (solid line) (A). However, there was a significant improvement in the incidence of neutrophil engraftment from 50% up to 75% in patients treated with FM regimen and received an expanded CB unit (p=0.03) (B). This benefit was not observed in patients treated with TCF who had a higher incidence of neutrophil engraftment as 78% even with the infusion of two unmanipulated CB units (C).
We did not observe a significant difference for the median time to platelet recovery between the comparison groups; MPC group had platelet recovery with a median of 31 days (range 9–52) and control with a median of 37 days (range 10–64), p=0.3. Similarly, the cumulative incidence of platelet engraftment by day 60 was 74% in both MPC and control groups (HR 1.1, 95% CI, 0.6–1.9, p=0.7) (figure 3a) and did not differ by the type of conditioning regimen used as shown in Table 2 and figures 3b and 3c.
Figure 3:
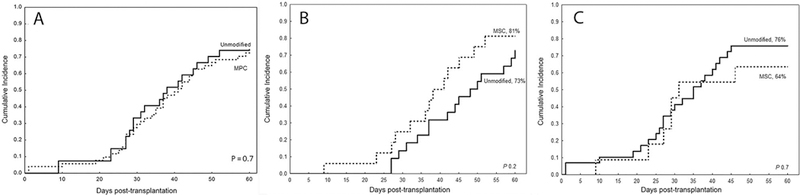
The cumulative incidence of platelet engraftment by day 60 was 74% in both MPC (dotted line) and control groups (solid line) (A) and did not differ by the type of conditioning regimen used; it was 81% vs 73% in MPC and control groups, respectively among those treated with FM regimen (B) and 64% vs 76%, respectively in those treated with TCF regimen (C)
Table 2:
Neutrophil and Platelet Engraftment
| MPC Expanded (N=27) |
Control (N=51) |
P value | |
|---|---|---|---|
| Median (range) days to neutrophil engraftment | |||
| All patients | 12 (1–28) | 16 (5–48) | 0.02 |
| FM regimen | 14 (9–27) | 22 (11–48) | 0.001 |
| Flu/Cy/TBI regimen | 6 (1–28) | 11 (5–31) | 0.04 |
| Cumulative incidence (95% C.I.) of neutrophil engraftment by day 26 | |||
| All patients | 78% (64–95) | 67%(55–81) | 0.1 |
| Flu/Mel regimen | 75% (56–99) | 50%(33–76) | 0.03 |
| Flu/Cy/TBI regimen | 82% (62–100) | 79%(66–95) | 0.4 |
| Median (range) days to platelet engraftment | |||
| All patients | 31(9–52) | 37(10–64) | 0.3 |
| Flu/Mel regimen | 37(9–52) | 44(27–64) | 0.1 |
| Flu/Cy/TBI regimen | 29(9–46) | 31(10–45) | 0.5 |
| Cumulative incidence (95% C.I.) of platelet engraftment by day 60 | |||
| All patients | 74%(59–93) | 74%(63–87) | 0.7 |
| Flu/Mel regimen | 81%(64–100) | 73%(56–94) | 0.2 |
| Flu/Cy/TBI regimen | 64%(41–99) | 76%(62–93) | 0.7 |
Abbreviations: C.I., confidence interval; Flu, fludarabine; Mel, melphalan; Cy, cyclophosphamide; TBI, total body irradiation.
Higher TNC cell dose infused increased the incidence of engraftment with faster count recovery
Considering the main effect of the CB expansion was the increase in the cell dose infused, we investigated the impact of increasing cell dose (quartiles) on the rate of neutrophil and platelet recovery.
In the entire study cohort, infused TNC dose of >7 × 107/kg was associated with a cumulative incidence of 84% for neutrophil engraftment by day 26 as compared with 67% observed in lower TNC doses (HR 1.8, 95% CI, 1–3.3, p=0.048). The impact of higher TNC cells on neutrophil engraftment was in the same direction with a similar magnitude in patients treated with FM (HR=1.9, 95%CI=0.7–5, p=0.2) and TCF (HR=2.1, 95%CI=1.1–4.2, p=0.025) but due to smaller sample size the impact of cell dose on neutrophil engraftment was not statistically significant in the FM group. As expected, not only the incidence of neutrophil engraftment improved with higher TNC dose, but also median time to engraftment was shorter. Patients with TCF had median time to neutrophil recovery within 6 days versus 10 days and patients with FM as early as 12 days versus 17 days with higher and lower TNC doses infused respectively.
