Abstract
Background/Aims
The aim of present study was to assess the protective effects of Shenfu injection (SI) on the intestinal mucosa and its regulation on the mucosal immune responses in rats with sepsis.
Materials and Methods
Sprague-Dawley rats were randomly divided into the sham, model, low-dose SI (LSF), and high-dose SI (HSF) groups. Sham animals underwent laparotomy only, whereas sepsis was modeled by cecal ligation and puncture in the remaining groups. At 2 h post-surgery, the LSF and HSF groups were intraperitoneally administered 5 and 20 mL/kg SI, respectively, whereas other animals with saline. At 12 h and 24 h post-surgery, eight rats per group were sacrificed, and blood and intestinal tissues were collected. The intestinal mucosa was analyzed by hematoxylin and eosin staining. Serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 concentrations, as well as secretory immunoglobulin A (sIgA) content in the intestinal mucosa, were evaluated by enzyme-linked immunosorbent assay. CD3 and γδT lymphocytes were quantified by flow cytometry. Animal survival until 72 h was also recorded.
Results
Intestinal mucosal injury was significantly higher in model animals than in sham animals at postoperative 12 h and 24 h. Serum TNF-α and IL-6 levels were markedly increased, whereas sIgA and CD3 and γδT cell amounts were overtly decreased (p<0.01). The LSF and HSF rats showed lower mortality, intestinal mucosal injury, and serum TNF-α and IL-6 levels (p<0.05), as well as higher sIgA levels and CD3 and γδT cell amounts, than the model group (p<0.01), with a dose-dependent manner.
Conclusion
SI dose-dependently prolongs survival and protects the intestinal mucosa in rats with sepsis, possibly through strengthening innate immunity instead of acquired immunity.
Keywords: Shenfu injection, sepsis, intestinal mucosa, secretory immunoglobulin A, γδT lymphocytes
INTRODUCTION
Sepsis refers to an infection-based systemic inflammatory response syndrome (SIRS) and is a common complication in critically ill patients with various infections or severe burn wounds (1). It also occurs after major surgery, with a fatality rate of up to 30%–50% (2,3). Therefore, sepsis has become one of the major health problems worldwide. The pathophysiology of sepsis results from inadequate regulation of the normal immune and physiological reactions to eliminate the invading pathogens (4). Lymphocyte costimulatory molecules are upregulated in sepsis, whereas apoptosis is fast and delayed in lymphocytes and neutrophils, respectively; overall, this results in elevated tissue necrosis (5).
The mucosal epithelium is central to the interactions between the mucosal immune system and the luminal contents, such as dietary antigens and microbial products (6). Enterogenic infections and their relationship with multiple organ dysfunction syndromes (MODSs) have been studied thoroughly (7). In addition, intestinal translocation of bacteria in sepsis and the related defense through the intestinal mucosal barrier attract increasing attention. Interestingly, mucosal immune impairment was shown to contribute to intestinal barrier injury during endotoxemia, with elevated regulatory T cells and lymphocyte apoptosis (8). Meanwhile, patient death in the intensive care unit (ICU) following sepsis is associated with immunosuppression (9).
The gastrointestinal tract is the body’s largest immune organ. Secretory immunoglobulin A (sIgA), γδT cells, and cytokines contribute to the functions of the intestinal mucosal barrier (10,11). IgA is critical to the intestinal mucosal barrier and is the most represented mucosal immunoglobulin, with crucial roles in mucosal protection. Indeed, mice with acute liver necrosis have less intestinal IgA+ plasma cells and IgA amounts, suggesting the presence of mucosal immune barrier dysfunction in acute liver necrosis (12). CD3+ cells are T lymphocytes; γδT cells amount to 1%–10% of circulating T lymphocytes (13). The γδT cells express either the V δ1 (GD1) or the V δ2 (GD2) receptor, with γδT cells predominantly found in the mucosal surfaces to maintain epithelial tissue integrity (13,14). It was proposed that γδT cells, expanded in response to specific antigens, increase IgA class switching on B cells. In addition, previous studies (15–17) have revealed that γδT cells play important roles in anti-infectious immunity and autoimmune diseases, as well as the production and maintenance of immune tolerance in organ grafting, thereby effectively regulating the types and strengths of body immune response; they also assist αβT cells to exert their functions in specific immune responses. A previous study (18) demonstrated that deficiency of γδT cells may result in high mortality and immunosuppression in rats with sepsis. Therefore, changes of T cell subsets are often considered an indicator of body immune function variation after sepsis.
