Abstract
OBJECTIVES
The aim of this study is to assess magnetic resonance imaging (MRI), diffusion-weighted imaging (DWI), T2-weighted image (T2WI), and apparent diffusion coefficient (ADC) maps’ threshold values before computed tomography (CT)-guided transthorasic biopsy in solitary pulmonary nodules (SPN) by describing tumoral cell density.
MATERIALS AND METHODS
Patients who had SPN were prospectively evaluated with MRI (T1WI, T2WI) and DWI (b=0, b=500, b=1000).The ADC maps were created for each patient. Before the biopsy, lesion muscle ratios (LMR) at T2WI, ADC value, and lesion spinal cord ratio at each b values were noted. The measurements were correlated with the histopathological results.
RESULTS
A total of 53 patients were included in the study: 30.2% (n=16) were female, and 69.8% (n=37) were male. Among them, 17 lesions (32.1%) were benign, and 36 lesions (67.9%) were malignant. The age varied between 40 and 82 years, with a mean of 61.7±9.1 years. The SPN diameters were between 10 and 30 mm, and the median was 24 mm. The LSR0 and LMR values were not statistically significant in detecting malignancy. LSR500 >0.53 value can predict malignancy with 100% sensitivity and 70.6% specificity. LSR1000 >0.53 can predict malignancy with 88.9% sensitivity and 88.2% specificity. Setting the cut-off value at 0.9×10−3, the ADC values had a sensitivity of 72.2% and a specificity of 88.2% for predicting malignancy.
CONCLUSION
For SPN follow-up, a new following-up protocol can be safely established using DWI and ADC mapping. Using these MRI parameters might decrease unnecessary biopsy rates and complications of biopsies.
Keywords: Benign, DWI, malignant, MRI, solitary pulmonary nodule
INTRODUCTION
Solitary pulmonary nodule (SPN) is a relatively well-defined round or oval pulmonary parenchymal lesion, surrounded by pulmonary parenchyma and/or visceral pleura, and it is not associated with lymphadenopathy, atelectasis, or pneumonia, measured equal or smaller than 30 mm [1]. SPNs are detected by pulmonary plain graphy with an incidence of 0.2%. With the increasing use of computerized tomography (CT), the rate of SPN presence increases [2].
Multiple pathologies (granulomas, lung cancer, hamartomas, etc.) can cause SPN [3]. Detecting and identifying SPNs is very crucial because of the possibility of lung cancer. Mortality rates of lung cancer can increase up to 85%. Therefore, its early diagnosis is very important. On the other hand, approximately 40–60% of SPN cases are benign nodules [4]. Correctly differentiating benign and malign nodules provides early diagnosis, and it prevents unnecessary biopsy procedures.
Diffusion-weighted imaging (DWI) is a magnetic resonance imaging (MRI) type based on measuring the random Brownian motion of water molecules. In general, highly cellular tissues or those with cellular swelling show restricted diffusion coefficients, so that DWI can be useful in tumor characterization and cerebral ischemia.
Apparent diffusion coefficient (ADC) is a measurement for the magnitude of water molecule diffusion. DWI represents the random motion of water molecules. Diffusion of water molecules can be expressed quantitatively by using the ADC value. The ADC values are generally acquired by using different b values (0,500, or 1000) via changing gradient amplitude. The DWI and ADC parameters are used in combination to effectively detect diffusion restriction [5,6]. Lately, the diagnostic success of DWI and ADC in differentiating benign and malign SPNs has been evaluated [7,8].
The aim of this study is to evaluate diagnostic performance of the DWI and ADC maps in the differentiation of malign and benign SPN cases.
MATERIALS AND METHODS
Approval for the study was granted by the institutional ethics review board (Ankara Training and Research Hospital, decision number: 5348). Written informed consents were taken from the participants. The study was performed between March 2016 and September 2017.
Fifty-three patients having an SPN detected with CT were included in this prospective study. We excluded the patients with nodules less than 1 cm or more than 3 cm in diameter; the ones with calcified nodules or ground glass nodules; and the ones without pathologic diagnosis.