The TNC cell dose that increased the likelihood of platelet engraftment by day 60 was >4 × 107/kg (HR=2.0, 95% CI, 1.1–5.0, p=0.03). With TNC >4 × 107/kg, the incidence of platelet recovery increased from 53% to 82% with a median time to recovery of 33 (range, 19–60) days vs. 35 (range, 9–57) days compared with lower doses. Transplantation with smaller doses of TNC led to lower incidence of platelet recovery both in FM and TCF groups, 56% and 50%, respectively (HR=0.3, 95%CI=0.1–0.9, p=0.04 and HR=0.5, 95%CI=0.2–1.6, p=0.3).
Infusion of one CB unit expanded ex vivo with co-culture of mesenchymal precursor cells leads to unit predominance with the un-manipulated CB unit
Of 23 engrafted patients with CB expanded, 19 were evaluable for chimerism analyses as early as day 30 and 18 were evaluable by day 100 after the transplantation. In MCP group, 10 of 19 (52.6%) patients had evidence of hematopoiesis solely from the unmanipulated CB unit as shown in Figure 4. On day 100, still the majority, 11 of 18 (61%) evaluable patients had 100% hematopoiesis solely from the unmanipulated unit. Interestingly, 2 evaluable (10%) patients had hematopoiesis completely from the ex-vivo expanded CB units at both time points. Six patients (30%) had hematopoiesis derived from both units at day 30, with 4 of the 5 evaluable having still both CB units present at day 100.
Figure 4:
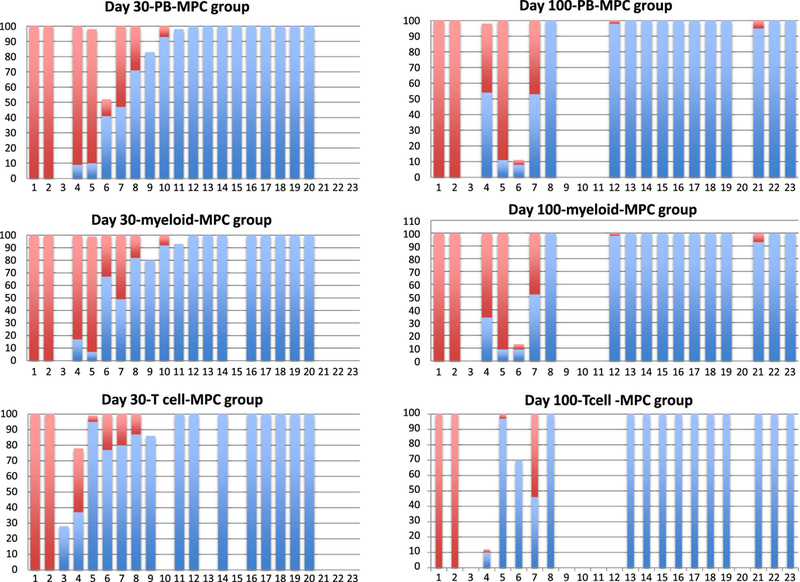
Of 19 evaluable patients on day 30, 10 (52.6%) had evidence of hematopoiesis solely from the unmanipulated CB unit. On day 100, still the majority, 11 of 18 (61%) evaluable patients had 100% hematopoiesis solely from the unmanipulated unit.
In the control group with 2 unmanipulated CB units infused, 37 of 46 engrafted patients were evaluable on day 30 and 17 (45.9%) had evidence of hematopoiesis solely from one CB unit as shown in supplementary figure 1. This rate was not different from the 50% observed in MPC group (p=0.8). On day 100, 15 of 23 (65.2%) evaluable patients had evidence of hematopoiesis only from one CB and that rate was still comparable to 61% observed in MPC group (p=0.5).
Infusion of one CB unit expanded ex vivo with co-culture of mesenchymal precursor cells is not associated with increased incidence of GvHD
The cumulative incidence of grade II-IV acute GVHD was 28% in MPC group and that was comparable to 27% in control group (HR 0.96, 95% CI, 0.4–2.5, p=0.9) as presented in Table 3. Similarly, the incidence of chronic GVHD at 2 years did not differ in comparison groups with 29% incidence in MPC and 20% control groups (HR 1.1, 95% CI, 0.4–2.9, p=0.9).