A previous study (19) found that Shenfu injection (SI) regulates oxidative stress in the intestinal mucosal epithelial cells, suppresses apoptosis, and repairs tight junctions between epithelial cells to maintain the mechanical integrity of the intestinal mucosa in rats with sepsis. However, its protective effects on the intestinal mucosal immune system in rats with sepsis are undefined. Therefore, the aim of the present study was to evaluate the impacts of SI on the intestinal mucosal immune (humoral and cellular) responses at the background of sepsis. Our findings provide a solid basis for the clinical application of SI in sepsis.
MATERIALS AND METHODS
Animals and grouping
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals. The study was approved by the ethics committee of our institution (2012-K-004-02). A total of 96 male specific-pathogen-free Sprague-Dawley rats (180–220 g) were provided by the Experimental Animal Center of our university (SCXX [Hu] 2008-0016). Before the experiments, the animals were fed adaptively for 2 weeks in the Experimental Animal Center under the following conditions: temperature 25°C, humidity 45%–55%, light and dark alternating every 12 h, and ventilation 8–15 times/h. Using the random number method, the rats were assigned to the sham (n=18), model (n=26), low-dose SI (LSF, n=26), and high-dose SI (HSF, n=26) groups.
Immediately after sham operation (sham group) or cecal ligation and puncture (CLP) (model and SI groups), the rats received percutaneous injection of 10 mL saline for anti-shock. Two h after the operation, the sham and model groups were administered intraperitoneal injection of 20 mL/kg saline. Meanwhile, the LSF and HSF groups received 5 and 20 mL/kg SI, respectively (red ginseng and monkshood, polysorbate 80; Ya’an 39 Pharmaceutical Co., Ltd., Chengdu, Sichuan, China; National License Medical no. Z20043117).
All rats had free access to food and water. At 12 h post-surgery, vital sign monitoring was performed. At 12 h and 24 h post-surgery, a 2 mL abdominal aortic blood was collected for analysis. Blood and peritoneal exudate samples were also collected for bacterial culture.
In addition, at 12 h and 24 h after the operation, eight rats were selected in each group using a random number table and sacrificed. Samples of a 35 cm distal ileum tissue were collected for analysis. The remaining 10 rats, respectively, in the model, LSF, and HSF groups, as well as 2 sham animals, were assessed for survival once every 12 h for a total of 72 h.
Sepsis modeling
A rat model of sepsis was established by CLP operation, except for sham animals. Briefly, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (3 mL/kg), followed by fixation, skin preparation, disinfection, and draping with aseptic hold-towel. Then, a 1.5 cm abdominal median incision was made to expose the cecum. Ligation was performed at one-thirds of the cecum, and the middle position of the ligated cecum was pierced twice using a 16-gauge needle. A small amount of stool was extruded, and the cecum was repositioned, followed by layer by layer suture of the abdominal incision and disinfection of the peripheral skin. Rats in the sham group underwent the same procedures except for CLP.
Vital sign monitoring and sample collection for bacterial culture
Heart rate (HR) and rectal temperature (T) were monitored as follows. Electric acupuncture poles were inserted, respectively, into the upper limbs, whereas the left lower limb was connected to the RM-6280 physiologic recorder (Dongguan, Guangdong Province, China) to measure the HR. A thermometer was directly used to monitor T.
Heart blood was sampled using the following procedure. After hair removal and skin disinfection beneath the xiphoid, a syringe was used to extract 2 mL blood directly from the heart. Then, the abdominal cavity was opened, and abdominal exudate samples were collected using aseptic cotton swabs. The collected blood and abdominal exudate samples were sent for bacterial culture to the causative bacteria laboratories of our institution.
