Abstract
Background/Aims
As the most common liver disease in hemodialysis patients, chronic hepatitis C (CHC) can cause cirrhosis and hepatocellular carcinoma, even increase in renal-related mortality. In Turkey, the frequency of anti-hepatitis C virus (HCV) antibodies in hemodialysis patients ranged from 31.4% to 51%. Until recently, the mainstay of the CHC treatment for these patients was pegylated interferon with potential toxicities and low sustained virological response. The 3D regimen, a combination of four drugs (ombitasvir, paritaprevir, dasabuvir, and ritonavir), has recently been used for patients with chronic kidney disease infected with genotype 1a and 1b HCV. The aim of the present study was to present results of 3D treatment for patients with hemodialysis-dependent chronic renal failure (CRF) who were chronically infected with HCV.
Materials and Methods
Overall, 25 patients with hemodialysis-dependent CRF who were infected with genotype 1a/1b HCV have been treated using the 3D regimen in our gastroenterology clinic between July 2016 and October 2017. Three patients were administered additional ribavirin 200 mg/day. Serum HCV RNAs, blood chemistry, blood count, and side effects were recorded at 0, 4, and 12 weeks.
Results
All 25 patients completed and well tolerated their planned treatment. At the end of 4 weeks, the viral response (defined as HCV RNA clearance) rate was 92%. At the end of 12 weeks of treatment and 3 months after treatment, viral response rates were both 100%.
Conclusion
We observed that the treatment with 3D regimen in hemodialysis patients infected with genotype 1 hepatitis C is highly effective and well tolerated.
Keywords: Chronic hepatitis C, chronic renal failure, direct-acting antiviral agent
INTRODUCTION
Chronic hepatitis C (CHC) is the most common liver disease in hemodialysis patients. The worldwide prevalence of chronic hepatitis C virus (HCV) infection in hemodialysis patients ranges from 2.6% to 22.9%. In Turkey, the frequency of anti-HCV antibodies in hemodialysis patients ranged from 31.4% to 51% (1,2). The natural course of hepatitis C in dialysis patients is unclear. However, in a multicenter prospective study, it has been shown to increase the risk of death due to hepatic cirrhosis and hepatocellular carcinoma (HCC) in such patients (4). Furthermore, hepatitis C infection increases renal-related mortality (5). In prospective studies, anti-HCV and HCV RNA positivity were shown to be risk factors for mortality. HCV positive recipients have an increased risk of chronic liver disease and mortality following renal transplantation compared with those negative recipients (6).
Before the routine use of direct-acting antiviral (DAA) agent-based regimens, the standard treatment for CHC in hemodialysis patients was pegylated interferon (Peg-IFN) with or without ribavirin. Unfortunately, the efficacy of this regimen was suboptimal, and the potential toxicity of therapy was high (7,8).
We aimed to present the results of our 3D treatment with patients with hemodialysis-dependent chronic renal failure (CRF) who were chronic infected with HCV.
MATERIALS AND METHODS
Direct-acting antivirals have been used in Turkey since June 18, 2016 for the treatment of CHC, since they have been reimbursed by the state healthcare system. We evaluated the treatment outcomes of patients with antiviral therapy who are treated between July 2016 and October 2017. All patients were treated with 12-week ritonavir-boosted paritaprevir, ombitasvir, and dasabuvir combination. The daily doses were dasabuvir 2×250 mg and ritonavir 100 mg/day, paritaprevir 150 mg, and ombitasvir 25 mg. Only three patients were administered additional ribavirin 200 mg/day. Ribavirin was discontinued if the hemoglobin values were reduced by 2 g/dL or more in less than 4 weeks or if the hemoglobin value was below 10 g/dL. Patients continue to use other medications and dialysis sessions. They were invited for follow-up visits on a monthly basis as long as there were no additional complaints. Serum HCV RNAs, blood chemistry, blood count, and side effects were recorded.
These evaluations were performed at 4, 12 (end of treatment), and 24 (to evaluate sustained viral response (SVR) 12 weeks after treatment) weeks.
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS®) software program version 21 (IBM Corp.; Armonk, NY, USA). Student’s t-test was used for comparison of mean values. Ethical committee approval was not needed since this was a retrospective study from the records of the gastroenterology department.