Table 3:
Secondary outcomes
| MPC Expanded (N=27) |
Control (N=51) |
H.R. | 95% C.I. | P-value | |
|---|---|---|---|---|---|
| Acute GVHD II-IV, day 100 | 28% (14–56) | 27% (16–44) | 0.99 | 0.4–2.7 | 0.9 |
| Acute GVHD III-IV, day 100, N=2 | - | - | N.A | - | - |
| Chronic GVHD, 2 years | 29% (16–54) | 20% (11–36) | 1.1 | 0.4–2.9 | 0.9 |
| NRM, day 100 | 7% (2–28) | 12% (5–24) | 0.6 | 0.1–3.2 | 0.6 |
| NRM, 6 months | 30% (17–53) | 24% (14–40) | 1.26 | 0.5–3.0 | 0.6 |
| NRM, 1 year | 37% (23–61) | 32% (22–49) | 1.2 | 0.5–2.6 | 0.7 |
| NRM, 2 years | 45% (30–69) | 38% (26–56) | 1.3 | 0.6–2.6 | 0.5 |
| DFS, 2 years | 28% (12–46) | 25% (13–39) | 0.9 | 0.5–1.5 | 0.6 |
| OS, 2 years | 32% (16–50) | 34% (21–48) | 1.1 | 0.6–1.9 | 0.8 |
Abbreviations: C.I., confidence interval; GVHD, graft-versus-host disease; HR, hazards ratio; NRM, non-relapse mortality; OS, overall survival.
The incidence of NRM was 7% and 30% at day 100 and 6th month after transplant in MPC group. Patients in the control group had similar NRM with day 100 incidence of 12% and 6th month incidence of 24% (HR 0.6, 95% CI, 0.1–3.2, p=0.6 and HR 1.26, 95% CI, 0.5–3, p=0.6 respectively).
DFS and OS at 2 years were also comparable between groups, with 28% versus 25% (HR 0.9, 95% CI, 0.5–1.5, p=0.6) and 32% versus 34% respectively (HR 1.1, 95% CI, 0.6–1.9, p=0.8) for HPC and control groups.
Overall, 18 deaths were observed in the MPC and 34 deaths in the control group during the study period. Disease relapse or persistence was the major cause of mortality, with 6 deaths (33%) in the MPC group and 15 (44%) in the controls. Cause of death was infection in 5 patients (28%) in the MPC group and 11 patients (32%) in control group followed by GvHD in 3 (17%) and 2 patients (6%) in MPC and control groups (supplemental table 2). There was no difference in the length of hospitalization between MPC (median 29, range 19–114 days) and the controls (median 34, range 15–79 days), p=0.9.
DISCUSSION
Our results show that the infusion of one CB unit ex vivo expanded with MPCs in addition to an unmanipulated CB unit after RIC regimen served its purpose for increased cell dose to be infused with improvement in time to engraftment compared with infusion of two unmanipulated CB units. To our knowledge this is the first demonstration of faster hematopoietic engraftment with the use of ex vivo expanded hematopoietic progenitors in the RIC setting. Most of the clinical trials investigating strategies to improve engraftment either by expansion to increase the number of cells or improving homing has focused on patients treated with MAC regimens who are reported to have neutrophil engraftment usually beyond day 24(27, 28). The strategies to expand CB progenitor cells ex vivo, such as using the engineered Notch ligand(10), copper chelators(29), nicotinamide(11), aryl hydrocarbon receptor antagonist(30) or the use of “off-the-shelf” MPCs which was pioneered at our institution(14) demonstrated remarkable improvement in engraftment after CBT in the setting of myeloablative conditioning. The need for strategies to improve engraftment after RIC regimens has not been well-defined considering that most patients experience neutrophil engraftment within 15–25 days depending on the intensity of the regimen used(31–33).
In our study, the TNC dose in the expanded CB unit increased by a median factor of 12. When combined with the unmanipulated CB unit, the median of TNC dose infused was 7.9×107/kg in the HPC group compared with 4.25×107/kg in the control group. Similarly the CD34+ cell dose was approximately four fold higher; 19.7×105/kg vs. 4.3×105/kg, in patients receiving an ex-vivo expanded CB unit in addition to an unmanipulated one. This approach accelerated the time to neutrophil engraftment with a median of 12 days, faster than would be expected using two unmanipulated CB units (median time of 16 days in the control group) following RIC regimes. The engraftment was faster in the MPC group regardless of the intensity of the RIC regimen used, although this impact was more pronounced particularly in those who received the more intensive FM conditioning where the engraftment occurred by a median of 8 days earlier in the MPC group than the controls.
