Abstract
OBJECTIVES
Asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO) is defined as a persistent airflow limitation with features of both asthma and COPD. However, in Turkey, there are limited data about ACO. The aims of the present study were to determine the prevalence of ACO among patients with asthma, to compare the clinical characteristics of patients with ACO and asthma, and to determine the threshold values for the diagnosis of ACO.
MATERIALS AND METHODS
The study group comprised 338 patients admitted to the outpatient clinics between 2010 and 2017 and who had undergone at least three pulmonary function tests within the last 2 years. Patients aged >40 years with a smoking history of >10 pack-years or biomass exposure, with at least three features of both diseases, and with reversible and persistent airflow limitation were diagnosed with ACO. The study is a retrospective study so we did not get informed concent.
RESULTS
Asthma-chronic obstructive pulmonary disease overlap was diagnosed in 40 (11.8%) patients. Patients with ACO had fewer allergic comorbidities, worse spirometric parameters, and required higher doses of inhaled corticosteroids than patients with asthma only (p<0.05). No significant differences were observed between the groups regarding survival or number of hospitalizations and attacks (p>0.05). Threshold values were determined as age ≥57.5 years, smoking history ≥14 pack-years, and diagnosis at age ≥40.5 years.
CONCLUSION
The frequency of ACO was observed to be very high in patients with asthma. In patients >57.5 years old, with a smoking history of >14.5 pack-years, and diagnosed with asthma at >40.5 years old, the probability of ACO diagnosis increases.
Keywords: Asthma, asthma-COPD overlap, prevelance, risk factors
INTRODUCTION
Asthma and chronic obstructive pulmonary disease (COPD) are airway diseases with different clinical features, but both are characterized by airflow limitation. Patients with both COPD and asthma could also have a diagnosis of asthma-COPD overlap (ACO), where the clinical and functional features of both diseases overlap. Various national and international guidelines started to include ACO in 2000 [1–4]. ACO should be considered in patients with asthma with a persistent airflow limitation [post-bronchodilator (BD) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70%] with a smoking history or in patients with COPD with a history of childhood asthma, eosinophilia, and reversibility in the pulmonary function tests (PFTs) [5]. The prevalence of cases with ACO has been reported at varying rates of 15%–55% in different studies. Patients with ACO have more frequent and severe attacks resulting in a greater number of hospitalizations, emergency visits, and higher health costs than patients with asthma and COPD [6,7].
The aims of the present study were to determine the prevalence of ACO in patients previously diagnosed with asthma and to compare these patients with patients with asthma in terms of comorbidities, general clinical features, and number of attacks and hospitalizations.
MATERIALS AND METHODS
This was a descriptive retrospective study. The study included patients who were diagnosed with asthma according to the guideline of the Global Initiative for Asthma (GINA) and were followed up in the outpatient clinic of the Department of Pulmonary Diseases between 2010 and 2017, who underwent PFT at least three times over a 2-year period, and who had no other pulmonary diseases (interstitial lung disease, lung cancer, etc.) [8]. The Gazi University Clinical Research Ethics Committee (Date: 13/03/2017, Decision No: 47) approved the study. After evaluation of 2742 patients’ charts, 338 patients who met the inclusion criteria were included in the study. According to the guidelines of GINA and the Turkish Thoracic Society, patients diagnosed with ACO were those who were >40 years old with at least 10 SPY or biomass exposure, at least three features of both asthma and COPD, had a post-BD FEV1/FVC <70% in reversible PFT and/or had a FEV1/FVC <70% in at least three PFTs during the previous 2 years of follow-up, and the others were assessed as the asthma group [8,9]. The comorbidities were recorded from patient medical records based on the patient history. The skin prick tests of the subjects were evaluated by an allergy specialist. The skin prick test was performed using allergen extracts and positive and negative controls and was interpreted 15–20 min after application. A positive test was defined as a weal ≥3 mm in diameter [10].