RESULTS
A total of 25 (15 male and 10 female, mean age: 56.03±11.83 years, age range between 37 and 72 years) patients with hemodialysis-dependent CRF were treated in our department. The mean Modification of Diet in Renal Disease value was 10.37 mL/min. Of the patients, eight had already received Peg-IFN therapy, but HCV recurrence had developed after treatment. Six patients were Peg-IFN-experienced, and two patients were both Peg-IFN- and boceprevir-experienced. All of the patients were genotype 1. Genotype 1 (subtype unknown) was found in 6 patients, genotype 1a in 3 patients, and genotype 1b in 16 patients. The pretreatment median HCV RNA level (log 10 IU/mL) was 6.28. There was no cirrhosis among the 25 patients. The median hemodialysis duration of the patients was 6 (2–25) years.
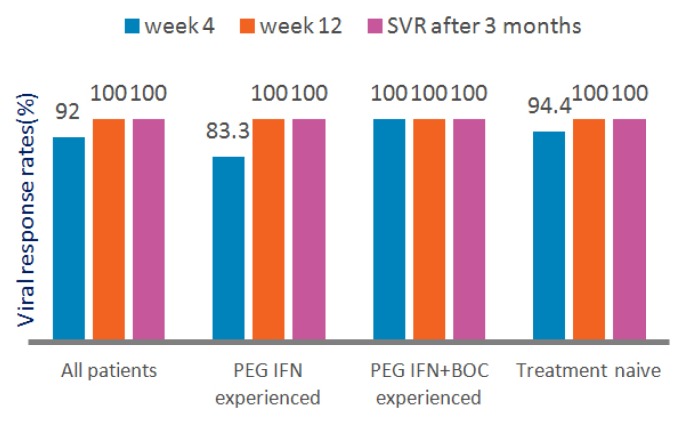
All of the patients completed their planned treatment. At the end of 4 weeks, the viral response (defined as HCV RNA clearance) rate was 92%. At the end of 12 weeks of treatment, the viral response rate was 100%. Viral responses were similar in both treatment-naive and Peg-IFN, Peg-IFN, and boceprevir-experienced patients (Figure 1).
Figure 1.
Viral responses to 3D combination treatment
The mean serum alanine transaminase levels decreased from 16.6 to 8.7 U/l after treatment. The mean serum bilirubin levels increased from 0.49 to 0.56 mg/dL after treatment. There was no difference in serum albumin levels.
All 25 patients well tolerated the treatment regimen. Serious side effects causing treatment interruption were not observed. The most frequent side effects were fatigue and itching, and they disappeared after completion of therapy (Table 1).
Table 1.
Adverse effects during 3D treatment
| Adverse effect, n (%) | 3D (n=22) | 3D+ribavirin (n=3) |
|---|---|---|
| Fatigue | 2 | 1 |
| Itching | 2 | 0 |
| Worsening in hypertension | 1 | 0 |
| Insomnia | 1 | 0 |
| Amnesia | 1 | 0 |
In one patient, previously known systolic hypertension worsened, and additional antihypertensive drug had to be administered. After 12 weeks of DAA treatment was completed, the additional antihypertensive drug was ceased because of normalization of blood pressure to pretreatment values. There was no significant elevation in serum bilirubin concentrations.
DISCUSSION
Hemodialysis patients are at risk of contact with HCV. Risk factors include duration of hemodialysis, number of transfusions, dialysis type, and noncompliance with internationally recognized measures (9). Therefore, the prevalence of chronic HCV infection in hemodialysis patients is high. It ranges from 2.6% to 22.9%. In Turkey, the frequency of anti-HCV antibodies in hemodialysis patients ranged from 31.4% to 51% (10). HCV infection is associated with a decline in renal function (11–13).
In a multicenter prospective study, HCV has been shown to increase the risk of death due to hepatic cirrhosis and HCC in such patients (14). Furthermore, hepatitis C infection increases renal-related mortality (14). In prospective studies, anti-HCV and HCV RNA positivity were shown to be risk factors for mortality (14). There is a negative effect of HCV on survival in hemodialysis patients (15).