Our results support the notion that strategies to increase cell dose infused in CB unit by manipulation may lead to a lower minimal TNC dose required for a safe CB transplantation and increase the number of acceptable CB units available in public CB banks. This is especially important for patients considered for RIC who are often older and have a less likelihood of finding a matched related sibling alive and/or eligible. In addition, it is well-known that despite the more than 21 million adult volunteer donors in the National Marrow Donor Program and affiliated registries, at least 50% of Caucasians and 80% of patients from diverse racial and ethnic backgrounds cannot identify a suitably matched unrelated volunteer donor in the required time period(34). In addition to providing the advantage of increasing the number of eligible CB units in the inventory by lowering the minimum cell dose required for a safe transplant, expansion can also improve transplant outcomes but increasing the likelihood of identifying better HLA matched units; that is shown to improve CBT transplant by lowering transplant-related mortality(35).
In our study, we observed that hematopoiesis as early as day 30 was derived mostly from the unmanipulated CB unit and on longer follow-up, this finding remained the same. While it is possible that this observation was a consequence of loss of long-term repopulated cells during ex vivo culture, it is also likely that the T cell replete unmanipulated unit mounted a cellular immune response leading to rejection of the expanded graft(36, 37). However, the expanded CB units provided the hematopoiesis solely in 2 patients and partially in 5 patients. These findings support the notion that expanded cell population may preserve a longer-term repopulating stem/progenitor cells. Further support of our hypothesis for the long-term hematopoietic potential of expanded CB units may provide basis for eliminating the need for transplantation of a second unmanipulated CB unit and proceeding with single ex-vivo expanded CBT unit.
We acknowledge limitations of our study inherent in the retrospective design and limited conclusions can be drawn from the clinical data presented. In the absence of chimerism data available prior to day 30, the possibility of early engraftment from autologous reconstitution could not be excluded. Also, despite improvement in the time to neutrophil engraftment in both RIC regimens, there was no improvement in the rates of graft failures (3 in the study group and 4 in the controls). As compared to 33% of controls, 22% of patients in the MPC group did not engraft neutrophils by day 26. Although this difference was negligible in those who received the lesser intensive TCF regimen, yet among those who received more intensive FM regimen, there was 25% statistically significant improvement in neutrophil engraftment by day 26 in the MPC group than in the controls. Similar to other published CB expansion trials, our study did not find differences in GVHD, relapse risk, TRM, DFS or OS as compared to controls, signifying that this approach is safe to use even in older population. The ongoing multicenter randomized clinical trial (NCT01854567) investigating the efficacy and safety of CB cells expanded with MPCs for hematopoetic recovery in patients with hematologic malignancies after myeloablative treatment twill determine the role of using MPC expanded CB at least in the myeloablative setting. In the ongoing trial, patients with lesser intensive regimens including FM are also included and those observations would be relevant to our reported results. However, we believe that our results confirming the safety and potential efficacy of using CB units expanded ex-vivo with MPCs with less intensive regimens including TCF deserves to be investigated for its potential to broaden the application of CBT to older adults with hematological malignancies who are in need of urgent unrelated donor transplant.
Supplementary Material
Highlights:
RIC DCBT with one MPC-expanded CB unit leads to faster neutrophil engraftment
Time to platelet engraftment is not improved with MPC expansion after RIC CBT
MPC-expansion is safe and does not add to any toxicities after RIC CBT
Acknowledgements
This work was supported in part by NCI RO1 CA061508–23, CPRIT RO1 RP100469), NCI P01 CA148600–02, HRSA HHSH250201000011C and Cancer Center Core Grant CA016672 (MD Anderson investigators).
