Abstract
True primary mucinous ovarian carcinomas are rarer than originally thought and their clinical behavior and treatment response are different than more common epithelial ovarian carcinomas. Secondary ovarian neoplasms often mimic the clinical and histological features of mucinous ovarian cancer making their diagnosis, and therefore treatment, more difficult. Misdiagnosis can have a significant impact on both treatment and prognosis. The majority of these secondary ovarian neoplasms arise from the gastrointestinal tract, with mucinous histology often of pancreaticobiliary origin. Our study objective was to review current evidence distinguishing pancreaticobiliary ovarian metastasis from primary mucinous ovarian carcinoma. We utilized a PubMed search using MeSH terms and selected articles were reviewed, synthesized and summarized. Thirty-nine articles were included in the review. The clinical, gross, histological and immunohistochemical features distinguishing primary mucinous ovarian carcinomas from pancreaticobiliary ovarian metastasis were identified. Compared to primary mucinous ovarian carcinoma, metastatic pancreaticobiliary tumors are more often bilateral, <10 cm, have irregular external surface and surface implants, display an infiltrative pattern of invasion and stain for MUC1 and CK17. Primary ovarian mucinous tumors rarely (<3%) have signet ring cells or involvement of the hilum. Metastatic mucinous tumors mimic their primary mucinous ovarian counterparts and their clinical and histopathological features overlap in many ways. However, these metastatic tumors have features that can help differentiate them from primary mucinous carcinoma. With a high index of suspicion and knowledge of the reviewed features, distinguishing these tumors will continue to become easier.
Keywords: Metastatic pancreaticobiliary tumors, Ovarian mucinous tumors, Immunohistochemistry
Highlights
-
•
Primary ovarian and metastatic pancreaticobiliary tumors present similarly.
-
•
Histologic findings can differentiate primary from metastatic ovarian tumors.
-
•
Bilaterality, size, surface appearance can differentiate primary versus metastatic.
1. Introduction
Surveillance, Epidemiology and End Results Program (SEER) from the National Cancer Institute estimates approximately 22, 440 new cases of ovarian cancer per year. However, the ovary is a common site for metastasis with approximately 15% of ovarian cancers noted to be secondary malignancies (Noone et al., 2015). Metastases most commonly arise from the colon, breast, uterus and stomach. Their clinical and histological features often mimic those of primary ovarian neoplasms, especially in endometrioid and mucinous type neoplasms.
Approximately 5–10% of primary epithelial ovarian cancers are reportedly mucinous adenocarcinomas. However more recent literature acknowledges that this may be an overestimate, as many “primaries” are actually undiagnosed metastases from the gastrointestinal (GI) tract (Seidman et al., 2003). The most common primary sites of the GI tract that give rise to metastatic mucinous carcinoma within the ovary are colon/rectum, appendix, pancreas, biliary tract, and stomach. Distinction of primary versus secondary ovarian neoplasms can be especially difficult when a patient presents with metastatic disease. In a review by Petru et al., researchers reported that ovarian involvement may precede detection of the primary neoplasm in up to 38% of cases (Petru et al., 1992).
Ovarian metastases from the GI tract have been well-studied, the notorious example being the Krukenberg tumor. This tumor most commonly arises from the stomach and is defined by the histological identification of “signet ring cells”. Less common causes of ovarian metastasis are pancreaticobiliary tract cancers including cancers of the pancreas, gallbladder and bile ducts (cholangiocarcinoma), comprising roughly 6% of metastatic cancers to the ovary (Seidman et al., 2003). However, mucinous metastases from the pancreaticobiliary tract often create diagnostic confusion as their histology and immunohistochemistry profiles are similar to that of primary mucinous ovarian carcinomas.
There are several case reports in the literature of mucinous ovarian neoplasms metastatic from the pancreaticobiliary tract that presented clinically as primary ovarian neoplasms (Seidman et al., 2003; Petru et al., 1992; Alvarado-Cabrero et al., 2013; Corr et al., 2013; Di Marco et al., 2012; Garcia et al., 2004; Goldstein et al., 2001; Guerriero et al., 2012; Hibner and Greenspan, 2004; Jain et al., 2006; Jarvi et al., 2006; Ji et al., 2002; Khangura et al., 2013; Khunamornpong et al., 2008; Khunamornpong et al., 2007; Khunamornpong et al., 2006; Kim et al., 1999; Kiyokawa et al., 2006; Kumar et al., 2010; Kurt et al., 2016; Lashgari et al., 1992; Lee and Young, 2003; Lee et al., 2015; Lyra et al., 2015; McCluggage and Young, 2008; Meriden et al., 2011; Okamoto et al., 2011; Sun et al., 2012; Taranto et al., 2006; Testa et al., 2007; Vang et al., 2006a; Vang et al., 2006b; Wang and El-Bahrawy, 2014; Yemelyanova et al., 2008; Young and Hart, 1989; Young and Scully, 1990; Park and Kim, 2018). Many of these patients did not present with the typical symptoms of jaundice and back pain, but instead with vague symptoms often associated with ovarian malignancy (abdominal pain, abdominal-pelvic mass). The variable clinical presentations, radiologic findings, tumor serum markers and gross appearance of the ovarian neoplasms make an accurate histologic diagnosis essential to establish the treatment plan and prognosis. This review will highlight case reports and literature reviews that address the presentation and diagnosis of pancreaticobiliary metastases to the ovary compared to primary mucinous ovarian carcinomas. Our review will focus on the clinical and pathological features that help differentiate one from the other.