Hepatitis C virus positive recipients have an increased risk of chronic liver disease and mortality following renal transplantation compared with those negative recipients (16). Even if they had kidney donors, many patients missed the chance of kidney transplantation. In addition, the main sequelae of chronic HCV infection include cirrhosis and HCC. Eradication of HCV before the development of decompensated cirrhosis results in decreased liver-related deaths (3).
According to the recommendations of the international renal and liver authorities, including the European Association for the Study of the Liver, people who have chronic renal disease and are infected with HCV should be treated regardless of the fibrosis level (17). Antiviral therapy should be considered in patients with renal disease and mixed cryoglobulinemia associated with HCV infection (17). Early recognition and treatment of individuals with chronic kidney failure who are infected with HCV prevents long-term negative outcomes for both the liver and the kidney (18).
Before the routine use of DAA-based regimens, the standard treatment for CHC in hemodialysis patients was Peg-IFN with or without ribavirin. Unfortunately, the efficacy of this regimen was suboptimal, with sustained virological response in only one-third of patients on maintenance dialysis with HCV, and the potential toxicity of therapy was high (7,8). Interferon and ribavirin could not be administered at effective doses and durations for these patients due to intolerance and complications which reduced the success of treatment (7,19). Ribavirin metabolites cause hemolysis by accumulating in the erythrocytes due to low renal clearance. It can be used in patients with stage 1 and 2 CRFs with dose adjustment but is not recommended for use in stages 3, 4, and 5 and hemodialysis patients (20).
There are many studies showing the inadequate viral responses with both interferon and Peg-IFNs with or without ribavirin treatment for patients with CRF with CHC in the literature (7,21–28). Among them, a retrospective cohort study showed that the SVR rate is approximately 40% with PEG-IFN monotherapy, and the addition of ribavirin does not lead to improved virological response (7).
Direct-acting antiviral drugs caused a revolution in CHC treatment, especially 3D regime has also led to very effective and reliable results especially in patients with CRF. Daclatasvir, simeprevir, and ombitasvir-paritaprevir-ritonavir+dasabuvir, which are metabolized in the liver, can be used in CRF. Sofosbuvir is excreted by the kidney; therefore, sofosbuvir-containing regimens are not recommended in patients with glomerular filtration rate (GFR) <30 mL/min or hemodialysis patients (29,30). For patients with GFR <30 mL/min, ombitasvir-paritaprevir-ritonavir+dasabuvir can be used without dose adjustment. For genotype 1a, ribavirin 200 mg/day can be added if the hemoglobin level is >10 g/dL. Ombitasvir is a nonstructural (NS) protein 5A inhibitor, paritaprevir an inhibitor of the NS3/4A serine protease, and dasabuvir a non-nucleoside NS5B polymerase inhibitor. They all have antiviral activity in HCV both genotype 1a and 1b. Ritonavir, without a direct effect on HCV, is a potent inhibitor of cytochrome P450 3A4 enzymes and used as a pharmacologic booster for paritaprevir. The regimen can be used with or without ribavirin (31).
According to the American Association for the Study of Liver Diseases guidelines for treatment-naive patients with HCV genotype 1 without cirrhosis and with creatinine clearance rates <30 mL/min, treatment with the daily fixed-dose combination of paritaprevir (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg) plus twice daily dose of dasabuvir (250 mg) with (1a) or without (1b) ribavirin (200 mg) once daily is recommended (32).
In a clinical trial with 20 patients with CRF (6 patients with stage 4 and 14 patients with stage 5 CRF), the combination of ombitasvir, paritaprevir, and ritonavir administered with dasabuvir led to an SVR of 12 in 90% of patients with HCV genotype 1 infection and stage 4 or 5 CRF. The regimen is well tolerated though ribavirin use may require a reduction or interruption to manage anemia (33).
In another study evaluating the efficacy and safety of ombitasvir/paritaprevir/dasabuvir and ribavirin in patients with CRF (a total of 33 patients), it is reported that the efficacy of DAAs and ribavirin in patients with advanced CRF and HCV infection is similar to that observed in those without renal disease. Ribavirin use was also reported to be rarely associated with severe adverse effects in this study (34).
All of our patients had 3D regimen with HCV clearance and SVR. In the group with hemodialysis-dependent end-stage renal failure, intolerance and side effects were not observed, which would otherwise require cessation of the oral 3D regimen.
