Abstract
As Ixodes ticks spread to new regions, the incidence of Lyme disease (LD) in companion animals and humans will increase. Preventive strategies for LD in canines center on vaccination and tick control (acaricides). Both subunit and bacterin based LD veterinary vaccines are available. Outer surface protein C (OspC), a potent immunogen and dominant early antigen, has been demonstrated to elicit protective antibody (Ab) responses. However, a single OspC protein elicits a relatively narrow range of protection. There are conflicting reports as to whether the immunodominant epitopes of OspC reside within variable or conserved domains. A detailed understanding of the antigenic determinants of OspC is essential for understanding immune responses to this essential virulence factor and vaccinogen. Here, we investigate the contribution of the conserved C-terminal C10 motif in OspC triggered Ab responses. Using a panel of diverse recombinant full length OspC proteins and their corresponding C10 deletion variants (OspCΔC10), we demonstrate that the C10 motif does not significantly contribute to immunization or infection induced Ab responses in rabbits, rats, canines, horses and non-human primates. Furthermore, the C10 motif is not required to trigger potent bactericidal Ab responses. This study provides insight into the antigenic structure of OspC. The results enhance our understanding of immune responses that develop during infection or upon vaccination and have implications for interpretation of LD diagnostic assays that employ OspC.
INTRODUCTION:
Lyme disease (LD) is a growing public health threat in N. America, Europe, and Asia. It is caused by spirochetes of the genus Borreliella (formerly classified within the genus Borrelia) [1]. In N. America, LD is caused primarily by B. burgdorferi while B. burgdorferi, B. garinii, and B. afzelli cause disease in Europe [2–4]. The LD spirochetes are transmitted by Ixodes scapularis ticks in the eastern half of N. America and I. pacificus along the west coast. In Europe and Asia, I. ricinus and I. persulcatus serve as vectors for the LD spirochetes. Regions that support Ixodes ticks are expanding [5–7] and additional Ixodes species (I. angustus, I. affinis and I. auritulus) are emerging as possible B. burgdorferi vectors [7–9].
Routine screening for tick-borne diseases in veterinary medicine has proven invaluable in monitoring the spread and risk of LD. The Companion Animal Parasite Council (CAPC) reported 303,000 canine LD positive Ab test results in the US in 2017 (https://www.capcvet.org). Since data are collected for ~30% of the tests that are run, the number of positive Ab tests in 2017 may be closer to 900,000. The incidence of LD in horses is increasing as well [10]. One study reported that 77% of horses tested in Virginia were LD Ab positive [11]. A study from the CDC reported that the number of clinician diagnosed, human LD cases each year in the US is ~329,000 [12].
LD prevention in humans consists of tick avoidance, physical barriers, and the application of various commercially available tick repellents [13]. While human LD vaccines are not available, several bacterin and subunit LD vaccines have been approved by the USDA for use in canines [14]. Current subunit vaccines consist of Outer surface protein (Osp) A or OspA in combination with a modified form of OspC. These proteins are produced during different stages of the enzootic cycle [15]. OspA production is high in unfed ticks (and during cultivation), low in fed ticks, and off in mammals [16]. OspC production is low in unfed ticks (and during cultivation) but high in fed ticks and mammals [16]. Ab to OspA targets spirochetes within ticks, inhibiting transmission. However, Ab to OspA cannot bind to spirochetes in mammals since they are not producing OspA [17,18]. Ab to OspC targets spirochetes during transmission and upon entrance into a host. The production of OspC in mammals suggests that OspC has the potential to trigger anamnestic responses in animals vaccinated with OspC.
Understanding the antigenic structure of OspC and its inherent diversity is essential for interpreting Ab responses induced by vaccination or during infection. While the structure of OspC is well conserved [19,20], OspC proteins vary considerably in amino acid sequence [21]. OspC variants or “OspC types”of OspC are differentiated by letter designations [21,22]. While OspC is a protective antigen [23,24], due to its variation, protection is generally strain specific [25,26]. The narrow protection provided by any one given OspC type protein suggests that the protective epitopes of OspC reside within variable regions of the protein [27,28]. Two linear epitopes, referred to as L5 and H5, have been localized within residues 136 to 150 and 168 to 203, respectively, and demonstrated to elicit OspC type-specific bactericidal Ab responses [21,29,30]. In a separate study, H5 residues 179–188 were concluded to be the epitope responsible for OspC type-specific Ab responses [28]. To develop a broadly protective OspC based subunit vaccine antigen, Earnhart and Marconi developed unique recombinant proteins, referred to as chimeritopes (chimeric epitope based proteins), that consist of diverse L5 and H5 epitopes. OspC chimeritopes were demonstrated to elicit bactericidal and protective Ab responses [27,29–31]. VANGUARD®crLyme (Zoetis), the most recent LD vaccine to receive USDA approval, consists of OspA and a multivalent OspC chimeritope [32].