The MRI examinations were performed with 1.5 Tesla MRI systems (Magnetom Aera, Siemens Healthcare GmbH, Erlangen, Germany). We used DWI, axial T1-weighted images (T1WI), coronal T2-weighted images (T2WI), and the ADC maps (axial T1WI vibe: TR/TE: 4.4 msn/2,1 msn, thickness 3 mm, FOV 276×340 mm, matrix 196×320, bandwidth 400 Hz/pixel, NEX: 1, distance factor 20%, scan time 20 s; coronal T2WI HASTE P2 mbh: TR/TE: 1200 msn/91 ms, thickness 3 mm, FOV 400×400, matrix 256×256, bandwidth 700Hz/pixel, NEX: 1, distance factor 20%, scan time 38 s; DWI: TR/TE: 5500 ms/90 ms, thickness 6 mm, FOV 285×380, matrix 116×192, bandwidth 1735 Hz/pixel, NEX: 4, distance factor 20%, scan time 3 m, 2 s, b values 0, 500, 1000 mm2/s).
We used TIWI for image detection of the localization and for measurement of dimensions. On coronal T2WI, we placed a region of interest (ROI) on both the lesion and neighboring muscles (on a muscle part without containing fat signal), and then we calculated the lesion muscle intensity ratio (LMR).On DWI, we placed an ROI on both the lesion and spinal cord. Lesion spinal cord intensity ratio (LSR) was calculated for all b values (LSR0, LSR500, LSR1000). The ADC values for all lesions were also recorded (Figure 1–3).
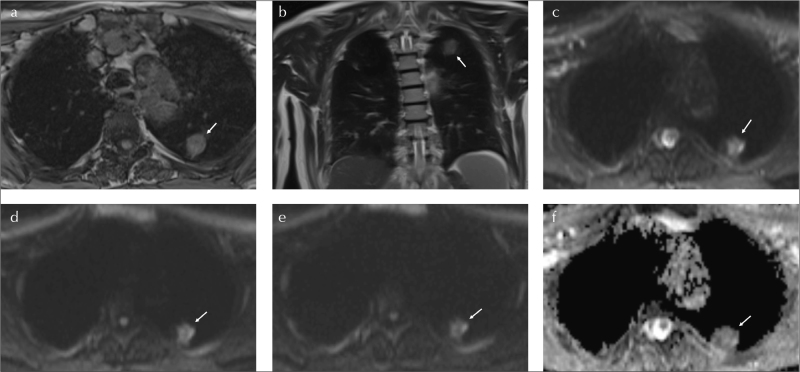
Figure 1. a–f.
A 58-year-old male with 40 pack-year smoking. SPN at left lung, upper lobe, posterior segment (arrows). SPN is measured 25×17 mm on axial T1WI (a). LMR on coronal T2WI is 2.8 (b).The LSR500 and LSR1000 values are 0.55 and 0.73, consequently on axial DWIs (c–e).The ADC value is 0.7 mm2/s (f). Histopathological diagnosis is adenocarcinoma
SPN: solitary pulmonary nodule; LSR: lesion spinal cord ratio; LMR: lesion muscle ratio; ADC: apparent diffusion coefficient
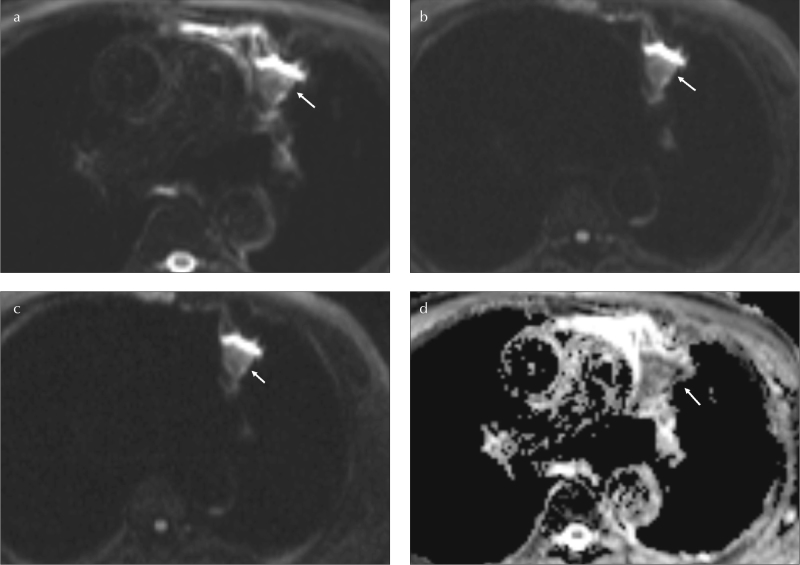
Figure 2. a–d.