2. Materials and methods
This systematic review was conducted in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement (Moher et al., 2009).
2.1. Search strategy
A literature review of published literature on differentiating primary versus secondary mucinous ovarian neoplasms with a focus on ovarian metastases of pancreaticobiliary cancers was performed. Inclusion criteria included MeSH major terms “mucinous ovarian cancer”, “panreaticobiliary tract cancer”, “pancreatic”, “bile duct”, “gallbladder AND “neoplasm”, “cancer”, “metastases/metastasis”. PubMed, Scopus, Embase, World Wide Science, and National Cancer Institute Grey Literature and Clinical trials were searched with the above stated criteria. All English-language articles published between 1985 and 2018 were included.
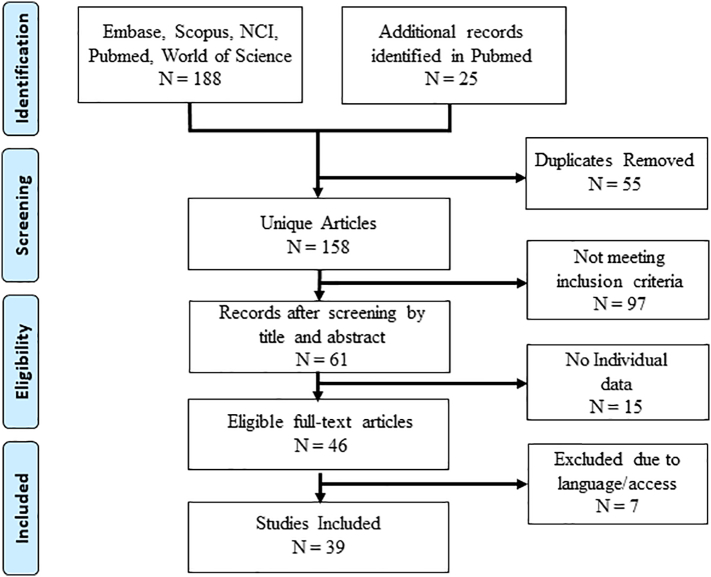
The electronic search identified 188 articles and 25 additional records found by manual search for a total of 213 articles (Fig. 1). After 55 duplicate articles were removed 158 articles remained. The articles were screened by title and abstract and 98 did not meet the inclusion criteria. The remaining 61 unique citations were further screened. Fifteen articles addressed a variety of metastatic cancers to the ovary and provided limited individual useful information about pancreaticobiliary carcinomas. These 15 articles were also excluded. Seven additional articles were removed due to inability to access the article and only non-English language versions of the article were available. Thirty-nine unique citations were selected for inclusion.
Fig. 1.
PRISM diagram of article selection process (Moher et al., 2009).
2.2. Inclusion/exclusion criteria
Inclusion was determined after review of article title and abstract, followed by full text review. If the full text was unavailable the article was excluded. Included articles were in the English language, involving human subjects, and published between 1985 and 2018. Included articles addressed a variety of features (as discussed within this review) used to facilitate the distinction of primary versus secondary ovarian neoplasms, specifically secondary mucinous carcinomas arising from the pancreaticobiliary tract. Exclusion criteria included articles with a primary focus on other sources of secondary mucinous carcinoma of the ovary including colon/rectum, appendix, stomach, breast and cervix. Articles were also excluded if they were primarily about treatment or management of the neoplasms and did not provide any contributing information to this review.
2.3. Data extraction
For each included article information that could help in differentiating primary versus secondary mucinous ovarian carcinoma to include clinical presentation, gross and microscopic pathology findings, as well as immunohistochemistry staining profile was extracted.