To add to our understanding of antigenic structure of OspC, we investigated the potential contribution of the conserved C10 [33] motif (also referred to as the C7 motif [34]) in OspC directed Ab responses during natural infection and upon immunization. It has been suggested that the C10 domain is immunodominant [34] and is the primary protective epitope of OspC [35–38]. The C10 motif (PVVAESPKNP; B. burgdorferi B31), which consists of the C-terminal 10 amino acids of OspC, is well conserved among LD isolates. In spite of its conservation, it is of unknown functional or structural significance. We conclude that the C10 motif is not an immunodominant epitope and does not contribute significantly to bactericidal Ab responses. The data suggest that a single OspC protein delivered in the context of either a bacterin or subunit vaccine is insufficient to convey protection against diverse strains. To generate an OspC based antigen that elicits broad protection, the diversity and antigenic structure of OspC must be considered.
Materials and methods.
Cultivation of the LD spirochetes.
B. burgdorferi strains (LD spirochetes) used in this study are described in supplementary Table 1. Spirochetes were cultivated in BSK-H media supplemented with 6% rabbit serum at 34°C with 5% CO2. Growth was monitored using wet mounts and dark-field microscopy. Cells were harvested from cultures by centrifugation.
Generation of recombinant proteins.
ospC genes from strains producing different OspC types (as indicated in supplementary Table 1) were PCR amplified from genomic DNA with or without the C10 encoding segment of each gene. PCR primers were designed with ligase independent cloning (LIC) tails to allow for annealing into linearized pET46-Ek/LIC vector as described by the supplier (Novagen). All methods pertaining to cloning, protein production and purification were exactly as decribed in an earlier study [39].
SDS-PAGE and immunoblot analyses.
Recombinant proteins (500 ng) or cell lysates of LD spirochete strains were subjected to SDS-PAGE using precast AnykD Criterion Gels (Biorad) and transferred to PVDF membranes using a Trans-blot Turbo Transfer system as detailed by the supplier (Biorad). Immunoblot analyses were conducted as previously described [40]. Antiserum dilutions are indicated below for each experiment. Detection of Ab binding was accomplished using Clarity Max Western ECL substrate and chemiluminescence (Biorad). Images were captured using a Biorad ChemiDoc imaging System (Biorad). Note that in some cases, images were cropped to remove blank spaces. Equal loading of proteins was verified by immunoblot with anti-His-tag antisera (Novagen). Equivalent loading of cell lysates was demonstrated by staining gels with Coomassie Brilliant Blue. Screening with preimmune serum served as the negative control.
Generation of antiserum and description of serum samples.
Anti-OspC type A (derived from B. burgdorferi B31) and anti-OspCΔC10 (type A) antisera were generated in Sprague-Dawley rats as previously described [41]. In brief, recombinant proteins were delivered (50 μg; Freunds Complete Adjuvant; Sigma) with a boost 14 days later (25 μg; Freunds Incomplete Adjuvant; Sigma). On day 21, the rats were euthanized, bled by cardiac puncture and sera harvested using Z Serum Sep Clot Activator columns as instructed by the supplier (Vacuette). Titers were determined by standard end-point dilution as previously described [40]. The rat-anti-OspC and rat-anti-OspCΔC10 antisera were used at dilutions of 1:1000. Serum from rabbits immunized with OspC proteins of different OspC types [39] were used at a dilution of 1:30000. Serum from infected dogs were obtained from North Carolina State University. The canine serum samples were determined to be LD Ab positive (C6) using the SNAP4Dx test (IDEXX) and the results confirmed through immunoblotting (primary serum dilution of 1:1000) using B. burgdorferi B31 cell lysate as the immobilized antigen and by the Global Lyme Disease ELISA test (https://glymedx.com). Serum samples from horses that were LD Ab positive were used at dilutions ranging from 1:250 to 1:1000. Serum samples from Rhesus macaques were derived from an earlier study in which the monkeys were infected by tick bite. The ticks were laboratory raised and infected with B. burgdorferi B31[by the capillary tube feeding approach [42]. Non-human primate sera were used at 1:1000 dilution. Each animal was confirmed to be LD negative by screening for antibody reactivity to B. burgdorferi 5 antigen serological assay [43]. Practices in the housing and care of animals conformed to the regulations and standards of the PHS Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals. The Tulane National Primate Research Center is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care-International. The Institutional Animal Care and Use Committee of the Tulane National Primate Research Center approved all animal-related protocols, including the infection, treatment, and sample collection from nonhuman primates.
ELISA.
ELISA wells (96 well plates) were coated with 500 ng of recombinant protein in bicarbonate buffer as previously described [44]. Primary and secondary Ab dilutions were 1:100 and 1:15000, respectively. ABTS substrate was added, the plates were incubated for 20 min and absorbance read at 405nm. The factor H binding protein (FhbB) of the periodontal disease pathogen, Treponema denticola, served as a negative control antigen [45]. Statistical significance, was assessed using an unpaired, two-tailed student’s t-test (95% CI, p < 0.05). All calculations were performed on GraphPad Prism 5 (GraphPad). All assays were performed in triplicate at least twice.