A 45-year-old female without history of smoking. SPN at left lung, upper lobe, inferior lingular segment (arrows). Axial TIWI image (a).The LSR500 and LSR1000 values are 0.69 and 0.82, consequently on axial DWIs (b,c). The ADC value is 0.73 mm2/s (d). Histopathological diagnosis is squamous cell carcinoma
LSR: lesion spinal cord ratio; SPN: solitary pulmonary nodule
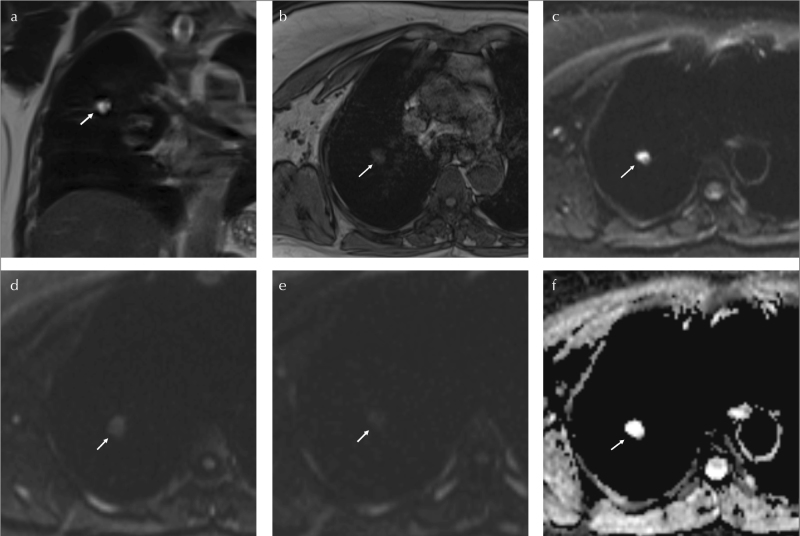
Figure 3. a–f.
A 50-year-old male, without smoking history, working at coal mine. SPN at right lung, upper lobe, posterior segment (arrows). On coronal T2WI, LMR is 2.5 (a). On axial T1WI, SPN is measured 16×11 mm (b).The LSR500 and LSR1000 values are 0.41 and 0.6, consequently on axial DWIs (c–e). The ADC value is 1.6 mm2/s (f). Histopathological diagnosis is anthracosis
SPN: solitary pulmonary nodule; LMR: lesion muscle ratio; LSR: lesion spinal cord ratio
Some patients (22 patients) had positron emission tomography-computed tomography (PET-CT) results. However, we could not acquire the PET-CT results of the other patients. Hence, we could not involve the PET-CT results into the statistical evaluation.
Histopathological diagnosis was noted.
Statistical Analysis
Statistical analyses were performed using Statistical Package for the Social Sciences Statistics software (Version 21.0; SPSS IBM Corp.; Armonk, NY, USA). Continuous parameters were stated as mean ± standard deviation, and skewed continuous parameters were evaluated with the Mann-Whitney U test. Categorical data were stated as frequencies (n) and percentages (%), and they were compared using the Fisher exact or chi-square tests where appropriate. Student’s t test and Mann-Whitney U test were used to analyze numerical variables in two groups. The power and success of diagnostic tests were defined by using the ROC curve analysis, and expressed with positive/negative predictive value, sensitivity, and specificity. Prediction values were calculated with Youden index.
