Main Text
Chimeric antigen receptor (CAR) T cells represent a revolutionary therapy for combatting cancers.1 This novel immunotherapy was only approved in 2017 for treatment of advanced blood cancers in the US and Europe. The most frequently used vehicles for CAR gene delivery in autologous T cells are lentiviral vectors (LVs) pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G-LV). Ex vivo gene delivery employing these broad spectrum LVs might, however, lead to off-target transduction of contaminating cancer or antigen-presenting cells with these CARs leading to severe health risks and adverse events.2 In a new study published in Molecular Therapy – Methods and Clinical Development, Jamali et al.3 limit these risks by using CD4+ or CD8+ T cell targeted LVs for CAR gene transfer. The authors engineered human CD4+ and CD8+ T cell-targeted LVs capable of selective gene delivery into human CD4+ or CD8+ T cells, respectively.4, 5
The targeting of LVs is based on the principle that fusion-activation of a chimeric envelope should be triggered by the interaction of the ligand displayed on the vector surface with its specific receptor on the target cell. Buchholz and co-workers3 designed a novel strategy based on the fact that paramyxoviruses separated the two functions, cell binding and virus-cell fusion, into separate glycoproteins. In this case, redirection of the glycoprotein that confers binding to the target cell leaves the fusion glycoprotein (gp) “untouched” and functional. This strategy was initially successful for LVs pseudotyped with measles virus (MV-LVs) retargeted glycoproteins and more recently LVs pseudotyped with receptor-targeted Nipah virus glycoproteins (NiV-LVs). These effectively enter into cells when using cell surface proteins as receptors that bring them sufficiently close to the cell membrane.6
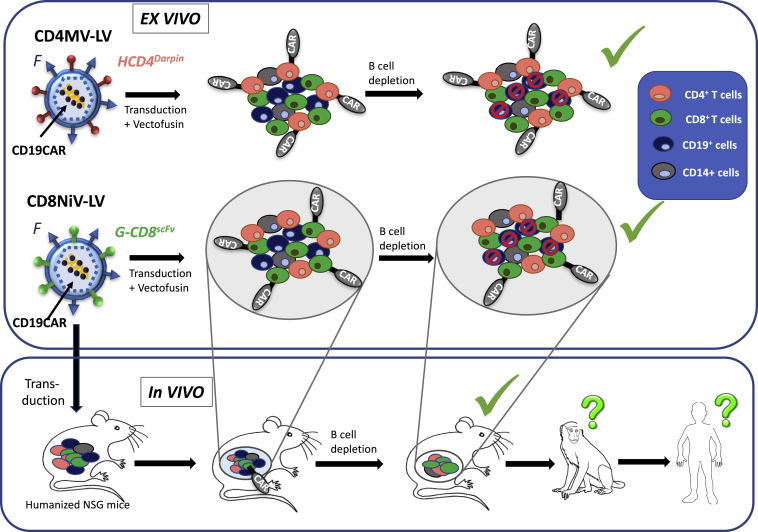
Jamali et al.3 demonstrated exclusive transduction of CD4+ or CD8+ T cells in total peripheral blood mononuclear cells (PBMCs) with an anti-CD19 CAR encoding CD4MV- or CD8NiV-retargeted LVs, respectively. Moreover, in the presence of an LV facilitating agent, Vectofusin, transduction with CD19-CAR-targeted LVs was comparable to T cell transduction levels achieved with VSVG-LVs currently used in the clinic. This was achieved without compromising the specificity for the target T cells (Figure 1). Although such a targeted approach will make ex vivo CAR T cell therapy safer, it remains a highly personalized treatment that likely requires a costly clinical manufacturing grade and labor-intensive ex vivo T cell manufacturing process. These considerations highlight the obstacles associated with manufacturing of this kind of patient-specific therapy. Therefore, implementation of CAR T cell therapy for routine use in the clinic remains a real challenge and this new therapeutic product may be out of reach for many cancer patients in need of this novel therapy. A future approach avoiding the above concerns may be in vivo CAR T cell generation.
Figure 1.