Patients were divided into two groups as the asthma group and the ACO group, and the features of these two groups were compared.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 15.0 software (SPSS Inc.; Chicago, IL, USA). Normal distribution of continuous variables was evaluated using visual (histogram and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). The continuity correction chi-square test and Fisher’s exact test were used in the comparison of the frequency rates of categorical variables between the two groups (asthma/ACO). The Mann-Whitney U test and independent samples t-test were utilized in the comparison analysis. The effect of ACO on mortality was assessed using the log-rank test. The mortality rate was computed using the Kaplan-Meier survival analysis. When estimating survival time, the start date was determined as the date of asthma diagnosis and the end date as July 1, 2017. The interval was reported in months. Receiver operating curve (ROC) analysis was used to determine whether smoking pack-year (SPY), age, and age at the onset of asthma were optimum cut-off values in distinguishing patients with ACO. The sensitivity, specificity, positive predictive value, and negative predictive value of significant limit values were estimated. Univariate binary logistic regression analyses were used to assess the effect of independent predictors on ACO. A p-value <0.05 was considered as statistically significant.
RESULTS
Of 2742 patients initially screened, 338 met the study inclusion criteria. ACO was determined in 40/338 (11.8%) patients with asthma. The ACO group comprised 27 (67.5%) male and 13 (32.5%) female patients with a mean age of 63.78±10.23 years. The asthma group comprised 64 (21.5%) male and 234 (78.5%) female patients with a mean age of 53.86±13.04 years. When the mean age of asthma onset (MAO) was examined in both groups, it was higher in patients with ACO. There was no significant difference between the groups in terms of disease duration.
When the smoking history was examined, the mean SPYs was 25.83±14.24 pack-years, the median value was 22.5 (0–50) pack-years in the ACO group, and the mean SPYs of 6.44±13.87 pack-years with a median value of 0 (0–100) pack-year in the asthma group. After evaluating the first-line treatment, it was determined that higher doses of inhaled corticosteroids (ICS) were started in patients with ACO than in patients with asthma (p<0.05). There were no significant differences between the groups in terms of the average number of hospitalizations and attacks. Table 1 shows the demographic data of the patients.
Table 1.
Demographics of the study population
| ACO (n=40) (11.8%) | Asthma (n=298) (88.2%) | p | |
|---|---|---|---|
| Age (years), mean±SD | 63.78±10.23 | 53.86±13.04 | 0.0011 |
| Gender, n (%) | |||
| Female | 27 (67.5%) | 64 (21.5%) | 0.0012 |
| Male | 13 (32.5%) | 234 (78.5%) | |
| Mean age of asthma onset, mean±SD | 48.73±13.01 | 38.81±13.42 | 0.0011 |
| Smoking pack-years, median (min-max) | 22.5 (0–50) | 0 (0–100) | 0.0011 |
| Asthma duration (years), mean±SD | 15±7.88 | 15.07±10.73 | 0.4533 |
| Treatment ICS dose, n (%) | |||
| Low-medium dose | 19 (47.5%) | 208 (69.8%) | 0.0081 |
| High dose | 21 (52.5%) | 90 (30.2%) | |
| Hospitalizations, median (min-max) | 0 (0–5) | 0 (0–5) | 0.7883 |
| Attacks, median (min-max) | 0 (0–3.75) | 0 (0–3.33) | 0.9523 |
Independent samples t-test
Continuity correction chi-square test
Mann-Whitney U
ACO: asthma-COPD overlap; ICS: inhaled corticosteroids; SD: standard deviation
The comorbidities of the patients were grouped as renal, endocrine, cardiac, neurological, and liver diseases. No significant differences in the number of comorbidities or frequency were detected between patients with ACO and asthma (p>0.05).
The presence of urticaria, nasal polyps, allergic rhinitis, or sinusitis was evaluated as an allergic comorbidity. The presence of any allergic comorbidities in patients with ACO was lower than that in patients with asthma (p<0.05) (Table 2).
Table 2.