Differences between primary mucinous ovarian and primary pancreaticobiliary groups were calculated using the independent group t-test for continuous variables and chi-squared for nominal variables. All statistical analysis was performed using IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
3. Results
The reported diagnosis from the cases extracted from the 39 articles reviewed included 394 primary mucinous ovarian carcinomas (Seidman et al., 2003; Goldstein et al., 2001; Ji et al., 2002; Khunamornpong et al., 2006; Lee and Young, 2003; McCluggage and Young, 2008; Okamoto et al., 2011; Vang et al., 2006a; Vang et al., 2006b; Wang and El-Bahrawy, 2014; Yemelyanova et al., 2008), 171 primary pancreatic carcinoma (Seidman et al., 2003; Petru et al., 1992; Alvarado-Cabrero et al., 2013; Di Marco et al., 2012; Goldstein et al., 2001; Ji et al., 2002; Meriden et al., 2011; Okamoto et al., 2011; Testa et al., 2007; Vang et al., 2006a; Wang and El-Bahrawy, 2014; Young and Hart, 1989; Kim et al., 1999), 108 primary biliary tract carcinoma (Petru et al., 1992; Corr et al., 2013; Garcia et al., 2004; Jain et al., 2006; Jarvi et al., 2006; Khangura et al., 2013; Khunamornpong et al., 2008; Khunamornpong et al., 2007; Khunamornpong et al., 2006; Kumar et al., 2010; Kurt et al., 2016; Lashgari et al., 1992; Lee et al., 2015; Lyra et al., 2015; Meriden et al., 2011; Okamoto et al., 2011; Sun et al., 2012; Taranto et al., 2006; Testa et al., 2007; Vang et al., 2006a; Wang and El-Bahrawy, 2014; Young and Scully, 1990; Park and Kim, 2018; Sharma et al., 1997; Low et al., 2003; Majumdar, and D S, S K, A R, R G, 2007), and 48 unspecified pancreaticobiliary carcinoma (Guerriero et al., 2012; Meriden et al., 2011; Vang et al., 2006b; Yemelyanova et al., 2008; Park and Kim, 2018). All tumors were classified as mucinous.
3.1. Clinical characteristics
Primary mucinous ovarian tumors generally present with increasing abdominal girth, symptoms of abdominal distension which may be described as bloating abdominal pressure or pain, pelvic pressure or pain, changes in bowel or bladder habits, or other sequela of abdominal disease. Table 1 summarizes the reported clinical characteristics. The mean age of patients with primary ovarian mucinous carcinoma was 48.8 years compared to 54.2, 55.4, and 60.5 years for patients with primary pancreatic, primary biliary tract, or primary unspecified pancreaticobiliary cancer, respectively. Of those cases with case-specific data, 65.6% and 60.9% primary pancreatic and primary biliary tract carcinoma, respectively, presented with pelvic and/or abdominal pain. GI related symptoms were the presenting complaint in 25% of primary pancreatic and 23.4% of primary biliary tract carcinomas, while jaundice was the presenting symptoms in 15.6% in both groups. Other reported symptoms included pelvic mass/pressure, abdominal distention/ascites, vaginal bleeding, and weight loss.
Table 1.
Clinical characteristics of women with primary mucinous ovarian carcinoma and those with ovarian metastasis from a pancreaticobiliary cancer.
| Primary mucinous ovarian | Primary pancreatic | Primary biliary tract | Primary pancreaticobilary (unspecified) | |
|---|---|---|---|---|
| Median age (range) (years) | 48 (18–78) | 54 (29–87) | 54 (33–80) | 65 (17–68) |
| Presentation [N and (%)] | NA | 32 | 64 | 7 |
| Pelvic/Abdominal Pain | 21 (65.6) | 39 (60.9) | 1 (14.3) | |
| Abdominal Distention/ascites | 1 (3.1) | 9 (14.1) | 0 | |
| Pelvic Mass | 3 (9.4) | 9 (14.1) | 1 (14.3) | |
| Vaginal Bleeding | 1 (3.1) | 3 (4.7) | 0 | |
| Jaundice Weight Loss |
5 (15.6) | 10 (15.6) 7 (20.9) |
0 4 (57.1) |
|
| GI Symptoms (RLQ pain, nausea, vomiting, epigastric pain, anorexia, obstruction)a | 8 (25.0) | 15 (23.4) | 0 | |
| Laboratory Values (mean, N) | NA | NA | ||
| Alkaline Phosphatase | NP | 892 (14)b | ||
| Amylase | 45 (1) | 953 (1) | ||
| CA 19–9 | 337 (2) | 2360 | ||
| CA 125 | 92 (5) | (17)c | ||
| CEA | 13.1 (1) | 236 (24)d N/A |
NA = data not available
1 case each of large bowel obstruction, rectal bleeding, 2 cases of epigastric pain
all but 1 value elevated above 137 IU/L
2 cases of outliers- 1 value of 20,000, one value of 30,000; 16 of 17 values elevated above 37 U/ml
3 of 27 cases with reported normal levels
Ovarian mucinous neoplasms are known to produce abnormal serum levels of certain substances. Carbohydrate antigen 19–9 or cancer antigen 19–9 (CA 19–9) is produced by mucin cells. It is commonly used in the evaluation of patients suspected to have exocrine pancreatic cancer with a reported sensitivity and specificity of 70–92% and 68–92%, respectively (Pleskow et al., 1989). The marker itself is a mucin protein. While mostly used to evaluate patients with pancreatic tumors, an elevated CA 19–9 level can also be found in cancers of other sites including liver, biliary, and GI tract, and in inflammatory conditions of those sites. Elevated levels have been reported in some primary ovarian mucinous tumors.