A two-tailed value of p<0.05 was considered statistically significant.
RESULTS
Population
The study population consisted of 53 patients (17 benign, 32.1%; 36 malign, 67.9%). There were16 females (30.2%) and 37 males (69.8%). Patients’ age varied between 40 and 82 years, with the mean age 61.7±9.1 years.
Diameters of SPNs varied between 10 and 30 mm, with median 24mm. LSR0 varied between 0.2 and 1.3, with median 0.5. LSR500 varied between 0.2 and 2.0, with median 0.7; and LSR1000 varied between 0.4 and 4.6, with median 0.9. The ADC values of the lesions were between 0.4 and 1.6×10−3, with mean 1.0±0.3 ×10−3. The LMR values were between 1.6 and 5.9, with mean 3.1±0.8 (Table 1).
Table 1.
Population characteristics according to variables
| Whole population n=53 |
|
|---|---|
| Sex | |
| Female | 16 (30.2%) |
| Male | 37 (69.8%) |
| Age (years) | 61.7±9.1 |
| SPN diameter | 24 (10–30) |
| <20 mm | 19(35.8) |
| 20–30 mm | 34(64.2) |
| LSR | |
| LSR0 | 0.5 (0.2–1.3) |
| LSR500 | 0.7 (0.2–2) |
| LSR1000 | 0.9 (0.4–4.6) |
| ADC (10−3) | 1.0±0.3 |
| LMR | 3.1±0.8 |
SPN: solitary pulmonary nodule; LSR: lesion spinal cord ratio; ADC: apparent diffusion coefficient; LMR: lesion muscle ratio
According to the pathology results, there were 16 adenocarcinomas (30.2%), 9 metastasis (17%), 8 anthracosis (15.1%), 8 squamous cell carcinoma (15.1%), 4 fibrotic nodule (7.5%), 3 small cell carcinoma (5.7%), 2 abscess (3.8%), 1 hamartoma (1.9%), 1 organized pneumonia (1.9%), and 1 tuberculoma (1.9%) (Table 2).
Table 2.
Histopathological results
| Histopathology | Whole population n=53 |
|---|---|
| Adenocarcinoma | 30.2%; n=16 |
| Metastasis | 17.0%; n=9 |
| Anthracosis | 15.1%; n=8 |
| Squamous cell cancer | 15.1%; n=8 |
| Fibrotic nodule | 7.5%; n=4 |
| Small cell lung cancer | 5.7%; n=3 |
| Abscess | 3.8%; n=2 |
| Hamartoma | 1.9%; n=1 |
| Organized pneumonia | 1.9%; n=1 |
| Tuberculoma | 1.9%; n=1 |
Malignant and Benign Subgroups
Mean age was higher in malignant subgroup (64.6±6.7 vs 55.5±10.4; p<0.001). Sex was not different between the two groups. The mean SPN diameter was higher in malignant subgroup (25.5 mm vs 18 mm; p=0.026). Median LSR500 and LSR1000 values were higher in patients in the malignant subgroup. The mean ADC values were fewer in patients in the malignant subgroup (0.9±0.3 ×10−3 vs 1.3±0.3 ×10−3; p<0.001).
Patients in the malignant subgroup had mostly the diagnosis of adenocarcinoma (44%). Metastasis (25%), squamous cell carcinoma (22.2%), and small cell carcinoma (8.3%) diagnosis were also present.
Patients in the benign subgroup consisted of anthracosis (47.1%), fibrotic nodule (23.5%), hamartoma (5.9%), organized pneumonia (5.9%), abscess (11.8%), and tuberculoma (5.9%) (Table 3).
Table 3.