The atopy features and the frequency of allergic comorbidity of the study population
| ACO (n=40) (11.8%) | Asthma (n=298) (88.2%) | p | |
|---|---|---|---|
| Skin prick positive, n (%) | 4/16 (25%) | 75/189 (39.7%) | 0.3732 |
| Total IgE (IU/dL) | 33.3 (12–233) | 70 (6–2432) | 0.2461 |
| Blood eosinophils, n (min-max) | 100 (0–600) | 170 (0–1017) | 0.8301 |
| Blood eosinophils, % (min-max) | 1.42 (0–6.5) | 2.1 (0–15.5) | 0.5301 |
| Presence of any allergic comorbidity, n (%) | 17 (42.5%) | 194 (65.1%) | 0.0092 |
Kruskal-Wallis test
Continuity correction Chi-square test
ACO: asthma-COPD overlap
No significant differences were identifed between the groups with respect to skin prick test positivity, serum total IgE, and the number and percentage of blood eosinophils at the last examination, in order to evaluate the atopic properties of the patients (p>0.05) (Table 2).
Table 3 shows the spirometric data of the patients. FEV1, FVC, and FEV1/FVC values were worse in patients with ACO than in patients with asthma. Forced expiratory flow 25–75 (FEF25–75) % values were found to be lower in patients with ACO than in patients with asthma (p<0.05).
Table 3.
Pulmonary function test results of the study population
| ACO (n=40) (11.8%) | Asthma (n=298) (88.2%) | p | |
|---|---|---|---|
| Post-BD FEV1 % | 70.44±15.94 | 101.83±98.03 | 0.0441 |
| Post-BD FEV1 L | 2.077±0.675 | 2.509±0.861 | 0.0031 |
| Post-BD FVC % | 87.93±17.37 | 99.79±16.89 | 0.0011 |
| Post-BD FVC L | 3.244±1.015 | 3.155±0.96 | 0.6021 |
| Post-BD FEV1/FVC | 64.09±5.64 | 79.02±8.41 | 0.0011 |
| Post-BD FEF25-75 % | 36.73±13.91 | 64.18±26.81 | 0.0011 |
| Post-BD FEF25-75 L/sn | 1.274±0.570 | 2.272±1.18 | 0.0011 |
Independent samples t-test
ACO: asthma-COPD overlap; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity
In the mortality assessment, no statistically significant relationship was identified between the presence of ACO and mortality (p=0.221). Survival was seen in 37 (92.5%) out of 40 patients with ACO and 288 (96.8%) out of 298 patients with asthma. The average survival of the patients with ACO was 376.7±22.5 [95% confidence interval (CI): 332.5–420.9] months, whereas that of patients with asthma was 680.1±12.3 (95% CI: 655.9–704.3) months.
ROC analysis was performed to determine whether SPY, age, and age of asthma onset were optimum cut-off values in discriminating patients with an ACO diagnosis. Area under the curve (AUC), 95% confidence interval, and p-values were estimated as follows: AUC SPY: 0.859, 95% CI: 0.799–0.918, p<0.001/AUC age: 0.723, 95% CI: 0.643–0.803, and p<0.001/0.704, 95% CI: 0.618–0.791, p<0.001. According to the estimated results, AUC was determined to be statistically significant (p<0.01) for all three values (Figure 1). As a result of the assessment with ROC analysis, SPY, age, and age of asthma onset were determined as diagnostic values in predicting ACO diagnosis.
Figure 1.
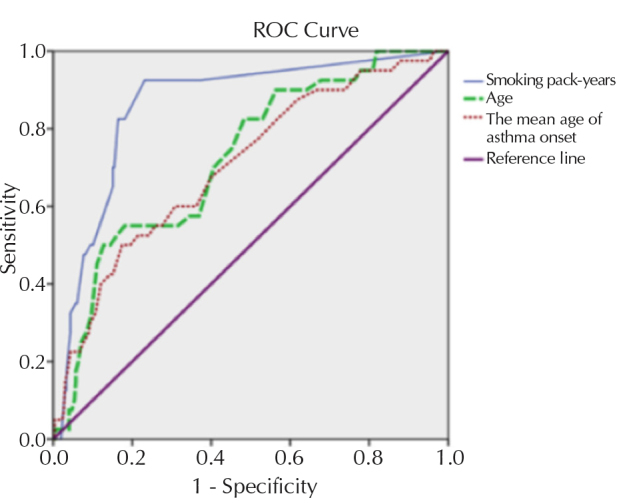
ROC curve analysis of age, smoking pack-years, and age of asthma diagnosis in 338 patients with asthma
ROC: receiver operating curve
The cut-off value of 57.5 years yielded a sensitivity of 70% and a specificity of 59.4%, and the cut-off value of 14 SPYs had a sensitivity of 82.5% and a specificity of 83.6% for the prediction of ACO diagnosis. In addition, the MAO at 40.5 years as a cut-off value demonstrated a sensitivity of 70% and a specificity of 57% (Table 4).