Detection indexes in abdominal aortic blood samples
The levels of white blood cells (WBCs), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine (Cr), as well as the activity of creatine kinase-muscle/brain (CK-MB), were assessed by routine biochemical methods using a Hitachi Automatic Analyzer (Hitachi, Ltd., Japan). In addition, serum TNF-α and IL-6 concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (Shanghai Westang BIO-TECH Inc., Ltd., Shanghai, China). The lowest tested concentrations are <4.0 pg/mL TNF-α for ELISA and <4.0 pg/mL IL-6.
Intestinal mucosal injury scoring and pathological observation
A 5 cm intestinal tissue was harvested from 5 cm above the ileocecum, fixed in 10% formaldehyde solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Then, the pathological changes were assessed by optical microscopy independently by two expert pathologists blinded to the treatments. Intestinal mucosal injury was classified using the Chiu’s method (20): 0, normal intestinal mucosal villus; 1, cystic gaps appearing under the epithelium at the villus apex, with capillary hyperemia; 2, expanded cystic gaps under the epithelium, moderate edema in the lamina propria, and dilation of the central cheliferous vessels; 3, significant edema in the lamina propria, degeneration and necrosis of the intestinal mucosal epithelial cells, and abscission of a few villus apex; 4, degeneration, necrosis, and exfoliation of the epithelial cells, abscission of some villus, exposure of the lamina propria, capillary dilation, and hyperemia; and 5, abscission of the villus, disintegration of the lamina propria, and bleeding or ulceration.
Measurements of sIgA in the intestinal mucosa
Samples of 15 cm distal intestine tissues were collected. The intestinal lumen was quickly washed with 4 °C brine ice. Then, the tissues were cut open along the midline, and the mucosa was scrapped, placed in a freezing tube, and stored at −80 °C until use. A 100 mg intestinal tissue sample was homogenized in cold phosphate-buffered saline (PBS) using an ultrasonic cell disruptor and centrifuged at 8000 rpm for 10 min. The resulting supernatant was collected and placed at −20 °C until sIgA detection by ELISA. The procedures were strictly in accordance with the instructions of the rat sIgA ELISA kit (Shanghai Westang BIO-TECH Inc., Ltd.).
Quantification of CD3 and γδT cells in intestinal mucosa samples
Samples of 15 cm distal intestinal specimens were obtained. The intestinal lumen was quickly washed with 4 °C brine ice. Then, the tissues were cut open along the midline, and the mucosa was scrapped and placed in a 5 mL PBS containing collagenase. After incubation at 37 °C for 30–60 min with shaking, the cell suspension was obtained and filtered. Then, a 5 mL lymphocyte separation medium was added to another tube and overlayed with a 5 mL cell suspension, followed by density gradient centrifugation at 1900 rpm for 25 min. Subsequently, the middle layer containing lymphocytes was collected, and a volume of 10 mL PBS was added. After cell washing and counting, a 100 μL cell suspension was transferred into a flow cytometry tube, and anti-rat CD3-FITC or anti-rat γ/δ T-PE antibodies (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) were added for 30 min in the dark. Then, the cells were washed, placed at 4°C in the dark, and assessed for the quantification of CD3 and γδT cells by flow cytometry (Epics Altra; Beckman Coulter, USA) within 1 h.
Statistical analysis
Statistical analyses were performed by the Statistical Package for Social Sciences 15.0 software (SPSS Inc.; Chicago, IL, USA). Data are expressed as mean±standard deviation. Intergroup comparisons were performed by one-way analysis of variance with Scheffe’s method as post hoc test. Survival data were evaluated by the Kaplan-Meier method and compared by the log-rank test. A p value <0.05 was considered statistically significant.
RESULTS
Shenfu injection improves the general conditions of sepsis model rats
Sham animals had normal temperature and ate well. They were active and showed normal grooming and stool.