Serum cancer antigen 125 (CA 125) is a serum marker that has been associated with epithelial ovarian cancer. It is a mucin protein (MUC16) that is produced by mesoderm-derived cells and can be found in ovarian, pleural, peritoneal, and pericardial cells (Yin and Lloyd, 2001; Jacobs and Bast Jr., 1989). Due to its widespread production and presence in these epithelia, CA 125 provides limited value in the context of cancer, as inflammation or irritation of these epithelial surfaces can produce elevated levels.
Our review includes limited data on serum tumor markers as many of the available articles did not include this information. In patients with primary pancreatic carcinoma levels of CA 19–9 ranging from 20 to 654 U/mL were reported, while reported CA 125 levels ranged from 90 to 98 U/mL. In patients with primary biliary tract very elevated levels of alkaline phosphatase (mean 892 IU/L, range 128–2, 620 IU/L), CA 19–9 (mean 2360 U/mL, range 119–30,000 U/mL) and CA 125 (mean 236 U/mL, range 9–730 U/mL) were reported. Elevated levels of alkaline phosphatase and significantly high levels of CA 19–9 may be useful in differentiating primary ovarian mucinous carcinoma from metastatic pancreaticobiliary cancer.
3.2. Gross characteristics
Table 2 summarizes the gross characteristics of the various cancers reviewed. Primary ovarian mucinous tumors were largely unilateral (3/107, 96.3%), cystic in appearance (25/28, 89.3%), with solid components (21/28, 75%), had a smooth surface (20/25, 80%) without surface implants (43/44, 97.7%), and were larger in size (mean 20.3 cm) when compared to metastatic tumors. The majority of pancreaticobiliary tumors metastatic to the ovaries were bilateral, cystic with solid components, with tumor surface implants, and without a smooth appearing surface.
Table 2.
Gross characteristics of primary ovarian mucinous tumors and pancreaticobiliary carcinoma metastatic to the ovaries.
| Proportion N (%), Mean/range | Primary Ovarian Mucinous | Primary Pancreatic | Primary Biliary Tract | Primary Pancreaticobiliary (unspecified) | P-value (primary v. metastatic) |
|---|---|---|---|---|---|
| Bilateral | 4/108 (3.7) |
42/47 (89.4) |
62/79 (78.5) |
24/31 (77.4) |
<0.001 |
| Cystic Appearance | 25/28 (89.3) |
11/13 (84.6) |
58/69 (84.1) |
NA | 0.757 |
| Nodularity | NA | 16/28 (57.1) |
22/49 (44.9) |
3/6 (50) |
NP |
| Size (cm, range) | 20.3 (5–48) |
9.2 (1.5–22) |
10.0 (0.22–22.0) |
9.8 (2.5–21) |
<0.001 |
| Smooth Surface | 20/25 (80.0) |
7/27 (25.9) |
15/64 (23.4) |
NA | <0.001 |
| Solid Components | 21/28 (75.0) |
6/6 (100) |
54/67 (80.6) |
NA | 0.417 |
| Surface Implants | 1/44 (2.3) |
26/33 (78.8) |
45/64 (70.3) |
2/3 (66.7) |
<0.001 |
NA = data not available
NP = not performed
3.3. Histology
A summary of the histological characteristics described in the articles reviewed are included in Table 3. Compared to primary ovarian mucinous tumors, pancreaticobiliary carcinoma metastatic to the ovary exhibits a higher proportion of infiltrative histologic pattern (p < .001) and a higher rate of signet ring cell presence, particularly in biliary tract tumors (p < .001). Some of the articles reported histologic involvement of the ovarian hilum for primary ovarian and biliary tract cases, of which a higher proportion of hilum involvement was noted in biliary tract tumors (42.3% versus 2.4%, p < .001).
Table 3.