Table 4.
Predictive values of smoking pack-years, age, and mean age of asthma onset for ACO
| Cut-off values | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| ≥14 SPY | 82.5 | 83.6 | 40.2 | 97.3 |
| ≥57.5 years | 70 | 59.4 | 19 | 93 |
| ≥40.5 years MAO | 70 | 57 | 18 | 93.4 |
SPY: smoking pack-year; MAO: mean age of asthma onset; ACO: asthma-COPD overlap
Table 5 shows the results of the univariate binary logistic regression analysis. According to these results, the risk of ACO diagnosis was 7.5 times higher in males than in females (odds ratio (OR)=7.59; 95% CI: 3.70–15.55; p=0.001) (Table 5). After the same analysis was performed for cut-off values obtained in the ROC analysis, the risk of diagnosis of ACO in cases aged ≥57.5 years and ≥14 SPYs was 18.2 times higher (OR=18.2; 95% CI: 8.51–38.91; p=0.001), and in addition to the values described above, when the MAO was ≥40.5 years, the risk was increased 12.5-fold (OR=12.54; 95% CI: 5.88–26.73; p=0.001).
Table 5.
Univariate binary logistic regression analysis of the demographic characteristics for ACO
| ACO n |
ACO (%) | OR (95% CI) | p | |
|---|---|---|---|---|
| Gender | ||||
| Female | 13/247 | 5.3 | 1.00 | 0.001 |
| Male | 27/91 | 29.7 | 7.59 (3.70–15.55) | |
| SPY | ||||
| <14 SPY | 7/256 | 2.7 | 1.00 | 0.001 |
| ≥14 SPY | 33/82 | 40.2 | 23.95 (10.02–57.25) | |
| MAO | ||||
| <40.5 | 12/182 | 6.6 | 1.00 | 0.002 |
| ≥40.5 | 28/156 | 17.8 | 3.09 (1.51–6.32) | |
| Age | ||||
| <57.5 | 12/189 | 6.3 | 1.00 | 0.001 |
| ≥57.5 | 28/149 | 18.8 | 3.41 (1.67–6.97) | |
| SPY and MAO | ||||
| SPY <14 and MAO <40.5 | 18/282 | 6.4 | 1.00 | 0.001 |
| SPY ≥14 and MAO ≥40.5 | 22/56 | 39.3 | 9.49 (4.62–19.45) | |
| SPY and age | ||||
| SPY <14 and age <57.5 | 15/288 | 5.2 | 1.00 | 0.001 |
| SPY ≥14 and age ≥57.5 | 25/50 | 50 | 18.2 (8.51–38.91) | |
| SPY, MAO, and age | ||||
| SPY <14, MAO <40.5, age <57.5 | 20/296 | 6.8 | 1.00 | 0.001 |
| SPY ≥14, MAO ≥40.5, age≥57.5 | 20/42 | 47.6 | 12.54 (5.88–26.73) | |
N: number of patients with asthma; CI: confidence interval; SPY: smoking pack-year; MAO: mean age of asthma onset; OR: odds ratio; ACO: asthma-COPD overlap
DISCUSSION
Asthma-COPD overlap, where both clinical and functional features of ACO in patients with COPD and asthma, has become one of the most debated obstructive pulmonary diseases over the last few years. The aims of the present study were to determine the frequency of ACO in patients with asthma and to compare the general characteristics of patients with asthma and ACO.