Bactericidal Ab assays.
Bactericidal Ab assays were performed as previously described [44]. Anti-OspA antiserum incubated with B. burgdorferi B31 served as a positive control for complement dependent Ab mediated killing [44]. Negative controls consisted of cells incubated in media alone, cells incubated in media with 20% complement certified guinea pig serum (GPS; Complement Technologies, Inc.) and cells incubated with the corresponding heat inactivated (HI) hyperimmune serum (anti-OspA, anti-OspC type A or anti-OspCΔ10; 20%) with 20% HI-GPS. Heat inactivation of serum was accomplished by incubation at 56°C (30 min). Statistical significance was assessed using an unpaired, two-tailed student’s t-test (95% CI, p < 0.05). All calculations were performed on GraphPad Prism 5 (GraphPad). All assays were performed in triplicate at least twice and the percent killing was determined by counting the number of dead cells versus the total number of cells in 5 fields of view using dark-field microscopy.
RESULTS
Analysis of Ab responses elicited by immunization with OspC types A, B and K in rabbits.
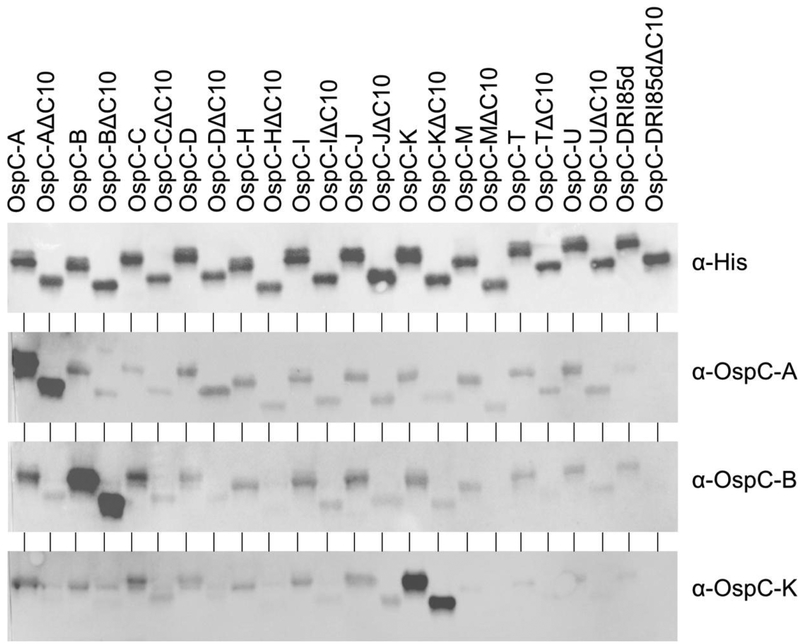
To gain further insight into the antigenic determinants of OspC and its potential to induce broadly protective Ab responses, 13 different OspC types, with or without the C10 motif, were screened with hyperimmune sera raised in rabbits against OspC types A, B and K. Each individual antiserum reacted strongly with the OspC protein that was used to generate the serum but not with heterologous OspC types demonstrating that IgG responses to recombinant OspC are type-specific (Figure 1). Detection specificity was not affected by the presence or absence of the C10 motif and no apparent differences in the level of IgG binding was evident as assessed by immunoblotting. It is clear from these analyses that conserved domains such as the C10 motif are not immunodominant and that a single OspC type protein when delivered as a vaccinogen will not convey broad protection.
Figure 1. Immunization with recombinant OspC type proteins A, B and K induces OspC type-specific, C10-independent Ab responses in rabbits.
Recombinant proteins were generated, purified and used to vaccinate New Zealand white rabbits as detailed in the text and in an earlier study [39]. The isolate of origin for each gene used to produce the recombinant proteins is listed in supplementary Table 1. Proteins (500 ng) were prepared for SDS-PAGE, electrophoresed and transferred to PVDF membranes using standard techniques. Immunoblots were screened with anti-His Ab (loading control) or with serum from the hyperimmunized rabbits (as indicated). The letter designation following OspC indicates the type identity of the OspC protein. The ΔC10 designation indicates that the protein was produced without the C10 motif. Note that irrelevant blank areas of the immunoblots images were cropped. Exposure times for the blots varied. Hence, the data are not suggested to be quantitative when comparing one image with another.
Ab responses to OspC in infected dogs, horses, and non-human primates are restricted and OspC type-specific.