Toward In Vivo CAR T Cell Therapy
Exclusive transduction of CD4+ or CD8+ T cells in total PBMCs with an anti-CD19 CAR encoding CD4darpinMV- and CD8scFvNiV-retargeted lentiviral vectors, respectively. CAR T cells were able to specifically deplete healthy and cancer CD19+ cells ex vivo. In vivo injection of CD8scFvNiV-retargeted lentiviral vectors into non-obese diabetic/severe combined immunodeficient (NOD/SCID) γC−/− mice that were engrafted with hCD34+ stem and progenitor cells resulted in expression of the CD19 directed CAR solely on CD8+ cells in vivo which, upon encounter with CD19+ B cells, were strongly expanded and resulted in B cell depletion in vivo. This represents a first step toward in vivo CAR therapy but evaluation in non-human primates will be required before these tools can enter the clinic for patient treatment.
The Buchholz team7 has very recently performed a first step toward in vivo reprogramming of CAR T cells using CD8NiV-targeted LVs. A single systemic injection of CD19-CAR-encoding CD8-LVs into immune-deficient mice engrafted with a human blood system generated in vivo CAR T cells, which effectively wiped out the human B cells (Figure 1).7 This outcome supports the feasibility of in vivo CAR T cell therapy. Remarkably, NiV-LVs could be produced at up to two orders of magnitude higher titers compared to their MV-based counterparts and were at least 10,000-fold less effectively neutralized than MV glycoprotein pseudotyped LVs by human serum, underlining their potential as a medicine that is applicable for in vivo delivery.4
However, some of the CD8NiV-LV injected humanized mice developed a cytokine storm equivalent to some CAR T cell treated patients, which warrants further preclinical testing of the in vivo approach. A next logical step will be to test the performance of in vivo CAR T cells therapy in mice carrying patient derived tumor xenografts (PDX mice models). However, humanized mice do not recapitulate a fully functional immune system and evaluation in large animal models such as non-human primates may be needed before a first clinical trial on in vivo CAR T cell gene therapy can commence (Figure 1). Nonetheless, once these steps have been successfully completed future CAR T cell therapy might consist of a single injection of a vectorized medicine into the blood stream, circumventing the current cost-intensive ex vivo production of CAR T cells, and thus making this therapy more broadly available to patients.
References
- 1.Guedan S., Calderon H., Posey A.D., Jr., Maus M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2018;12:145–156. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruella M., Xu J., Barrett D.M., Fraietta J.A., Reich T.J., Ambrose D.E., Klichinsky M., Shestova O., Patel P.R., Kulikovskaya I. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018;24:1499–1503. doi: 10.1038/s41591-018-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamali A., Kapitza L., Schaser T., Johnston I.C.D., Buchholz C.J., Hartmann J. Highly efficient and selective CAR-gene transfer using CD4- and CD8-targeted lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2019 doi: 10.1016/j.omtm.2019.03.003. Published online March 16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender R.R., Muth A., Schneider I.C., Friedel T., Hartmann J., Plückthun A., Maisner A., Buchholz C.J. Receptor-Targeted Nipah Virus Glycoproteins Improve Cell-Type Selective Gene Delivery and Reveal a Preference for Membrane-Proximal Cell Attachment. PLoS Pathog. 2016;12:e1005641. doi: 10.1371/journal.ppat.1005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Q., Uhlig K.M., Muth A., Kimpel J., Lévy C., Münch R.C., Seifried J., Pfeiffer A., Trkola A., Coulibaly C. Exclusive Transduction of Human CD4+ T Cells upon Systemic Delivery of CD4-Targeted Lentiviral Vectors. J. Immunol. 2015;195:2493–2501. doi: 10.4049/jimmunol.1500956. [DOI] [PubMed] [Google Scholar]
- 6.Frank A.M., Buchholz C.J. Surface-Engineered Lentiviral Vectors for Selective Gene Transfer into Subtypes of Lymphocytes. Mol. Ther. Methods Clin. Dev. 2018;12:19–31. doi: 10.1016/j.omtm.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeiffer A., Thalheimer F.B., Hartmann S., Frank A.M., Bender R.R., Danisch S., Costa C., Wels W.S., Modlich U., Stripecke R. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 2018;10:10. doi: 10.15252/emmm.201809158. [DOI] [PMC free article] [PubMed] [Google Scholar]