In the present study, the frequency of ACO in patients with asthma was determined as 11.8%. In the literature, the overlap rate of doctor-diagnosed asthma-COPD was reported to be 1.6% at age 20–44 years, 2.1% at age 45–64 years, and 4.5% at age 65–84 years in the Gene-Environment Interactions in Respiratory Diseases study, which consisted of 8360 patients. In the same study, ACO in patients with asthma was determined as 16% at age 20–44 years, 30% at age 45–64 years, and 61% at age 65–84 years [11]. In a multicenter study in Latin American countries (Proyecto Latinoamericano de Investigation en Obstruccion Pulmonar), a total of 767 patients with asthma were classified as asthma, COPD, and ACO at the estimated ratios of 1.7%, 12%, and 1.8%, respectively [7]. In The Prevalence StUdy and Regular Practice, Diagnosis and TreatMent, Among General Practitioners in Populations at Risk of COPD in Latin America (PUMA) study, the rate of ACO in the overall population was reported as 5.3% in cases with a previous asthma diagnosis and as 2.3% in cases newly diagnosed during the study [12].
In another previous review, the frequency of ACO was reported as 12.1%–55.2% in patients with COPD and as 13.3%–61% in patients with asthma [13]. In epidemiological studies, the reason for the variability in the prevalence of ACO is accounted for by different diagnostic criteria used by researchers. Another important reason is the characteristics of the population under investigation. In other words, different ratios for the frequency of ACO are reported in the general population, whereas different ratios are presented for patients with either asthma or COPD.
In accordance with the literature, the mean age was higher in the ACO group than in other patients with asthma in the present study. It is thought that the increase in the incidence of ACO at an older age compared with asthma could be explained by increased airway remodeling, biomass exposure, and development of fixed airflow limitation due to the burden of smoking in addition to the age-related decline in FEV1 [14–16]. In the current study, serum total IgE and eosinophil percentages and counts were compared, and no significant differences were determined between the groups, similar to the results of the study by Tommola et al. [17]. In a study comparing patients with ACO and asthma, the number and percentage of eosinophils were found to be higher in patients with asthma than in patients with ACO [18]. In another study, the number and percentage of eosinophils in patients with ACO were lower than other values, whereas the IgE values were found to be higher [19].
The assessment of spirometric variables is an important step in the diagnosis of ACO. In a review of the literature, a comparison of spirometric variables of patients with asthma, COPD, and ACO revealed that the values of patients with ACO were lower than those of patients with asthma and higher than those of patients with COPD [5,8,9,11,12]. Small airways are defined as airways <2 mm in diameter. Both large and small airway dysfunctions due to inflammation has been observed in patients with asthma, and FEF25–75 % is used to evaluate small airway dysfunction. The assessment in the current study of the first and last FEF25–75 % values of patients revealed that patients with ACO had lower values. The dysfunctions were considered to be related to exposures, such as smoking and biomass, and were more severe in patients with ACO. In another study, it was suggested that a smaller airway dysfunction is related to uncontrollable asthma, and that these patients are more symptomatic and have more attacks [20]. In the current study, the lower values of spirometric measurement on the first visit compared with other patients with asthma were considered to be possibly due to the diminished pulmonary functions related to the presence of ACO at an older age, having lower values as a result of smoking, and persistent airflow restriction. The retrospective nature of the study, however, was a limitation.
In a previous study, the frequency of patients with allergic comorbidities was lower in patients with ACO, but not statistically significant [17]. In the current study, the presence of any allergic comorbidity was found to be significantly lower in patients with ACO than in patients with asthma. However, similar ratios of the presence of allergic comorbidities were found in patients with asthma and ACO and higher than in other healthy people and patients with COPD in a longitudinal study [21].
In many studies, after comparisons of asthma, ACO, and COPD, it has been suggested that patients with ACO have higher rates of attacks and hospitalizations than patients with asthma and COPD [5–8,22]. In the PUMA trial, when patients with asthma, COPD, and ACO were compared in terms of the number of asthma attacks and hospital admissions, the rate of patients who had an attack in the previous year was found to be higher in ACO than in asthma and COPD alone. The number of hospital admissions for any reason or for exacerbation of the disease was observed to be higher in ACO than in asthma and COPD alone [12]. In the current study, there was no significant difference between the groups with respect to hospital admissions and the annual mean number of attacks. This may have been due to the fact that the patients may have provided incomplete information during their admissions to the outpatient clinic of chest disease, and that they may not have been aware of the treatment they received after admission to the outpatient clinics and the visits to the emergency services for any ailments, and due to the absence of their medical records in the databases of other hospitals.