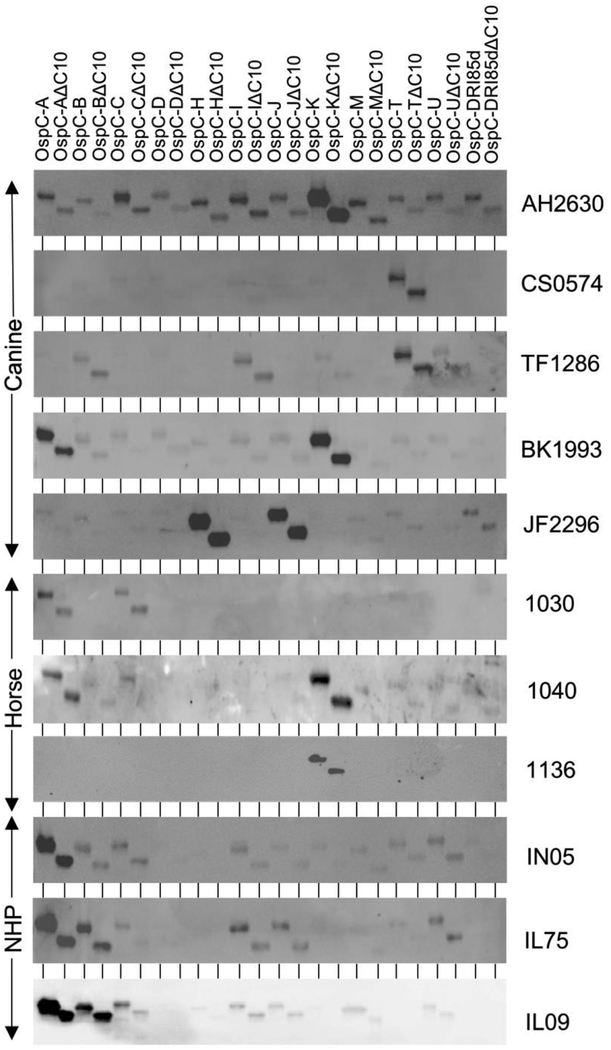
To assess IgG responses to OspC that develop during natural infection immunoblots of the OspC and OspCΔC10 protein panel were screened with serum from infected canines and horses (Figure 2). OspC type-restricted Ab responses were observed in all animals tested. For example, IgG from dog BK1993 bound to OspC types A and K but not to the remaining OspC types (Figure 2). Similarly, serum from dog CS0574 reacted specifically with OspC type T. Dog JF2296 reacted exclusively with OspC types H and J. Serum from horse 1136 reacted with a single OspC protein while horse 1040 reacted with OspC types A and K. It is likely that individual animals that developed Ab to multiple OspC types were infected with a heterogenous population of LD spirochetes. This could result from being fed upon by a single tick carrying multiple LD spirochete strains or from exposure to multiple ticks carrying individual strains. It has been demonstrated that ticks typically carry a genetically heterogenous population of LD spirochetes [46,47]. In addition, laboratory studies have demonstrated that canines infected by infestation with field collected ticks can be simultaneously infected with a diverse array of strains producing several different OspC type proteins [48]. In view of the exposure probability of canines to ticks in Lyme endemic areas, and the potential for individual ticks to harbor a genetically heterogenous population of LD spirochetes, either possibility is equally plausible. All immunoblot results are summarized in Table 1.
Figure 2. Ab responses to OspC in infected dogs, horses and non-human primates are OspC type restricted.
Immunoblot analyses and image preparation were conducted as detailed in Figure 1 and in the text. The proteins loaded in each lane are indicated across the top of the figure. Serum identifier numbers are indicated to the right of each blot. Serum samples AH2630, CS0574, TF1286, BK1993 and JF2296 were obtained from client owned dogs; samples 1030, 1040 and 1136 were from client owned horses; samples IN05, IL75 and IL09 were from experimentally infected rhesus macaques [42]. Note that data obtained with OspC type F are not shown due to limitations on the number of samples that could be analyzed in each gel.
Table 1.
Summary of immunoblot results.
| Animal/serum ID | Dominant immunoreactive OspC typea |
|---|---|
| Canine AH2630 | K |
| Canine CS0574 | T |
| Canine TF1286 T | |
| Canine BK1993A, K | |
| Canine JF2296 | H, J |
| Horse 1077 | I |
| Horse 1030 | A, C |
| Horse 1031 | A |
| Horse 1026 | F, I |
| Horse 1040 | A, K |
| Horse 1136 | K |
| Monkey IN05 | A |
| Monkey IL75 | A, B |
| Monkey IL09 | A, B |
Note that the table lists those OspC types that reacted the strongest using an immunoblot format with the serum samples indicated.
IgG responses to OspC in experimentally infected rhesus macaques were type-restricted but not completely type specific. The serum assessed in this study was from macaques that were infected under controlled laboratory conditions with I. scapularis ticks carrying B. burgdorferi B31 [42]. Strain B31 produces OspC type A and consistent with this, IgG from animal IN05 bound exclusively to OspC type A. However, serum from two infected macaques (IL75 and IL09) reacted with OspC type A and B. The basis for these cross-reactive Ab responses is unclear.
ELISA analyses of OspC Ab responses.