Histologic findings of primary mucinous ovarian carcinoma and pancreaticobiliary carcinoma metastatic to the ovary.
| Proportion N (%) | Primary Ovarian Mucinous | Primary Pancreatic | Primary Biliary Tract | Primary Pancreaticobiliary (unspecified) | p-value (primary vs. metastatic) |
|---|---|---|---|---|---|
| Infiltrative Pattern | 5/41 (12.2) |
26/40 (65.0) |
44/59 (74.6) |
5/7 (71.4) |
<0.001 |
| Involvement of Ovarian Hilum | 1/41 (2.4) |
NA | 22/52 (42.3) |
NA | <0.001 |
| Signet Ring Cells | 3/108 (2.8) |
2/28 (7.1) |
9/28 (32.1) |
1/20 (5.0) |
0.001 |
NA = data not available
3.4. Immunohistochemistry
Immunostains have been extensively studied in the distinction between primary and secondary mucinous ovarian neoplasms. Cytokeratin (CK) 7 and CK20 are most frequently helpful in distinguishing between colorectal (CK7−/CK20+) and ovarian primaries (CK7+/CK20− or CK20+) (Khunamornpong et al., 2008; Khunamornpong et al., 2007; Khunamornpong et al., 2006). CK7 is typically diffusely positive in primary ovarian mucinous carcinomas, while CK20 shows variable positivity (Tot, 1999). Table 4 lists the summary of immunostaining reported in studies reviewed. CK7 was positive in a majority of both primary ovarian mucinous and pancreaticobiliary tumors metastatic to the ovaries. MUC1 can be helpful in differentiating primary ovarian mucinous from metastatic pancreaticobiliary as our review found only 19.4% of primary ovarian mucinous carcinomas stained positive, while >90% of biliary tract and 100% of pancreatic tumors metastatic to the ovaries stained positive. MUC5AC was found to stain a high proportion of ovarian mucinous carcinomas and pancreatic carcinomas metastatic to the ovaries, but not metastatic biliary carcinomas (97.8 and 93.3% versus 16.7%, respectively). Although limited in number of cases, staining for CDX2 was more common in metastatic pancreaticobiliary tumors compared to primary ovarian mucinous carcinomas (64.3% versus 37.8%).
Table 4.
Immunohistochemistry staining pattern of primary mucinous ovarian carcinoma and pancreaticobiliary carcinoma metastatic to the ovaries.
| Proportion N (%) | CK7 | CK20 | CK17 | CEA | MUC1 | MUC5AC | DPC4 | CDX2 | Other (n/%) |
|---|---|---|---|---|---|---|---|---|---|
| Primary Mucinous ovarian | 195/199 (98.0) | 123/167 (73.7) | NA | 8/15 (53.3) |
7/36 (19.4) | 91/93 (97.8) | NA | 17/45 (37.8) |
CA125 5/15 (33.3) WT1 0/12 (0) |
| Primary pancreatic | 35/38 (92.1) | 46/102 (45.1) | 27/64 (42.2) | 58/72 (81.2) | 18/18 (100) | 28/30 (93.3) | 16/34 (47.1) |
NA | CA125 52/64 (81.3) |
| Primary Biliary Tract | 25/30 (83.3) |
17/30 (56.7) |
NA | NA | 11/12 (91.7) |
2/12 (16.7) |
3/4 (75) |
1/4 (25.0) |
CA19–9 1/1 (100) |
| Primary pancreaticobiliary (unspecified) | 3/3 (100) | 15/17 (88.2) | NA | NA | NA | NA | 0/7 (0.0) |
9/15 (60.0) |
NA = data not available
4. Discussion
Our review highlights features that could be used in distinguishing primary ovarian mucinous carcinoma from pancreaticobiliary carcinomas metastatic to the ovary. Laterality and size were notably different between primary ovarian mucinous metastatic pancreaticobiliary tumors (Table 2). Pancreaticobiliary carcinomas metastatic to the ovary are more frequently bilateral and smaller than 10 cm. Gross appearance was also different in primary ovarian mucinous and metastatic pancreaticobiliary tumors. The surface of the primary mucinous ovarian tumors are most often smooth and contain no surface implants, while ovarian tumors due to metastasis from pancreaticobiliary carcinoma most often have an irregular surface and surface implants. The presence of cystic and solid components was not found to be different between primary and secondary neoplasms.
Our results are consistent with other reports on differences between primary and metastatic ovarian mucinous carcinomas. Dr. Robert H Young produced the first report and a detailed review on metastatic tumors to the ovary in which he highlighted the mimicry of primary mucinous ovarian tumors by metastases (Young, 2006; Young, 2007). In this review he notes that the clinical presentation of primary versus secondary mucinous ovarian tumors can be variable, and many times the secondary ovarian neoplasm may present similar to the presentation of a primary neoplasm. Similarly, our study reported abdominal and pelvic pain as the most frequent symptom with secondary ovarian neoplasms, with variable GI complaints as the second. It is worth noting that secondary neoplasms of the ovary are often larger than their corresponding primary tumors in GI sites. Their large size is often responsible for a patient's initial clinical presentation and may contribute to misdiagnosis as an ovarian primary. The first paper to highlight this mimicry from GI sites other than the appendix and intestines, began with Dr. Young's own series on pancreatic primaries (Young and Hart, 1989).