The main limitation of the present study is the number of patients. There were only three patients in the ribavirin co-administered group.
We observed that the treatment with paritaprevir, ombitasvir, dasabuvir, and ritonavir in hemodialysis patients infected with genotype 1 hepatitis C is highly effective and well tolerated.
Footnotes
Ethics Committee Approval: The authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - O.S., E.A.; Design - S.Y., E.Ü.; Supervision - E.Ü., E.A.; Resource - F.A.; O.Ö., E.Ü.; Materials - F.A., O.Ö.; Data Collection and/or Processing - F.A., O.Ö.; Analysis and/or Interpretation - S.Y.; Literature Search - S.Y., O.S.; Writing Manuscript - S.Y., O.S.; Critical Review - E.A.
Conflicts of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Fissell RB, Bragg-Gresham JL, Woods JD. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;64:2335–42. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 2.Koksal I. Pegylated interferon for treatment in hemodialysis patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:491–4. doi: 10.1111/j.1440-1746.2006.04285.x. [DOI] [PubMed] [Google Scholar]
- 3.Backus L, Boothroyd DB, Phillips BR, et al. Impact of sustained virologc response to pegylated interferon/ribavirin on all-cause mortality by HCV genotype in a large real-world cohort: The US Department of Veterans Affairs’ experience. Hepatology. 2010;52:428A. [Google Scholar]
- 4.Nakayama E, Akiba T, Marumo F, et al. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11:1896–902. doi: 10.1681/ASN.V11101896. [DOI] [PubMed] [Google Scholar]
- 5.Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–77. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 6.Kes P, Basic-Jukic N. Hepatitis C in dialysed patients-what is the current optimal treatment? Kidney Blood Pres Res. 2007;30:156–61. doi: 10.1159/000101918. [DOI] [PubMed] [Google Scholar]
- 7.Espinosa M, Hernàndez J, Arenas MD, et al. Pegylated interferon (alone or with ribavirin) for chronic hepatitis C in haemodialysis population. Kidney Blood Press Res. 2015;40:258–65. doi: 10.1159/000368501. [DOI] [PubMed] [Google Scholar]
- 8.Sporea I, Popescu A, Sirli R, et al. Pegylated-interferon alpha 2a treatment for chronic hepatitis C in patients on chronic haemodialysis. World J Gastroenterol. 2006;12:4191–4. doi: 10.3748/wjg.v12.i26.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valadez JA, Juárez IG, Pedrero RR. Management of chronic hepatitis C virus infection in patients with end-stage renal disease: a review. Ther Clin Risk Manag. 2015;11:329–38. doi: 10.2147/TCRM.S74282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone M, Mutimer D, Neuberger J. Hepatitis C virus and nonliver solid organ transplantation. Transplantation. 2013;95:779–86. doi: 10.1097/TP.0b013e318273fec4. [DOI] [PubMed] [Google Scholar]
- 11.Senaka P. Hepatitis C and Renal Disease: Differences in Patient Characteristics and Clinical Outcomes in the United States. Hepatology. 2015;62:1120A. [Google Scholar]
- 12.Fabrizi F, Messa P, Martin P. The Unravelled Link between Chronic Kidney Disease and Hepatitis C Infection. New J Sci. 2014 doi: 10.1155/2014/180203. 180203. [DOI] [Google Scholar]
- 13.Tsui JI, Vittinghoff E, Shlipak MG, et al. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1168–74. doi: 10.1681/ASN.2005091006. [DOI] [PubMed] [Google Scholar]
- 14.Lee MH, Yang HI, Lu SN, et al. Chronic Hepatitis C Virus Infection Increases Mortality From Hepatic and Extrahepatic Diseases: A Community-Based Long-Term Prospective Study. J Infect Dis. 2012;206:469–77. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 15.Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat. 2012;19:601–7. doi: 10.1111/j.1365-2893.2012.01633.x. [DOI] [PubMed] [Google Scholar]