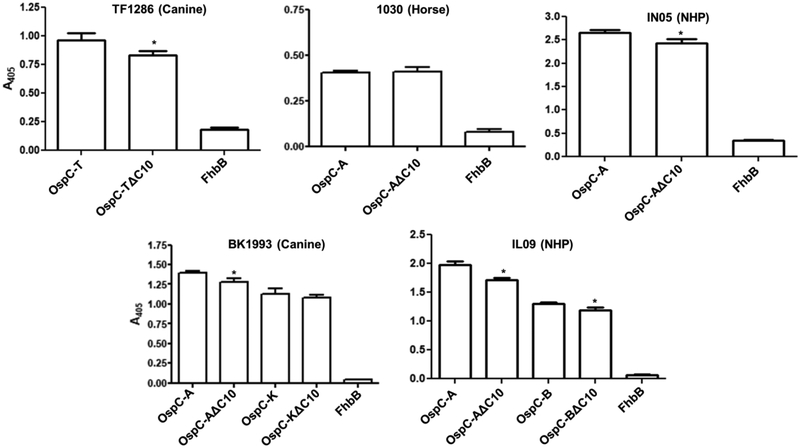
Epitope presentation can differ in denatured versus non-denatured proteins. To assess possible differential display of epitopes, the OspC and OspΔC10 proteins that bound Ab in the immunoblot assays were screened with the same serum using an ELISA format. Representative data are presented in Figure 3. Ab binding to some OspCΔ10 proteins was slightly lower than that observed with the corresponding full-length protein. The minor differences could indicate that the C10 motif is either weakly immunogenic or that it may contribute directly or indirectly to the presentation of a conformationally defined epitope. Several studies have provided evidence for the existence of conformational epitopes in OspC [23,26,49].
Figure 3. ELISA analyses of Ab responses to OspC in infected dogs, horses and non-human primates.
ELISAs were performed as detailed in the methods using the OspC proteins, and their OspCΔ10 counterparts, that reacted most strongly with the infection serum in the immuoblot assays presented in Figure 2. The serum samples tested are indicated above each graph. All assays were performed in triplicate. Standard deviation values are shown. The FhbB protein of Trepnema denticola served as a negative control [45]. The Student’s t-test was used to assess statistical significance (* p < 0.05).
The C10 motif is not required to elicit OspC mediated bactericidal Ab responses.
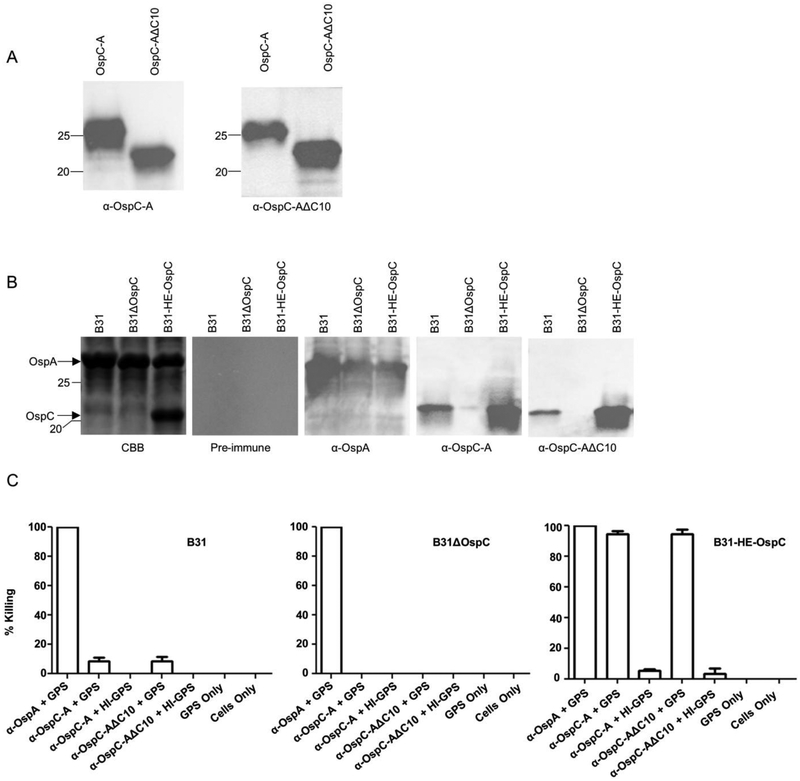
Several studies have demonstrated that OspC or OspC chimeritopes can elicit bactericidal or protective Ab responses [23,24,29,31]. To determine if the C10 motif contributes to bactericidal Ab responses elicited by immunization with recombinant OspC, hyperimmune antisera were generated to OspC type A and OspCΔC10. The OspC specific IgG log titers elicited by each protein were similar (4.8 and 4.4 for OspC and OspCΔC10, respectively) and anti-OspC and anti-OspCΔC10 displayed equal reactivity with both proteins as assessed by immunoblotting (Figure 4). Since OspC is produced at low and variable levels during cultivation [39,50], to measure bactericidal Ab activity, a strain (B31-HE-OspC) that constitutively produces OspC [51] was used as the assay strain. Controls included B. burgdorferi B31(wild type strain; low level OspC production) and B. burgdorferi B31ΔospC (negative control; no OspC production) [40]. The OspC production levels of all strains were confirmed by SDS-PAGE and by immunoblot using antisera against type A OspC (Figure 4). The anti-OspC and anti-OspCΔ10 antiserum displayed equivalent levels of Ab mediated-complement dependent killing of strain B31-HE. This observation indicates that the C10 domain is not required to elicit bactericidal Ab. These results are consistent with an earlier study that demonstrated that preabsorption of serum from LD infected humans with a peptide corresponding to the C10 peptide did not decrease the level of bactericidal activity [35]. It can be concluded that the potent bactericidal Ab responses induced by OspC are not dependent on the presence of the C10 motif.