Since then, various diagnostic algorithms involving tumor size and laterality have been created to help clinically differentiate between primary and metastatic tumors, often at the time of surgery. (Seidman et al., 2003; Jarvi et al., 2006; Jain et al., 2006) In 2003 Seidman et al. published the first algorithm based on size and laterality of ovarian neoplasms that correctly classified 90% of mucinous ovarian carcinomas in their series. In effect, bilateral tumors suggested metastases and unilateral tumors >10 cm favored primary tumors (Seidman et al., 2003). In 2006, Khunamornpong sought to further validate this algorithm and used it to classify 74 cases of mucinous adenocarcinomas with 84% correctly classified. Bilaterality and size <10 cm remained the strongest predictors of ovarian involvement with metastasis. However, they concluded that “the prediction of primary mucinous adenocarcinomas by unilaterality and size 10 cm or greater was less reliable than previously reported” (Khunamornpong et al., 2006). Yemelyanova et al. analyzed series of 194 mucinous tumors (52 primary, 142 metastases) and optimized the size standard to be >13 cm to identify primary tumors which resulted in a correct classification of 87% of tumors overall (98% of primary tumors, 82% of metastases). In their series 20 metastatic mucinous tumors were derived from the pancreaticobiliary tract and this algorithm correctly classified 100% of them (Yemelyanova et al., 2008). More recently, Simons et al. used similar characteristics to improve the sensitivity of an algorithm. In addition to laterality and size, they found that patient age and tumor histology (signet ring) were important components to increase sensitivity in classifying primary ovarian versus metastatic mucinous carcinomas (Simons et al., 2019).
We identified an infiltrative pattern as being more often seen in secondary ovarian tumors from pancreaticobiliary metastasis. Signet ring cells and hilar involvement are rarely seen in primary ovarian mucinous carcinomas (Table 3).
Lee and Young provided a useful summary of histologic features after reviewing a series of 50 mucinous ovarian tumors (25 primary, 25 metastatic). They concluded that surface involvement by tumor cells, extensive extraovarian tumor, an infiltrative and nodular pattern of invasion, and the presence of signet ring cells are all features that favor metastases. Histologic features favoring primary ovarian neoplasms include an expansive (pushing) pattern of invasion, and a complex papillary pattern (Lee and Young, 2003). It is important to note that this review included metastatic mucinous tumors from a variety of sites, not just the pancreaticobiliary tract.
Meriden et al. reviewed 35 cases of ovarian metastases from pancreaticobiliary tract adenocarcinomas found in the literature and summarized their findings (Meriden et al., 2011). In this series, surface involvement (40%) was not as prominent of a distinguishing feature of secondary tumors due to metastasis compared with nodular growth pattern (63%). The presence of tumor foci in the hilar region of the ovary was common and suggestive of metastases. This emphasizes the importance of adequate hilar tissue sampling if metastasis to the ovary is suspected.
Our review showed that immunohistochemical staining with MUC1 is the most helpful stain that could help differentiate mucinous carcinoma form pancreaticobilary metastasis to the ovary from primary mucinous ovarian carcinoma (Table 4).
Goldstein et al. evaluated additional immunostain panels including Wilm's tumor 1 (WT1), CK17, CK20, carcinoembryonic antigen (CEA), and CA-125 in pancreaticobiliary adenocarcinomas versus ovarian serous carcinomas versus primary ovarian mucinous neoplasms. Their results primarily distinguish ovarian serous carcinomas from the others. Of the ovarian serous carcinomas reviewed, 38 (93%) of 41 were reactive for WT1. In contrast, both metastatic pancreaticobiliary adenocarcinomas and primary mucinous ovarian cancers were WT1 negative. Interestingly this study identified a different potential marker for pancreaticobiliary carcinoma, CK17. In their study 27% of metastatic pancreaticobiliary carcinomas had immunoreactivity to CK17. Our study reported 42.2% CK17 immunoreactivity which is similar to other studies who report 42–83% CK17 immunoreactivity in cases of metastatic pancreatobiliary carcinoma. This is in stark contrast to primary mucinous ovarian cancers that exhibit no CK17 immunoreactivity (Goldstein et al., 2001). Immunohistochemical expression of DPC4 (deleted pancreatic carcinoma 4) was retained in 98% of primary ovarian mucinous tumors compared with only 45% to 50% of bile duct carcinomas (Ji et al., 2002). However, it is important to note that DPC4 expression is variable along the biliary tract; in pancreatic carcinoma only approximately 55% of tumor exhibits loss of DPC4 expression, limiting its use. The value of CK17 staining in the differential diagnosis of metastatic pancreaticobiliary carcinomas and primary mucinous ovarian carcinomas deserves further investigation as this marker is not widely used.