Distribution of variables according to malignant and benign SPNs
| Malignant n=36 |
Benign n=17 |
p | |
|---|---|---|---|
| Sex | |||
| Female | 11 (30.6) | 5 (29.4) | 0.933 |
| Male | 25 (69.4) | 12 (70.6) | |
| Age (Years) | 64.6±6.7 | 55.5±10.4 | <0.001* |
| SPN diameter (mm) | 25.5 (10–30) | 18 (12–29) | 0.026* |
| <20 mm | 8 (22.2) | 11 (64.7) | 0.005* |
| 20–30 mm | 28 (77.8) | 6 (35.3) | |
| LSR | |||
| LSR0 | 0.6 (0.2–1) | 0.4 (0.2–1.3) | 0.178 |
| LSR500 | 0.9 (0.6–1.9) | 0.4 (0.2–2) | <0.001* |
| LSR1000 | 1 (0.6–2.7) | 0.6 (0.4–4.6) | <0.001* |
| ADC (10x−3) | 0.9±0.3 | 1.3±0.3 | <0.001* |
| LMR | 3.2±0.8 | 3.1±0.6 | 0.669 |
SPN: solitary pulmonary nodule; LSR: lesion spinal cord ratio; ADC: apparent diffusion coefficient; LMR: lesion muscle ratio
xxxxxxxxxx
LSR Values
A positive correlation was observed betweentheLSR500 values and age (r=0.378; p=0.005). In the whole population, the LSR500 values and SPN diameters correlated positively. This correlation was present in the malignant subgroup (r=0.256; p=0.070), whereas there was no correlation in the benign subgroup (r=0.380; p=0.022).
A negative correlation was observedbetweentheLSR500 values and ADC values in whole population. This correlation was present in the malignant subgroup (r= −0.791; p<0.001), whereas there was no correlation in the benign subgroup (r= −0.213; p=0.277).
A positive correlation was observed between LSR1000 values and age (r=0.431; p=0.001). In whole population, theLSR1000 values and SPN diameters correlated positively. This correlation was present in the malignant subgroup (r=0.398; p=0.016), whereas there was no correlation in the benign subgroup (r=0.306; p=0.126).
A negative correlation was observed between the LSR1000 values and ADC values in whole population. This correlation was present in the benign subgroup (r= −0.591; p=0.012), whereas there was no correlation in the malignant subgroup (r= −0.277; p=0.102)
Predictive Values of the Parameters for Malignancy
The SPNs measuring more than 22 mm were found to be malignant with 72.2% of sensitivity and 70.6% of specificity (AUC±SE: 0.690±0.08; p=0.014). An LSR500 value of >0.53 predicts malignancy with 100% of sensitivity and 70.6% of specificity (AUC±SE:0.861±0.07; p<0.001). An LSR1000 value of >0.70 predicts malignancy with 88.9% of sensitivity and 88.2% of specificity (AUC±SE:0.875±0.07; p<0.001).The ADC values lesser than 0.9 predict malignancy with 72.2% of sensitivity and 88.2% of specificity (AUC±SE:0.828±0.07; p<0.001).
The lesion muscle intensity ratio values were not found to be successful in predicting malignancy (Table 4).
Table 4.
Diagnostic performance of variables in discriminating benign and malignant SPNs
| Variables | AUC | p | Sensitivity | Specificity | PPV | NPV | Predictive value |
|---|---|---|---|---|---|---|---|
| SPN diameter | 0.690+0.08 | 0.014* | 72.2 | 70.6 | 97.9 | 11.8 | >22.0 |
| LSR0 | 0.615+0.08 | 0.172 | 66.7 | 70.6 | 97.7 | 10 | >0.40 |
| LSR500 | 0.861+0.07 | <0.001* | 100 | 70.6 | 98.5 | 100 | >0.53 |
| LSR1000 | 0.875+0.07 | <0.001* | 88.9 | 88.2 | 99.3 | 29.5 | >0.70 |
| ADC | 0.828+0.07 | <0.001* | 72.2 | 88.2 | 99.1 | 14.3 | ≤0.90 |
| LMR | 0.490+0.08 | 0.908 | 11.1 | 100 | 100 | 5.6 | ≤2.0 |
p<0.05 statistical significance
SPN: solitary pulmonary nodule; LSR: lesion spinal cord ratio; ADC: apparent diffusion coefficient; LMR: lesion muscle ratio; AUC: area under curve; NPV: negative predictive value; PPV: positive predictive value
DISCUSSION
The incidence of cancer for SPNs varies between 5% and 70% in literature [9]. Because of the relatively small diameters, cancers presenting as SPN can be treated. Therefore, early diagnosis and differentiation of benign and malignant SPNs is an important point.