Figure 4. The C10 motif does not contribute to OspC directed bactericidal Ab responses.
Antisera were generated against type A OspC and OspCΔ10 in rats and used to screen immunoblots of the recombinant proteins. The left and right images of Panel A present the immunoblot results obtained with anti-OspC and anti-OspCΔC10, respectively. Panel B presents the result of SDS-PAGE and immunoblot analyses of the strains (indicated in the figure) employed in the bactericidal assays. All methods were as detailed in the text. Each panel is labeled to indicate the antiserum used to screen each blot. Note that B31-HE-OspC is a genetically engineered strain of B. burgdorferi B31 that constitutively produces OspC at a high level [51]. Panel C presents the results of bactericidal assays. The rationale for the strains tested and the controls used is presented in the text. The strains tested are indicated above the bar graphs in the figure. Abbreviations are as follows: GPS - guinea pig serum; HI - heat inactivated. All assays were performed in triplicate. The Student’s t-test was used to assess statistical significance (* p < 0.05).
CONCLUSIONS:
The type-specificity of the Ab response to OspC indicates that the immunodominant epitopes reside within segments of OspC that differ among OspC types. We did not find evidence to support the conclusions reached in earlier studies that the C10 motif is antigenic and elicits protective IgG responses [34,35]. The consensus sequence for C10 is P201VVAESPKNP210 (B. burgdorferi B31 OspC numbering). Polymorphisms in C10 tend to occur at either the V202 or N209 position. As examples, when compared with B31 type A OspC, OspC types N, I, C, B, and D share the same C10 sequence whereas OspC types K and E harbor a V202I substitution. Hence, the inability of Ab to detect the C10 motif in different OspC types is clearly not due to sequence divergence. Furthermore, since deletion of the C10 motif resulted in only a minor decrease in overall bactericidal activity it is evident that the C10 motif does not directly or indirectly influence the development of bactericidal Ab.
It is possible that the lack of a robust Ab response to C10 motif may be attributed to its spatial location on the OspC protein. OspC structural analyses place the C10 motif at the OspC-bacterial membrane interface [19,20,52]. An earlier study using immunogold labeling and transmission electron microscopy suggested that C10 is surface exposed and accessible to Ab [53]. However, no controls were included in that study to determine if preparation for microscopy resulted in disruption of the cell membrane which would potentially expose the normally buried C10 motif [53]. In summary, the data presented indicate that a single OspC protein, whether in the context of a bacterin or subunit vaccine formulation, is likely insufficient for eliciting broadly protective Ab responses directed at OspC. The inherent obstacles that OspC diversity has posed in term of vaccine and diagnostic antigen development have been recently overcome using laboratory designed OspC based chimeritopes [29,31,54]. Chimeritopes are recombinant proteins that consist of a series of linear epitopes derived from multiple OspC types. Proof of principle for the chimeritope approach was first established in mice [29,31]. Immunization with tetravalent or octavalent OspC chimeritope antigens triggered Ab responses against each component epitope that were bactericidal [29,31]. The biological role that the C10 motif plays, if any, in OspC function remains unclear. We previously demonstrated that LD spirochetes that were genetically manipulated to produce OspCΔC10 in place of full length OspC retained the ability to infect mice and disseminate [33]. Hence, the biological rationale for the relatively high conservation of this domain remains to be delineated. In closing, this study further enhances our understanding of the antigenic structure of OspC.