Meanwhile, model animals showed decreased activity, less eating, drowsiness, piloerection, and abdominal distension, which were gradually aggravated. Within 12 h of modeling, survival rats curled showed low fitness and reduced activity and were reluctant to eat and drink; they were also unresponsive. At 24 h after modeling, the rats started to have some level of activity, breathed more smoothly, and had a limited amount of diet and water. In addition, they showed fluffy and cloudy hair, eye discharge, and soft stool. The HR, T, WBC count, serum BUN, and Cr levels, as well as AST, ALT, and CK-MB activities, were significantly higher in the model group than in the sham group (Table 1). Furthermore, no bacteria were detected in blood and abdominal exudate specimens from sham animals, whereas Escherichia coli, Bacteroides, and Streptococcus faecalis were detected in the model group. Compared with model animals, the LSF or HSF groups showed no significant improvement in those detection indexes of abdominal aortic blood samples (data not shown). However, activity, reactivity, and eating and drinking habits in the LSF and HSF group animals were significantly improved compared with those in the model group.
Table 1.
HR, T, WBC, AST, ALT, BUN, Cr, and CK-MB at postoperative 12 h in the sham and model groups
| Group | HR (beats/min) | T (°C) | WBC (×109) | AST (IU/L) | ALT (IU/L) | BUN (mmol/L) | Cr (μmol/L) | CK-MB (IU/L) |
|---|---|---|---|---|---|---|---|---|
| Sham (n=8) | 318.3±33.4 | 37.1±0.3 | 6.3±1.6 | 35.7±4.5 | 39.4±3.8 | 6.9±1.5 | 58.6±3.1 | 20.7±4.2 |
| Model (n=8) | 526.7±24.5* | 38.8±0.2* | 13.5±1.3* | 91.8±3.6* | 96.1±3.1* | 12.1±1.9* | 117.4±7.9* | 42.5±3.7* |
HR: heart rate; T: rectal temperature; AST: aspartate aminotransferase; ALT: alanine aminotransferase; WBC: white blood cell count; BUN: blood urea nitrogen; Cr: creatinine; CK-MB: creatine kinase-muscle/brain
p<0.05 vs. sham group
Shenfu injection protects the intestinal mucosal structure in rats with sepsis
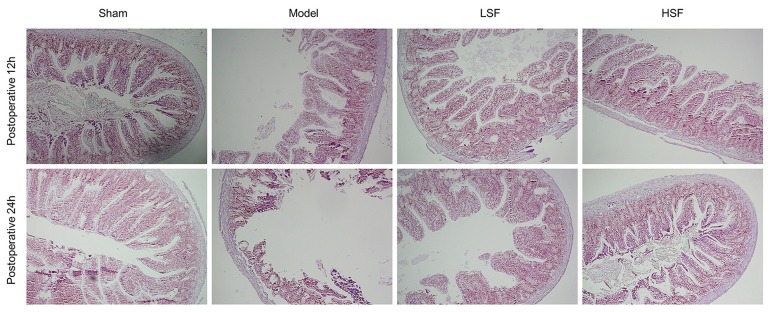
Sham rats had an intact intestinal mucosa, with tall columnar and well-arranged epithelial cells; there was no interstitial edema (Figure 1). Meanwhile, sepsis (model group) resulted in histological changes; rats had swollen intestinal mucosal villus, dilated central cheliferous vessels, and inflammatory cell infiltration at 12 h after modeling; at 24 h, model animals showed aggravated damage, with significantly swollen intestinal mucosal villus, overt necrosis and abscission of the epithelial cells, and severe edema of the lamina propria, as well as large amounts of infiltrated inflammatory cells. Treatment with SI (LSF and HSF groups) resulted in alleviated intestinal mucosal injury; the epithelial cells were loose and well-arranged, with reduced interstitial edema, compared with the model group.
Figure 1.
Pathological changes of intestinal mucosal tissue. Ileocecal tissue samples were collected 12 h and 24 h, respectively, after sepsis induction by cecal ligation and puncture (CLP) and submitted to hematoxylin and eosin staining. Representative images obtained under light microscopy were shown (×100 magnification)
The Chiu’s scores at different time points were significantly higher in the model animals than in the sham group (p<0.01). Meanwhile, the Chiu’s scores at postoperative 12 h and 24 h were significantly decreased after SI treatment in both LSF and HSF groups (p<0.01). The HSF group scores were further reduced compared with the LSF group (p<0.05) (Table 2).
Table 2.