The risk factors for a malignant SPN are age, sex, nodule diameters, smoking, localization, and history of cancer [10–12]. According to our data, malignant SPNs are seen mostly in aged patients. This observation is consistent with the literature. Different from the literature [13], we could not find any relationship between sex and the risk for malignancy. However, our female population is less than the male population. Different results can be obtained with a larger female population.
In a study from Turkey [14], tuberculosis has been found to be the most frequent cause for a benign SPN. However, according to our study, fibrotic nodules have been found to be the most frequent cause.
There are multiple studies in the literature emphasizing the positive correlation between the SPN diameter and malignancy risk [15–18]. Our results are consistent with those of the literature, stating the increased risk of malignancy for SPNs with increasing diameter. We showed that an SPN larger than 22 mm could be malignant with a sensitivity of 72.2% and specificity of 70.6%.
The apparent diffusion coefficient values provide quantitative information for malignant and benign SPN differentiation. Liu et al. [19] stated that the ADC values are significantly lesser in malignant lesions, whereas Mori et al. [20] said that there is no significant difference in the ADC values. The results of our study support those of Liu et al. We showed that the ADC values are significantly low in the malignant SPN subgroup. In Mori’s study, benign SPNs consisted of mainly active or chronic inflammatory lesions; these are mainly highly cellular lesions. Therefore, the ADC values might be decreased in the benign SPN group.
Bernardin et al. [21] stated that sensitivity of the ADC values is insufficient in characterization of lesions fewer than 2 cm in diameter. Differently, according to our results, the ADC, LSR500, and LSR1000 values can be useful in predicting malignancy both for the lesions lesser or more than 2 cm in diameter. Bernardin’s study examines not only SPNs, but also larger lung masses, and there is limited number of cases. These can be the causes of the mentioned difference.
Some studies stated that visual evaluation of diffusion characteristics is equally successful with the LSR assessment [22–24]. However, we believe that using ratios decreases the differences causing from the usage of different MRI devices. Consequently, we preferred using LSR instead of only visual assessment.
Koyama et al. [25] stated that the LSR values are more useful than the ADC values in predicting malignancy. Our results are consistent with those of the literature; all LSR values obtained from malignant SPNs are higher than those obtained from the benign ones. Both LSR500 and LSR1000 values are more sensitive than ADC in predicting malignancy. In the above mentioned study, the researchers said that different b values do not change the diagnostic power of the LSR values [25]. Consistent with the literature, the LSR500 and LSR1000 values have similar diagnostic performances. In the literature, it is generally advised to use higher b values for the SPN characterization, especially more than 600 s/mm2 [23, 26]. Our results are consistent with those of the literature; the LSR500 and LSR1000 values are more successful than the LSR0 values.
In the literature, we cannot find any study examining the relationship between LSR and lesion diameter. We showed a positive correlation between the LSR values and nodule diameter in the malignant SPN subgroup. We believe that the finding indicates that as larger the lesion gets, cellular volume and diffusion restriction increases. However, we do not have so many necrotic tumors; the results might change with the degree of necrosis presence.
In the literature, the T2 signal characteristics of SPNs are usually assessed visually [22]. Instead of this, we used the LMR values. We cannot find any study examining the LMR values. However, we could find any significant correlation between the LMR values and SPN histopathology. Further prospective studies using the LMR parameter might discover any potential relationships.