Supplementary Material
Highlights:
OspC of the Lyme disease spirochetes triggers highly specific IgG responses in a diverse mammals
IgG responses to OspC are not elicited by conserved regions of the protein
The C7/C10 motif does not play a central role in triggering IgG responses
The C7/C10 motif is not required for induction of bactericidal antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited:
- 1.Adeolu M & Gupta RS A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 2014, 105(6), 1049–1072. [DOI] [PubMed] [Google Scholar]
- 2.Benach JL, Bosler EM, Hanrahan JP et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med 1983, 308(13), 740–742. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Grodzicki RL, Kornblatt AN et al. The spirochetal etiology of Lyme disease. New England Journal of Medicine 1983, 308, 733–740. [DOI] [PubMed] [Google Scholar]
- 4.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E & Davis JP Lyme disease-a tick-borne spirochetosis? Science 1982, 216(4552), 1317–1319. [DOI] [PubMed] [Google Scholar]
- 5.Eisen RJ, Eisen L & Beard CB County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol 2016, 53(2), 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasmi S, Ogden NH, Lindsay LR et al. Surveillance for Lyme disease in Canada: 2009–2015. Can Commun Dis Rep 2017, 43(10), 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morshed MG, Lee MK, Man S et al. Surveillance for Borrelia burgdorferi in Ixodes Ticks and Small Rodents in British Columbia. Vector Borne Zoonotic Dis 2015, 15(11), 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott JD, Anderson JF & Durden LA Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J Parasitol 2012, 98(1), 49–59. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson N, Wong J & Foley J Host, habitat and climate preferences of Ixodes angustus (Acari: Ixodidae) and infection with Borrelia burgdorferi and Anaplasma phagocytophilum in California, USA. Exp Appl Acarol 2016, 70(2), 239–252. [DOI] [PubMed] [Google Scholar]
- 10.Divers TJ, Gardner RB, Madigan JE et al. Borrelia burgdorferi Infection and Lyme Disease in North American Horses: A Consensus Statement. J Vet Intern Med 2018, 32(2), 617–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk RA, Pleasant RS, Witonsky SG, Reeder DS, Werre SR & Hodgson DR Seroprevalence of Borrelia burgdorferi in Horses Presented for Coggins Testing in Southwest Virginia and Change in Positive Test Results Approximately 1 Year Later. J Vet Intern Med 2016, 30(4), 1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson CA, Saha S, Kugeler KJ et al. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg Infect Dis 2015, 21(9), 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paules CI, Marston HD, Bloom ME & Fauci AS Tickborne Diseases - Confronting a Growing Threat. N Engl J Med 2018, 379(8), 701–703. [DOI] [PubMed] [Google Scholar]
- 14.Littman MP, Gerber B, Goldstein RE, Labato MA, Lappin MR & Moore GE ACVIM consensus update on Lyme borreliosis in dogs and cats. J Vet Intern Med 2018, 32(3), 887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwan TG & Piesman J Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 2000, 38(1), 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwan TG Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans 2003, 31(Pt 1), 108–112. [DOI] [PubMed] [Google Scholar]
- 17.Tsao J, Barbour AG, Luke CJ, Fikrig E & Fish D OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis 2001, 1(1), 65–74. [DOI] [PubMed] [Google Scholar]
- 18.Fikrig E, Telford SR 3rd, Barthold SW, Kantor FS, Spielman A & Flavell RA Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci U S A 1992, 89(12), 5418–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumaran D, Eswaramoorthy S, Luft BJ et al. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. Embo J 2001, 20(5), 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eicken C, Sharma V, Klabunde T et al. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem 2001, 276(13), 10010–10015. [DOI] [PubMed] [Google Scholar]
- 21.Earnhart CG & Marconi RT OspC phylogenetic analyses support the feasibility of a broadly protective polyvalent chimeric Lyme disease vaccine. Clin Vaccine Immunol 2007, 14(5), 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM & Luft BJ Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 1999, 151(1), 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore RD Jr., Kappel KJ, Dolan MC, Burkot TR & Johnson BJ Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun 1996, 64(6), 2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preac-Mursic V, Wilske B, Patsouris E et al. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection 1992, 20(6), 342–349. [DOI] [PubMed] [Google Scholar]
- 25.Bockenstedt LK, Hodzic E, Feng S et al. Borrelia burgdorferi strain-specific OspC mediated immunity in mice. Infection and Immunity 1997, 65, 4661–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmondson DG, Prabhakaran S, Norris SJ et al. Enhanced Protective Immunogenicity of Homodimeric Borrelia burgdorferi Outer Surface Protein C. Clin Vaccine Immunol 2017, 24(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckles EL, Earnhart CG & Marconi RT Analysis of antibody response in humans to the type A OspC loop 5 domain and assessment of the potential utility of the loop 5 epitope in Lyme disease vaccine development. Clin Vaccine Immunol 2006, 13(10), 1162–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baum E, Randall AZ, Zeller M & Barbour AG Inferring epitopes of a polymorphic antigen amidst broadly cross-reactive antibodies using protein microarrays: a study of OspC proteins of Borrelia burgdorferi. PLoS One 2016, 8(6), e67445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earnhart CG, Buckles EL & Marconi RT Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine 2007, 25(3), 466–480. [DOI] [PubMed] [Google Scholar]