Chiu’s scores for intestinal mucosal injury at different time points
| Group | Time points | |
|---|---|---|
|
|
||
| 12 h | 24 h | |
| Sham (n=8) | 0.34±0.41 | 0.38±0.43 |
| Model (n=8) | 4.05±0.54** | 4.21±0.63** |
| LSF (n=8) | 3.21±0.75**,## | 3.05±0.71**,## |
| HSF (n=8) | 2.28±0.67**,##,† | 1.85±0.62**,##,† |
p<0.01 vs. sham group;
p<0.01 vs. model group;
p<0.05 vs. LSF group
Shenfu injection decreases serum TNF-α and IL-6 concentrations in rats with sepsis
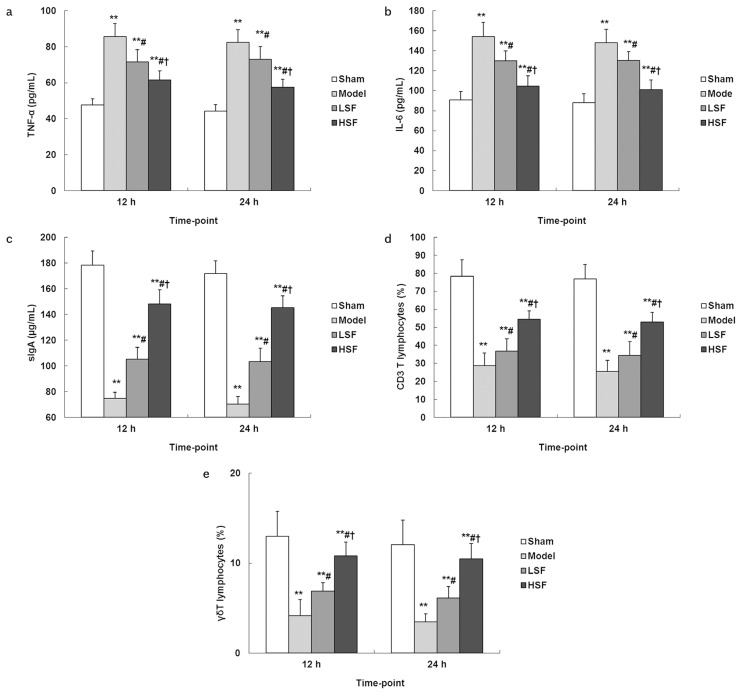
Sepsis increased serum TNF-α and IL-6 levels at postoperative 12 h and 24 h significantly (p<0.01) compared with sham operation. Meanwhile, treatment with SI (LSF and HSF groups) resulted in decreased serum TNF-α and IL-6 levels compared with model animals (p<0.05) (Figure 2a, b). The effect was more pronounced in the HSF group than in the LSF group (p<0.05).
Figure 2. a–e.
Concentrations of inflammatory factors, mucosal sIgA content, and percentages of intestinal mucosal CD3 and γδT cells in different groups. At different time points after CLP surgery, blood samples were collected from each group for serum preparation. Then, serum TNF-α (A) and IL-6 (B) concentrations were assessed by ELISA. Then, mucosal samples were obtained from each group; after homogenization, the lysates were submitted to ELISA for sIgA (C); the remaining samples were incubated with anti-rat CD3-FITC (D) or anti-rat γ/δ T-PE (E) antibodies and analyzed by flow cytometry. N=8, each group at each time. **p<0.01 vs. sham group; #p<0.05 vs. model group; †p<0.05 vs. LSF group
Shenfu injection increases intestinal mucosal sIgA content in rats with sepsis
Sepsis significantly reduced intestinal mucosal sIgA content at postoperative 12 h and 24 h (model group) compared with sham animals (p<0.01). Meanwhile, treatment with both low and high doses of SI resulted in significantly higher intestinal mucosal sIgA levels compared with the model group (p<0.05). On the other hand, higher sIgA amounts were obtained in the HSF group compared with values obtained for animals administered a low dose of SI (p<0.05) (Figure 2c).
Shenfu injection increases intestinal mucosal CD3 and γδT cell amounts in rats with sepsis
Sepsis caused important decreases in intestinal mucosal CD3 and γδT cells at postoperative 12 h and 24 h (model vs. sham group, p<0.01). Meanwhile, treatment with SI alleviated these changes. Indeed, the intestinal mucosal CD3 and γδT cell rates were significantly higher in the LSF and HSF groups than in the model animals (p<0.05), with a higher effect in the HSF group than in the LSF group (p<0.05) (Figure 2d, e).