The aim of the treatment of patients with ACO is to reduce both symptoms and the number of attacks. Eosinophilic inflammation is more prominent in patients with ACO than in patients with COPD, and ICS holds an important place in the treatment of these patients. When the initial treatments of the patients were examined, it was revealed that high dose ICS was used in the majority of patients with ACO, and low-mild dose ICS was started in patients with asthma. Studies have shown that patients with ACO are more symptomatic when the respiratory symptoms are compared among those with asthma, ACO, and COPD [17,21]. However, it leads to the use of high drug doses to control the disease. In another study, there was no significant difference in older age patients with asthma, COPD, and ACO in terms of the use of ICS and daily dose requirements [23].
In the current study, no significant differences were determined between the groups in terms of survival time. In a recent study, the mortality rates were lower in patients with ACO than in patients with asthma and COPD [24]. However, there was no significant difference between asthma, COPD, and ACO in terms of mortality over a 15-year period in patients >65 years old [25].
When the predictive values of demographics were examined, age of 60 years and smoking >20 pack-years were identified as the cut-off values in predicting the diagnosis of ACO in a previous study [26]. In that study, age of 60 years old showed 63.5% sensitivity and 59.4% specificity in anticipating ACO diagnosis, whereas in the current study, the age at diagnosis was detected as 57.5 years, which exhibited 70% sensitivity and 59.4% specificity. In the same study, sensitivity for 20 SPYs was found to be 80.8%, and specificity was 42.8%, whereas in the currrent study, the cut-off value of 14 SPYs revealed 82.5% sensitivity and 83.6% specificity [26].
The limitations of the current study were its retrospective design based on patient medical history and the reliability or accuracy of patient recall of symptoms and treatments.
In conclusion, the present study identified that there was a significant proportion of patients with ACO among patients with asthma. The presence of ≥14.5 SPY, age of ≥57.5 years, and age of asthma diagnosis at ≥40.5 years were determined as significant values in differentiating patients with ACO from patients with asthma. Physicians should consider the requirement of higher doses of ICS in these patients.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Gazi Universty Clinical Research (Date 13/03/2017, Decision No 47).
Informed Consent: Due to the retrospective design of the study, informed consent was not taken.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - İ.S.K.; Design - T.Ç., İ.S.K.; Supervision - T.Ç., İ.S.K.; Resources - T.Ç., İ.S.K.; Materials - T.Ç.; Data Collection and/or Processing - T.Ç.; Analysis and/or Interpretation - T.Ç., İ.S.K., Y.B.Ü., E.Ü., H.Ş.; Literature Search - T.Ç., İ.S.K., Y.B.Ü.; Writing Manuscript - T.Ç., İ.S.K.; Critical Review - T.Ç., İ.S.K., Y.B.Ü., E.Ü., H.Ş.; Other - T.Ç., İ.S.K., Y.B.Ü.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for diagnosis, management and prevention of COPD (Update 2017) Available from: http://goldcopd.org.
- 2.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease-2007 update. Can Respir J. 2007;14(Suppl B):5B–32B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee for the Third Edition of the COPD Guidelines of the Japanese Respiratory Society. Guidelines for the Diagnosis and Treatment of COPD. 3rd ed. Medical Review Co; Tokyo: 2009. Available date: 26, 03.2015. [Google Scholar]
- 4.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol. 2014;50(Suppl 1):S1–S16. doi: 10.1016/S0300-2896(14)70070-5. [DOI] [PubMed] [Google Scholar]
- 5.Şen E, Oğuzülgen IK, Bavbek S, et al. Asthma and COPD overlap syndrome. Tuberk Toraks. 2015;63:265–77. doi: 10.5578/tt.9885. [DOI] [PubMed] [Google Scholar]
- 6.Andersén H, Lampela P, Nevanlinna A, et al. High hospital burden in overlap syndrome of Asthma and COPD. Clin Respir J. 2013;7:342–6. doi: 10.1111/crj.12013. [DOI] [PubMed] [Google Scholar]
- 7.Menezes AMB, Montes de Oca MM, Pérez-Padilla R, et al. Increased risk of exerbation and hospitalization in subjects with an overlap phenotype. Chest. 2014;145:297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention (Update 2018) Available from http://www.ginasthma.org.