To our knowledge our study is the largest review of pancreaticobiliary tumors. Our study is however limited by exclusion criteria which excluded non-English studies as reviewers primary language is English and studies which analyzed metastatic subtypes as a group versus individually. Additionally, the study was limited by the inability to examine original samples. Our data was derived from each respective study and was therefore limited to the published data.
Metastatic mucinous carcinomas to the ovary mimic their primary mucinous ovarian counterparts and their clinical and histopathological features overlap in many ways. We reviewed the literature and found many distinctive clinical and pathological features that may help clinicians and pathologists decipher primary versus metastatic lesions of the ovary. Of those highlighted, for metastatic tumors to the ovary, our review continues to endorse bilaterality, size <10 cm, and involvement of the ovarian hilum and adds other defining features including the presence of surface irregularity (implants and texture), infiltrative pattern, and identification of signet ring cells. Additionally, the use of immunostains (CK17, MUC1) could help in making the correct diagnosis.
Clinicians who encounter the patient who presents with a large ovarian mass or masses should maintain a high index of suspicion for the possibility of metastatic carcinoma to the ovary. Thorough abdominal exploration and tissue sampling at the time of surgery could identify an occult non-ovarian cancer that presents as a secondary ovarian neoplasm.
Conflict of interest
All authors declare no financial relationships or conflicts of interest
Funding
No funding was received for this research
Author contributions
Drs. Sarah A Ackroyd and Lauren Goetsch conceived the subject, methods, completed the data acquisition and produced the primary draft of the manuscript. Additionally, Dr. Sarah A Ackroyd performed the statistical analysis and interpreted the data. Drs. Jennifer Brown, Enrique Hernandez and Karen Houck provided assistance with study design, critique for academic content, analysis and interpretation of the data, and thorough review and revision of the manuscript. Dr. Congli Wang provided critique for academic content, analysis and interpretation of the data, focusing on the pathological and immunohistochemical components of the review, and thorough review and revision of the manuscript. All authors agree with the manuscript results and conclusions.
References
- Alvarado-Cabrero I., Rodríguez-Gómez A., Castelan-Pedraza J., Valencia-Cedillo R. Metastatic ovarian tumors-A clinicopathologic study of 150 cases. Anal. Quant. Cytol. Histol. 2013;35(5):241–248. [PubMed] [Google Scholar]
- Corr B.R., Mantia-Smaldone G., Cantor J., Livolsi V.A., Furth E., Chu C.S. Metastatic cholangiocarcinoma to the ovary: a case series. Int. J. Gynecol. Pathol. 2013;32(6):562–565. doi: 10.1097/PGP.0b013e3182782b9f. [DOI] [PubMed] [Google Scholar]
- Di Marco M., Vecchiarelli S., Macchini M., Pezzilli R., Santini D., Casadei R. Preoperative gemcitabine and oxaliplatin in a patient with ovarian metastasis from pancreatic cystadenocarcinoma. Case Rep. Gastroenterol. 2012;6(2):530–537. doi: 10.1159/000341513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A., De la Torre J., Castellvi J., Gil A., Lopez M. Ovarian metastases caused by cholangiocarcinoma: a rare Krukenberg's tumour simulating a primary neoplasm of the ovary: a two-case study. Arch. Gynecol. Obstet. 2004;270(4):281–284. doi: 10.1007/s00404-003-0508-7. [DOI] [PubMed] [Google Scholar]
- Goldstein N.S., Bassi D., Uzieblo A. WT1 is an integral component of an antibody panel to distinguish pancreaticobiliary and some ovarian epithelial neoplasms. Am. J. Clin. Pathol. 2001;116(2):246–252. doi: 10.1309/8X4T-35B7-7529-QE7X. [DOI] [PubMed] [Google Scholar]
- Guerriero S., Alcazar J.L., Pascual M.A., Ajossa S., Olartecoechea B., Hereter L. 2012. Preoperative Diagnosis of Metastatic Ovarian Cancer is Related to Origin of Primary Tumor; pp. 581–586. [DOI] [PubMed] [Google Scholar]
- Hibner M., Greenspan D. Ovarian metastases from the biliary tract, pancreas and liver carcinomas. CME J. Gynecol. Oncol. 2004;9(2):125–128. [Google Scholar]