- 16.Morales JM, Marcén R, Andres A, et al. Renal transplantation in patients with hepatitis C virus antibody. A long national experience. NDT Plus. 2010;3(Suppl 2):41–6. doi: 10.1093/ndtplus/sfq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlotsky JM, Aghemo A, Back D, et al. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 18.KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of Hepatitis C in chronic kidney disease. Kidney Int. 2008;73(Suppl 109):1–99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 19.Russo MW, Goldsweig CD, Jacobson IM, et al. Interferon monotherapy for dialysis patients with chronic hepatitis C: an analysis of the literature on efficacy and safety. Am J Gastroenterol. 2003;98:1610–5. doi: 10.1111/j.1572-0241.2003.07526.x. [DOI] [PubMed] [Google Scholar]
- 20.Smolders EJ, Kante CTMM, van Hoek B, et al. Pharmacokinetics, Efficacy, and Safety of Hepatitis C Virus Drugs in Patients with Liver and/or Renal Impairment. Drug Saf. 2016;39:589–611. doi: 10.1007/s40264-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha CM, Perez RM, Ferreira AP, et al. Efficacy and tolerance of interferon-alpha in the treatment of chronic hepatitis C in end-stage renal disease patients on hemodialysis. Liver Int. 2006;26:305–10. doi: 10.1111/j.1478-3231.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 22.Degos F, Pol S, Chaix ML, et al. The tolerance and efficacy of interferon-alpha in haemodialysis patients with HCV infection: a multicentre, prospective study. Nephrol Dial Transplant. 2001;16:1017–23. doi: 10.1093/ndt/16.5.1017. [DOI] [PubMed] [Google Scholar]
- 23.Mousa DH, Abdalla AH, Al-Shoail G, et al. Alpha-interferon with ribavirin in the treatment of hemodialysis patients with hepatitis C. Transplant Proc. 2004;36:1831–4. doi: 10.1016/j.transproceed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Bruchfeld A, Lindahl K, Reichard O, et al. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat. 2006;13:316–21. doi: 10.1111/j.1365-2893.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 25.Rendina M, Schena A, Castellaneta NM, et al. The treatment of chronic hepatitis C with peginterferon alfa-2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol. 2007;46:768–74. doi: 10.1016/j.jhep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Kose S, Senger SS, Ersan G, et al. Virological responses of pegylated interferon alpha-2a treatment in hemodialysis patients infected with hepatitis C. Clin Exp Nephrol. 2013;17:115–9. doi: 10.1007/s10157-012-0663-x. [DOI] [PubMed] [Google Scholar]
- 27.Covic A, Maftei ID, Mardare NG, et al. Analysis of safety and efficacy of pegylated-interferon alpha-2a in hepatitis C virus positive hemodialysis patients: results from a large, multicenter audit. J Nephrol. 2006;19:794–801. [PubMed] [Google Scholar]
- 28.Kokoglu OF, Uçmak H, Hosoglu S, et al. Efficacy and tolerability of pegylated-interferon alpha-2a in hemodialysis patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:575–80. doi: 10.1111/j.1440-1746.2005.04008.x. [DOI] [PubMed] [Google Scholar]
- 29.Meyers CM, Seeff LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631–57. doi: 10.1016/S0272-6386(03)00828-X. [DOI] [PubMed] [Google Scholar]
- 30.Omata M, Kanda T, Yu ML, et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409–35. doi: 10.1007/s12072-012-9342-y. [DOI] [PubMed] [Google Scholar]
- 31.Kohli A, Alshati A, Georgie F, et al. Direct-acting antivirals for the treatment of chronic hepatitis C in patients with chronic kidney disease. Therap Adv Gastroenterol. 2016;9:887–97. doi: 10.1177/1756283X16665254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hepatitis C Guidance: AASLD-IDSA Recommendations for Testing, Managing, and Treating Adults Infected With Hepatitis C Virus. Hepatol. 2015;62:932–54. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 33.Pockros PJ, Reddy KR, Mantry PS, et al. Ombitasvir-Paritaprevir-Ritonavir and Dasabuvir in GT1 and Renal Disease RUBY-I. Gastroenterology. 2016;150:1590. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 34.Gomez RM, Rincon D, Hernandez E, et al. Ombitasvir/paritaprevir/ritonavir plus dasabuvir are safety and efficacy for treating HCV GT1 and 4 infection in patients with severe renal impairment or end-stage renal disease: a multicenter experience. J Hepatol. 2016;64:631–832. doi: 10.1016/S0168-8278(16)01588-9. [DOI] [Google Scholar]