- 9.Turkish Thoracic Society Asthma and Allergy Study Group. Asthma Diagnose and Treatment Guideline. Turk Thorac J. 2016;17(Supp 1):1–96. [Google Scholar]
- 10.Konstantinou GN, Bousquet PJ, Zuberbier T, et al. The longest wheal diameter is the optimal measurement for the evaluation of skin prick tests. Int Arch Allergy Immunol. 2010;151:343–5. doi: 10.1159/000250443. [DOI] [PubMed] [Google Scholar]
- 11.de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8:e62985. doi: 10.1371/journal.pone.0062985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montes de Oca M, Victorina Lopez Varela M, Laucho-Contreras ME, et al. Asthma-COPD overlap syndrome (ACOS) in primary care of four Latin America countries: the PUMA study. BMC Pulm Med. 2017;17:69. doi: 10.1186/s12890-017-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurst KE, Kelly-Reif K, Bushnell GA, et al. Understanding asthma-chronic obstructive pulmonary disease overlap syndrome. Respir Med. 2016;110:1–11. doi: 10.1016/j.rmed.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Zeki AA, Schivo M, Chan A, et al. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy (Cairo) 2011;2011 doi: 10.1155/2011/861926. 861926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Gao S, Zhu W, et al. Risk factors for persistent airflow limitation: Analysis of 306 patients with asthma. Pak J Med Sci. 2014;30:1393–7. doi: 10.12669/pjms.306.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding B, DiBonaventura M, Karlsson N, Ling X. Asthma-chronic obstructive pulmonary disease overlap syndrome in the urban Chinese population; prevalance and disease burden using the 2010, 2012, 2013 China National Health and Wellness Surveys. Int J Chron Obstruct Pulmon Dis. 2016;11:1139–50. doi: 10.2147/COPD.S103873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tommola M, Ilmarinen P, Tuomisto LE, et al. Differences between asthma-COPD overlap syndrome and adult-onset asthma. Eur Respir J. 2017;49 doi: 10.1183/13993003.02383-2016. pii:1602383. [DOI] [PubMed] [Google Scholar]
- 18.Lee HY, Kang JY, Yoon HK, et al. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014;55:980–6. doi: 10.3349/ymj.2014.55.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen M, Bårnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome-a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1443–54. doi: 10.2147/COPD.S85363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda T, Oga T, Niimi A, et al. Relationship between small airway function and health status, dyspnea and disease control in asthma. Respiration. 2010;80:120–6. doi: 10.1159/000242113. [DOI] [PubMed] [Google Scholar]
- 21.De Marco R, Marcon A, Rossi A, et al. Asthma, COPD and overlap syndrome: a longitudinal study in young European adults. Eur Respir J. 2015;46:671–9. doi: 10.1183/09031936.00008615. [DOI] [PubMed] [Google Scholar]
- 22.Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107:1053–60. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Fu JJ, Gibson PG, Simpson JL, et al. Longitudinal changes in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndrome. Respiration. 2014;87:63–74. doi: 10.1159/000352053. [DOI] [PubMed] [Google Scholar]
- 24.Baarnes CB, Andersen ZJ, Tjønneland A, et al. Incidence and long-term outcome asthma-COPD overlap compared to asthma and COPD alone: a 35-year prospective study of 57,053 middle-aged adults. Int J Chron Obstruct Pulmon Dis. 2017;12:571–9. doi: 10.2147/COPD.S123167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorino C, Pedone C, Scichilone N. Fifteen-year mortality of patients with asthma-COPD overlap syndrome. Eur J Intern Med. 2016;34:72–7. doi: 10.1016/j.ejim.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Kiljander T, Helin T, Venho K, et al. Prevalence of asthma-COPD overlap syndrome among primary care asthmatics with a smoking history a cross-sectional study. NPJ Prim Care Respir Med. 2015;25:15047. doi: 10.1038/npjpcrm.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]