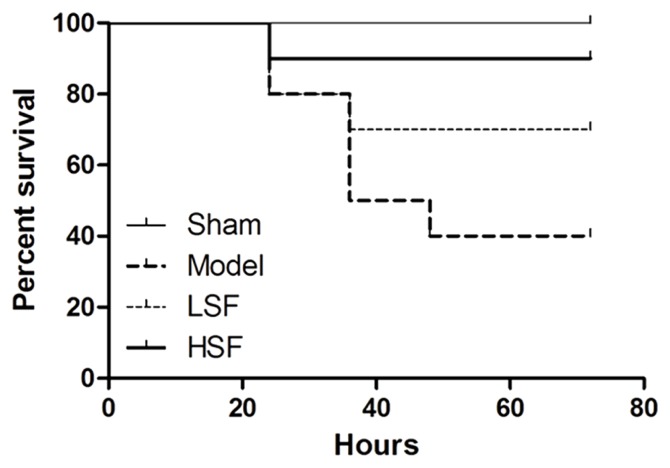
Shenfu injection increases survival rats with sepsis
As shown in Figure 3, sepsis significantly decreased animal survival. All animals survived in the sham group, whereas only 40% survival was recorded in the model group at 72 h postoperatively. Interestingly, treatment with SI resulted in increased survival compared with the model group. At 72 h, the survival rates of the LSF and HSF groups were 70% and 90%, respectively, indicating a more pronounced effect of the high drug dose (p<0.05).
Figure 3.
At 72 h survival of rats in different groups. In this experiment, 10 rats were assessed, respectively, in the model, LSF, and HSF groups, with 2 sham rats. The animals were checked every 12 h for a total of 72 h
DISCUSSION
The present study demonstrated that CLP-induced sepsis markedly decreased animal survival and increased Chiu’s scores, as well as serum TNF-α and IL-6 levels, while reducing intestinal mucosal sIgA content and CD3 and γδT cell amounts. All these effects were alleviated by SI treatment, in a concentration-dependent manner, although the drug at the tested concentrations showed no effects on the recovery of HR, T, WBC count, serum BUN, and Cr levels, as well as AST, ALT, and CK-MB activities.
Shenfu injection, which originated from Shenfu decoction, a well-known traditional Chinese medicine, is composed of red ginseng (Panax, family: Araliaceae) and aconite (Radix aconiti lateralis preparata, Aconitum carmichaelii Debx; family: Ranunculaceae). Its main active ingredients include ginsenoside and aconitum alkaloids. It has been widely used in China for more than two decades and is regarded as one of the most commonly used drugs for the assistant treatment of septic shock in emergency departments and ICUs. Previous studies (21,22) have revealed that ginsenoside plays multiple roles, such as antioxidation, free radical scavenging, vessel dilation, microcirculation improvement, anti-shock, anti-aging, and immune function enhancement, which can significantly improve immune function abnormalities.
Sepsis, an infection-derived SIRS, is a common severe complication in critically ill patients in the ICU. If sepsis is not effectively controlled, it easily evolves into septic shock and even causes MODSs (23), which greatly complicate clinical treatment. The intestinal canal is the central organ of sepsis; it is not only the initiator but also the damaged organ in sepsis (24). Thus, it is essential to protect the intestinal mucosal barrier function for sepsis prevention and treatment. As discussed above, sepsis caused significant morphological changes in the intestinal mucosal structure, with increased injury. However, treatment with SI of rats with sepsis resulted in remarkable alleviation of these symptoms.