- 30.Earnhart CG, Buckles EL, Dumler JS & Marconi RT Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect Immun 2005, 73(12), 7869–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earnhart CG & Marconi RT An octavalent lyme disease vaccine induces antibodies that recognize all incorporated OspC type-specific sequences. Hum Vaccin 2007, 3(6), 281–289. [DOI] [PubMed] [Google Scholar]
- 32.Ball EC Technical bulletin: VANGUARD crLyme: Chimeric recombinant vaccien technology for broad-spectrum protection against canine Lyme disease. Zoetis 2016, SAB-00193. [Google Scholar]
- 33.Earnhart CG, Rhodes DV, Smith AA et al. Assessment of the potential contribution of the highly conserved C-terminal motif (C10) of Borrelia burgdorferi outer surface protein C in transmission and infectivity. Pathog Dis 2014, 70(2), 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jobe DA, Lovrich SD, Schell RF & Callister SM C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clinical and Diagnostic Laboratory Immunology 2003, 10, 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovrich SD, Jobe DA, Schell RF & Callister SM Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clinical and diagnostic laboratory immunology 2005, 12, 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovrich SD, La Fleur RL, Jobe DA et al. Borreliacidal OspC antibody response of canines with Lyme disease differs significantly from that of humans with Lyme disease. Clin Vaccine Immunol 2007, 14(5), 635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaFleur RL, Dant JC, Wasmoen TL et al. Bacterin that induces anti-OspA and anti-OspC borreliacidal antibodies provides a high level of protection against canine Lyme disease. Clin Vaccine Immunol 2009, 16(2), 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rousselle JC, Callister SM, Schell RF et al. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J Infect Dis 1998, 178(3), 733–741. [DOI] [PubMed] [Google Scholar]
- 39.Oliver LD Jr, Earnhart CG, Virgina-Rhodes D, Theisen M & Marconi R Antibody profiling of canine IgG responses to the OspC protein of the Lyme disease spirochetes supports a multivalent approach in vaccine and diagnostic assay development. The Veterinary Journal 2016, (218), 27–33. [DOI] [PubMed] [Google Scholar]
- 40.Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD & Marconi RT Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol Microbiol 2010, 76(2), 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller DP, Oliver LD Jr., Tegels BK et al. The Treponema denticola FhbB Protein Is a Dominant Early Antigen That Elicits FhbB Variant-Specific Antibodies That Block Factor H Binding and Cleavage by Dentilisin. Infect Immun 2016, 84(7), 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Embers ME, Hasenkampf NR, Jacobs MB et al. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS One 2017, 12(12), e0189071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Embers ME, Hasenkampf NR, Barnes MB, Didier ES, Philipp MT & Tardo AC Five-Antigen Fluorescent Bead-Based Assay for Diagnosis of Lyme Disease. Clin Vaccine Immunol 2016, 23(4), 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izac JR, Oliver LD Jr., Earnhart CG & Marconi RT Identification of a defined linear epitope in the OspA protein of the Lyme disease spirochetes that elicits bactericidal antibody responses: Implications for vaccine development. Vaccine 2017, 35(24), 3178–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller DP, Bell JK, McDowell JV et al. Structure of factor H binding protein B (FhbB) of the periopathogen, Treponema denticola: Insights into the progression of periodontal disease. J Biol Chem 2012, 287, 12715–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girard YA, Travinsky B, Schotthoefer A et al. Population structure of the lyme borreliosis spirochete Borrelia burgdorferi in the western black-legged tick (Ixodes pacificus) in Northern California. Appl Environ Microbiol 2009, 75(22), 7243–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu WG, Dykhuizen DE, Acosta MS & Luft BJ Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics 2002, 160(3), 833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhodes DV, Earnhart CG, Mather TN, Meeus PF & Marconi RT Identification of Borrelia burgdorferi ospC genotypes in canine tissue following tick infestation: implications for Lyme disease vaccine and diagnostic assay design. Vet J 2013, 198(2), 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilmore RD Jr. & Mbow ML Conformational nature of the Borrelia burgdorferi B31 outer surface protein C protective epitope. Infect Immun 1999, 67(10), 5463–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang X, Yang Y, Du J et al. Investigation of ospC Expression Variation among Borrelia burgdorferi Strains. Front Cell Infect Microbiol 2017, 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tilly K, Bestor A, Jewett MW & Rosa P Rapid Clearance of Lyme Disease Spirochetes Lacking OspC from Skin. Infect. Immun 2007, 75(3), 1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumaran D, Eswaramoorthy S, Dunn JJ & Swaminathan S Crystallization and preliminary X-ray analysis of Borrelia burgdorferi outer surface protein C (OspC). Acta Crystallogr D Biol Crystallogr 2001, 57(Pt 2), 298–300. [DOI] [PubMed] [Google Scholar]
- 53.Mathiesen MJ, Holm A, Christiansen M et al. The dominant epitope of Borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect Immun 1998, 66(9), 4073–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Earnhart CG & Marconi RT Construction and analysis of variants of a polyvalent Lyme disease vaccine: approaches for improving the immune response to chimeric vaccinogens. Vaccine 2007, 25(17), 3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraser CM, Casjens S, Huang WM et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997, 390(6660), 580–586. [DOI] [PubMed] [Google Scholar]
- 56.Wang G, Ojaimi C, Wu H et al. Disease severity in a murine model of lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 2002, 186(6), 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.