Footnotes
Disclosure of Conflicts of Interest
Donna Skerrett and Elizabeth Burke are employed by Mesoblast.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood 2010. March 4;115(9):1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 2007. October 15;110(8):3064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010. November 25;116(22):4693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010. July;11(7):653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012. April;18(4):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majhail NS, Brunstein CG, Tomhlyn M, Thomas AJ, Miller JS, Arora M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: Unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Tr 2008. March;14(3):282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhal S, Powles R, Kulkarni S, Treleaven J, Sirohi B, Millar B, et al. Comparison of marrow and blood cell yields from the same donors in a double-blind, randomized study of allogeneic marrow vs blood stem cell transplantation. Bone Marrow Transpl 2000. March;25(5):501–5. [DOI] [PubMed] [Google Scholar]
- 8.Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematol-Am Soc Hemat 2012. December:215–22. [DOI] [PubMed] [Google Scholar]
- 9.Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 2013. October 24;122(17):3074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 2010. February;16(2):232–U143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz ME, Chao NJ, Rizzieri DA, Long GD, Sullivan KM, Gasparetto C, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest 2014. July;124(7):3121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popat U, Mehta RS, Rezvani K, Fox P, Kondo K, Marin D, et al. Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood 2015. May 7;125(19):2885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peled T, Mandel J, Goudsmid RN, Landor C, Hasson N, Harati D, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy 2004. August;6(4):344–55. [DOI] [PubMed] [Google Scholar]
- 14.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012. December 13;367(24):2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuji K, Miyakoshi S, Kato D, Miura Y, Myojo T, Murashige N, et al. Reduced-intensity unrelated cord blood transplantation for patients with advanced malignant lymphoma. Biol Blood Marrow Transplant 2005. April;11(4):314–8. [DOI] [PubMed] [Google Scholar]
- 16.Miyakoshi S, Yuji K, Kami M, Kusumi E, Kishi Y, Kobayashi K, et al. Successful engraftment after reduced-intensity umbilical cord blood transplantation for adult patients with advanced hematological diseases. Clin Cancer Res 2004. June 1;10(11):3586–92. [DOI] [PubMed] [Google Scholar]
- 17.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant 2007. January;13(1):82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunstein CG, Eapen M, Ahn KW, Appelbaum FR, Ballen KK, Champlin RE, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood 2012. June 7;119(23):5591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG. Effect of conditioning regimen intensity on acute myeloid leukemia outcomes after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2011. September;17(9):1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunstein CG, Eapen M, Ahn KW, Appelbaum FR, Ballen KK, Champlin RE, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood 2012. June 7;119(23):5591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majhail NS, Brunstein CG, Tomblyn M, Thomas AJ, Miller JS, Arora M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant 2008. March;14(3):282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011. July 14;118(2):282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponce DM, Zheng J, Gonzales AM, Lubin M, Heller G, Castro-Malaspina H, et al. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011. September;17(9):1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995. June;15(6):825–8. [PubMed] [Google Scholar]
- 25.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 1981. February;57(2):267–76. [PubMed] [Google Scholar]
- 26.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014. June 5;123(23):3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. New Engl J Med 2001. June 14;344(24):1815–22. [DOI] [PubMed] [Google Scholar]
- 28.Arcese W, Rocha V, Labopin M, Sanz G, Lori AP, de Lima M, et al. Unrelated cord blood transplants in adults with hematologic malignancies. Haematol-Hematol J 2006. February;91(2):223–30. [PubMed] [Google Scholar]
- 29.Stiff PJ, Montesinos P, Peled T, Landau E, Rosenheimer N, Mandel J, et al. StemEx (R)(Copper Chelation Based) Ex Vivo Expanded Umbilical Cord Blood Stem Cell Transplantation (UCBT) Accelerates Engraftment and Improves 100 Day Survival In Myeloablated Patients Compared To a Registry Cohort Undergoing Double Unit UCBT: Results Of a Multicenter Study Of 101 Patients With Hematologic Malignancies. Blood 2013. November 15;122(21). [Google Scholar]
- 30.Wagner JE, Brunstein C, McKenna D, Sumstad D, Maahs S, Laughlin M, et al. StemRegenin-1 (SR1) Expansion Culture Abrogates the Engraftment Barrier Associated with Umbilical Cord Blood Transplantation (UCBT). Blood 2014;124(21):728. [Google Scholar]
- 31.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood 2003. September 1;102(5):1915–9. [DOI] [PubMed] [Google Scholar]
- 32.Rocha V, Rio B, Garnier F, Renaud M, Sirvent A, Takahashi S, et al. Reduced intensity conditioning regimen in single unrelated cord blood transplantation in adults with hematological malignant disorders. An Eurocord-Netcord and SFGM-TC survey. Blood 2006. November 16;108(11):897a-a. [Google Scholar]
- 33.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Tr 2007. January;13(1):82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Appelbaum FR. Pursuing the Goal of a Donor for Everyone in Need. New Engl J Med 2012. October 18;367(16):1555–6. [DOI] [PubMed] [Google Scholar]
- 35.Oran B, Cao K, Saliba RM, Rezvani K, de Lima M, Ahmed S, et al. Better allele-level matching improves transplant-related mortality after double cord blood transplantation. Haematologica 2015. October;100(10):1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutman JA, Turtle CJ, Manley TJ, Heimfeld S, Bernstein ID, Riddell SR, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood 2010. January 28;115(4):757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moretta A, Andriolo G, Lisini D, Martinetti M, Pasi A, Rebulla P, et al. In Vitro Evaluation of Graft-versus-Graft Alloreactivity as a Tool to Identify the Predominant Cord Blood Unit before Double Cord Blood Transplantation. Biol Blood Marrow Tr 2012. July;18(7):1108–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