The study has some limitations. Only little amount of benign lesions are directed to biopsy, only the ones with suspicious imaging characteristics are examined histopathologically. Therefore, the malignant SPNs constitute the majority of the population. Calcified nodules are excluded from the study. Therefore, the study cannot provide information about calcified nodules. As stated in the literature, granulomas might show restricted diffusion because of high cellularity [23,25]. Our population contains only one granuloma case, so the study cannot give sufficient information about granuloma DWI characteristics. PET-CT provides valuable information about benign/malign nature of the SPNs. A malignant SPN greater than 8 mm in diameter is expected to uptake fluorodeoxyglucose (FDG) on PET-CT [27]. Unfortunately, we cannot acquire PET-CT results of all patients, so our result cannot give information about PET-CT correlations.
To conclude, ADC, LSR500, and LSR1000 values can be handy parameters for differentiation of benign-malignant SPNs. Using these MRI parameters might decrease unnecessary biopsy rates and complications of biopsies. The MRI results can also be useful in following the patients without sufficient PET-CT results. Also, they can be used for the follow-up of SPNs, instead of thorax CT, especially in patients who are more sensitive to ionizing radiation.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ankara Training and Research Hospital (decision number: 5348).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.F.; Design - S.A.; Supervision - P.K.; Materials - E.E., S.B.; Data Collection and/orProcessing - S.B., S.A.; Analysis and/orInterpretation - E.F.; LiteratureSearch - S.B.; WritingManuscript - S.A.; Critical Review - P.N.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Murthy SC, Rice TW. The solitary pulmonary nodule: a primer on differential diagnosis. Semin Thorac Cardiovasc Surg. 2002;14:239–49. doi: 10.1053/stcs.2002.34450. [DOI] [PubMed] [Google Scholar]
- 2.Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34–49. doi: 10.1148/radiol.2391050343. [DOI] [PubMed] [Google Scholar]
- 3.Zerhouni EA, Stitik FP, Siegelman SS, Naidich DP, et al. CT of the pulmonary nodule: a cooperative study. Radiology. 1986;160:319–27. doi: 10.1148/radiology.160.2.3726107. [DOI] [PubMed] [Google Scholar]
- 4.Siegelman SS, Zerhouni EA, Leo FP, et al. CT of the solitary pulmonary nodule. AJR Am J Roentgenol. 1980;135:1–13. doi: 10.2214/ajr.135.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Sener RN. Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph. 2001;25:299–326. doi: 10.1016/S0895-6111(00)00083-5. [DOI] [PubMed] [Google Scholar]
- 6.Kang Y, Choi SH, Kim YJ, et al. Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging--correlation with tumor grade. Radiology. 2011;261:882–90. doi: 10.1148/radiol.11110686. [DOI] [PubMed] [Google Scholar]
- 7.Tatli S, Gerbaudo VH, Feeley CM, et al. PET/CT-guided percutaneous biopsy of abdominal masses: initial experience. J Vasc Interv Radiol. 2011;22:507–14. doi: 10.1016/j.jvir.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Jin KN, Park CM, Goo JM, et al. Initial experience of percutaneous transthoracic needle biopsy of lung nodules using C-arm cone-beam CT systems. Eur Radiol. 2010;20:2108–15. doi: 10.1007/s00330-010-1783-x. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Cai YQ, Cai ZL, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–8. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 10.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003;348:2535–42. doi: 10.1056/NEJMcp012290. [DOI] [PubMed] [Google Scholar]