TNF-α is the most important inflammatory mediator in early inflammation and is mainly secreted by T helper 1 (Th1) cells, as well as activated by monocytes and macrophages; it is a key mediator of sepsis, and its expression is directly proportional to the severity of sepsis (25). TNF-α plays a significant role in the occurrence and development of septic inflammation, which can further promote the release of platelet-activating factor, IL-1, IL-8, and other inflammatory mediators, ultimately leading to SIRS (26). IL-6 plays a pivotal role in directing transition from innate to acquired immunity. Blockade of IL-6 signaling may contribute to the therapeutic management of Th1-driven inflammatory conditions, for example, in the chronic inflammatory disease of the gastrointestinal tract (27). As discussed above, sepsis significantly increased serum TNF-α and IL-6 concentrations at 12 h and 24 h after induction compared with sham values. Nevertheless, SI significantly decreased serum TNF-α and IL-6 concentrations in rats with sepsis, in a dose-dependent manner, corroborating the previous findings that SI regulates TNF-α expression (28). Previous studies had revealed that ginsenoside, an active component of SI, would exert strong inhibitory effects on TNF-α, which is secreted by monocytes and macrophages, thereby indirectly decreasing the release of platelet-activating factor, IL-1, IL-8, nuclear factor-κB, and other inflammatory mediators (29). Overall, these results indicated that SI may weaken the adaptive immune response as decreasing serum TNF-α and IL-6 levels.
sIgA prevents the intestinal mucosal epithelial cells from taking up toxins and other harmful molecules and removes pathogens and toxins through intestinal peristalsis. It prevents the immunoreactions in the intestinal mucosal barrier to intestinal probiotics. SI was shown to improve the internal environment in the intestinal canal and whole body, promote intestinal peristalsis, reduce colonization and reproduction of harmful bacteria, and alleviate intestinal mucosal damage (30). In the present study, we found that SI induced intestinal mucosal cells to secrete sIgA and increased γδT cells, which were major histocompatibility complex-unrestricted innate-like lymphocytes. Furthermore, the effects were directly proportional to SI dose. Therefore, we considered that SI may promote innate immune response to combat sepsis, as a compensation for suppressed adaptive immune response in the process of repairing the intestinal mucosa.
The present study had several limitations. First, sample size was relatively small, and observation time was short. Second, although SI did not show significant impacts on the liver, kidney, and heart functions in rats with sepsis, its side effects on the lung, spleen, and other organs were not evaluated. Finally, in-depth mechanisms for the current findings are required.
In summary, we found that in rats with sepsis, SI could improve the intestinal mucosa by inhibiting the production of TNF-α or IL-6, as well as by promoting the secretion of sIgA and increasing the amounts of γδT cells. The immunomodulation would alleviate intestinal mucosal injury resulting from adaptive immune response and eliminate the invading pathogens with strengthened innate immune response, thus decreasing the fatality rate.
Supplementary Data
The flow cytometry histogram of CD3 and γδT cells in the intestinal mucosa for the model and sham groups at 12 h and 24 h
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of First Affiliated Hospital, Zhejiang Chinese Medical University (Decision Number: 2012-K-004-02).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - R.J., L.J.; Design - S.J., R.J., S.L.; Supervision - R.J., S.L.; Resource - S.J., R.J., L.J.; Materials - S.J., C.Z., W.F.; Data Collection and/or Processing - S.J., W.F., Y.S.; Analysis and/or Interpretation - L.J., C.Z., Y.S.; Literature Search - S.J., C.Z., W.F., Y.S.; Writing Manuscript - S.J., R.J., S.L.; Critical Reviews - S.J., R.J., S.L., L.L.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This work was supported by grants from the Major Diseases Research Program of Prevention and Treatment of Zhejiang Traditional Chinese Medicine (2012ZGG001), the Scientific Research Foundation of Traditional Chinese Medicine of Zhejiang Province (2015ZA081 and 2015ZA085), and Zhejiang Provincial Natural Science Foundation of China (LY14H290006 and LY14H030006).
REFERENCES
- 1.Ma L, Zhang H, Yin YL, et al. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–35. doi: 10.1016/j.cyto.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–55. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 3.Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189:1204–13. doi: 10.1164/rccm.201310-1875OC. [DOI] [PubMed] [Google Scholar]
- 4.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Kebir D, Jozsef L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res. 2008;103:352–9. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- 6.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci. 2006;101:189–98. doi: 10.1254/jphs.CRJ06010X. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Li A, Weng YB, Duan ML, Wang BE, Zhang SW. Changes in intestinal mucosal immune barrier in rats with endotoxemia. World J Gastroenterol. 2009;15:5843–50. doi: 10.3748/wjg.15.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. Jama. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmeyer S, Kroger S, Vahjen W, Zentek J, Scharek-Tedin L. Impact of a probiotic Bacillus cereus strain on the jejunal epithelial barrier and on the NKG2D expressing immune cells during the weaning phase of piglets. Vet Immunol Immunopathol. 2014;161:57–65. doi: 10.1016/j.vetimm.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu JL, Wang ZH, Li GZ, Wang YR, Liu P. Decreased IgA+ plasma cells and IgA expression in acute liver necrosis mice. World J Gastroenterol. 2010;16:3827–33. doi: 10.3748/wjg.v16.i30.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcaide ML, Strbo N, Romero L, et al. Bacterial Vaginosis Is Associated with Loss of Gamma Delta T Cells in the Female Reproductive Tract in Women in the Miami Women Interagency HIV Study (WIHS): A Cross Sectional Study. PLoS One. 2016;11:e0153045. doi: 10.1371/journal.pone.0153045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–43. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han G, Geng S, Li Y, et al. gammadeltaT-cell function in sepsis is modulated by C5a receptor signalling. Immunology. 2011;133:340–9. doi: 10.1111/j.1365-2567.2011.03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WY, Hu YM, Ko TL, Yeh SL, Yeh CL. Glutamine modulates sepsis-induced changes to intestinal intraepithelial gammadeltaT lymphocyte expression in mice. Shock. 2012;38:288–93. doi: 10.1097/SHK.0b013e3182655932. [DOI] [PubMed] [Google Scholar]
- 18.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of gammadelta T lymphocytes contributes to mortality and immunosuppression in sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1338–43. doi: 10.1152/ajpregu.00283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu W, Jiang RL, Wang LC, et al. Effect of Shenfu injection on intestinal mucosal barrier in a rat model of sepsis. Am J Emerg Med. 2015;33:1237–43. doi: 10.1016/j.ajem.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 20.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–83. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, Cheng M, Yuan Z, Zhou S, Yu Z. Protective role of Shenfu on ischemia-reperfusion injury of rat liver grafts. Transplant Proc. 2012;44:978–81. doi: 10.1016/j.transproceed.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Ji XF, Yang L, Zhang MY, Li CS, Wang S, Cong LH. Shen-fu injection attenuates postresuscitation myocardial dysfunction in a porcine model of cardiac arrest. Shock. 2011;35:530–6. doi: 10.1097/SHK.0b013e31820e2058. [DOI] [PubMed] [Google Scholar]
- 23.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. Bmj. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 24.Puleo F, Arvanitakis M, Van Gossum A, Preiser JC. Gut failure in the ICU. Semin Respir Crit Care Med. 2011;32:626–38. doi: 10.1055/s-0031-1287871. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013;62:507–14. doi: 10.1007/s00011-013-0604-5. [DOI] [PubMed] [Google Scholar]
- 26.Castellheim A, Brekke OL, Espevik T, Harboe M, Mollnes TE. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol. 2009;69:479–91. doi: 10.1111/j.1365-3083.2009.02255.x. [DOI] [PubMed] [Google Scholar]
- 27.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang YG. Effect of Shenfu injection on TNF-α, IL-6, and IL-8 in patients with sepsis. J Emerg Tradit Chin Med. 2012;21:299–300. [Google Scholar]
- 29.Wu LL, Jia BH, Sun J, Chen JX, Liu ZY, Liu Y. Protective effects of ginsenoside Rb1 on septic rats and its mechanism. Biomed Environ Sci. 2014;27:300–3. doi: 10.3967/bes2014.053. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Liu L, Gao W, Liu K, Qi LW, Li P. Direct and comprehensive analysis of ginsenosides and diterpene alkaloids in Shenfu injection by combinatory liquid chromatography-mass spectrometric techniques. J Pharm Biomed Anal. 2014;92:13–21. doi: 10.1016/j.jpba.2013.12.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The flow cytometry histogram of CD3 and γδT cells in the intestinal mucosa for the model and sham groups at 12 h and 24 h