- 11.Tang AW, Moss HA, Robertson RJ. The solitary pulmonary nodule. Eur J Radiol. 2003;45:69–77. doi: 10.1016/S0720-048X(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 12.Lillington GA. Management of solitary pulmonary nodules. How to decide when resection is required. Postgrad Med. 1997;101:145–50. doi: 10.3810/pgm.1997.03.177. [DOI] [PubMed] [Google Scholar]
- 13.Bekci TT, Senol T, Maden E. The efficacy of serum carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 15-3 (CA15-3), alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) levels in determining the malignancy of solitary pulmonary nodules. J Int Med Res. 2009;37:438–45. doi: 10.1177/147323000903700219. [DOI] [PubMed] [Google Scholar]
- 14.Ors F, Deniz O, Bozlar U, et al. High-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivity. J Thorac Imaging. 2007;22:154–9. doi: 10.1097/01.rti.0000213590.29472.ce. [DOI] [PubMed] [Google Scholar]
- 15.Basile A, Gregoris A, Antoci B, et al. Malignant change in a benign pulmonary hamartoma. Thorax. 1989;44:232–3. doi: 10.1136/thx.44.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavrielides MA, Kinnard LM, Myers KJ, Petrick N. Noncalcified lung nodules: volumetric assessment with thoracic CT. Radiology. 2009;251:26–37. doi: 10.1148/radiol.2511071897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erasmus JJ, McAdams HP, Connolly JE. Solitary pulmonary nodules: Part II. Evaluation of the indeterminate nodule. Radiographics. 2000;20:59–66. doi: 10.1148/radiographics.20.1.g00ja0259. [DOI] [PubMed] [Google Scholar]
- 18.Hanley KS, Rubins JB. Classifying solitary pulmonary nodules. New imaging methods to distinguish malignant, benign lesions. Postgrad Med. 2003;114:29–35. doi: 10.3810/pgm.2003.08.1472. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Liu Y, Yu T, et al. Usefulness of diffusion-weighted MR imaging in the evaluation of pulmonary lesions. Eur Radiol. 2010;20:807–15. doi: 10.1007/s00330-009-1629-6. [DOI] [PubMed] [Google Scholar]
- 20.Mori T, Nomori H, Ikeda K, et al. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol. 2008;3:358–64. doi: 10.1097/JTO.0b013e318168d9ed. [DOI] [PubMed] [Google Scholar]
- 21.Bernardin L, Douglas NH, Collins DJ, et al. Diffusion-weighted magnetic resonance imaging for assessment of lung lesions: repeatability of the apparent diffusion coefficient measurement. Eur Radiol. 2014;24:502–11. doi: 10.1007/s00330-013-3048-y. [DOI] [PubMed] [Google Scholar]
- 22.Cakir C, Genchellac H, Temizoz O, et al. Diffusion Weighted Magnetic Resonance Imaging for the Characterization of Solitary Pulmonary Lesions. Balkan Med J. 2015;32:403–9. doi: 10.5152/balkanmedj.2015.15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yabuuchi H, Hatakenaka M, Takayama K, et al. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology. 2011;261:598–604. doi: 10.1148/radiol.11101503. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka R, Horikoshi H, Nakazato Y, et al. Magnetic resonance imaging in peripheral lung adenocarcinoma: correlation with histopathologic features. J Thorac Imaging. 2009;24:4–9. doi: 10.1097/RTI.0b013e31818703b7. [DOI] [PubMed] [Google Scholar]
- 25.Koyama H, Ohno Y, Seki S, et al. Value of diffusion-weighted MR imaging using various parameters for assessment and characterization of solitary pulmonary nodules. Eur J Radiol. 2015;84:509–15. doi: 10.1016/j.ejrad.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Koyama H, Ohno Y, Aoyama N, et al. Comparison of STIR turbo SE imaging and diffusion-weighted imaging of the lung: capability for detection and subtype classification of pulmonary adenocarcinomas. Eur Radiol. 2010;20:790–800. doi: 10.1007/s00330-009-1615-z. [DOI] [PubMed] [Google Scholar]
- 27.Umakoshi H, Iwano S, Yokoi K, et al. FDG PET/CT Overcomes Discordance Between Clinical and Pathologic TNM Classification of Small-size Primary Lung Cancer: Influence on Postoperative Prognosis. Clin Lung Cancer. 2018;19:e37–e45. doi: 10.1016/j.cllc.2017.05.021. [DOI] [PubMed] [Google Scholar]