- Jacobs I., Bast R.C., Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum. Reprod. 1989;4(1):1–12. doi: 10.1093/oxfordjournals.humrep.a136832. [DOI] [PubMed] [Google Scholar]
- Jain V., Gupta K., Kudva R., Rodrigues G.S. A case of ovarian metastasis of gall bladder carcinoma simulating primary ovarian neoplasm: diagnostic pitfalls and review of literature. Int. J. Gynecol. Cancer. 2006;16(Suppl. 1):319–321. doi: 10.1111/j.1525-1438.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- Jarvi K., Kelty C.J., Thomas W.E., Gillespie A. Bilateral ovarian metastases from carcinoma of the gallbladder. Gynecol. Oncol. 2006;103(1):361–362. doi: 10.1016/j.ygyno.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Ji H., Isacson C., Seidman J.D., Kurman R.J., Ronnett B.M. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int. J. Gynecol. Pathol. 2002;21(4):391–400. doi: 10.1097/00004347-200210000-00009. [DOI] [PubMed] [Google Scholar]
- Khangura D., Stefanovici C., Brahmania M., Moffatt D. Cholangiocarcinoma masquerading as an ovarian tumour. Can. J. Gastroenterol. 2013;27(2):72. doi: 10.1155/2013/159254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khunamornpong S., Suprasert P., Pojchamarnwiputh S., Na Chiangmai W., Settakorn J., Siriaunkgul S. Primary and metastatic mucinous adenocarcinomas of the ovary: evaluation of the diagnostic approach using tumor size and laterality. Gynecol. Oncol. 2006;101(1):152–157. doi: 10.1016/j.ygyno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Khunamornpong S., Siriaunkgul S., Suprasert P., Pojchamarnwiputh S., Na Chiangmai W., Young R.H. Intrahepatic cholangiocarcinoma metastatic to the ovary: a report of 16 cases of an underemphasized form of secondary tumor in the ovary that may mimic primary neoplasia. Am. J. Surg. Pathol. 2007;31(12):1788–1799. doi: 10.1097/PAS.0b013e3180674ded. [DOI] [PubMed] [Google Scholar]
- Khunamornpong S., Lerwill M.F., Siriaunkgul S., Suprasert P., Pojchamarnwiputh S., Chiangmai W.N. Carcinoma of extrahepatic bile ducts and gallbladder metastatic to the ovary: a report of 16 cases. Int. J. Gynecol. Pathol. 2008;27(3):366–379. doi: 10.1097/PGP.0b013e31815d6903. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Kim C.D., Lee H.S., Hyun J.H., Kim Y.S., Kim I.S. Bilateral ovarian carcinoma metastatic from the ampulla of Vater: a rare Krukenberg tumor. J. Korean Med. Sci. 1999;14(2):220–222. doi: 10.3346/jkms.1999.14.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa T., Young R.H., Scully R.E. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am. J. Surg. Pathol. 2006;30(3):277–299. doi: 10.1097/01.pas.0000190787.85024.cb. [DOI] [PubMed] [Google Scholar]
- Kumar Y., Chahal A., Garg M., Bhutani A. Occult gallbladder carcinoma presenting as a primary ovarian tumor in two women: two case reports and a review of the literature. J. Med. Case Rep. 2010;4:202. doi: 10.1186/1752-1947-4-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt S., Ulukuş Ç., Kazaz S.N., Astarcıoğlu İ. Bilateral ovarian metastasis of a Klatskin tumor: a rare case. Turk. J. Obstet. Gynecol. 2016;13(4):215–217. doi: 10.4274/tjod.40222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashgari M., Behmaram B., Hoffman J.S., Garcia J. Primary biliary carcinoma with metastasis to the ovary. Gynecol. Oncol. 1992;47(2):272–274. doi: 10.1016/0090-8258(92)90120-8. [DOI] [PubMed] [Google Scholar]
- Lee K.R., Young R.H. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am. J. Surg. Pathol. 2003;27(3):281–292. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Lee S., Suh S., Choi Y. Bilateral ovarian metastasis from common bile duct carcinoma with choledochal cyst masquerading as a primary ovarian neoplasm. HPB. 2015;17:193–194. [Google Scholar]
- Low J.J., Chew S.H., Chew S.P., Han C.C., Tay E.H., Ho T.H. A rare case of metastatic ovarian carcinoma originating from primary intrahepatic cholangiocarcinoma. Case report. Eur. J. Gynaecol. Oncol. 2003;24(1):85–88. [PubMed] [Google Scholar]
- Lyra T.C.B., Morbeck F., Guimaraes M.D., Franco L.F.S., Marchiori E., Siqueira G. Rare diagnosis of Krukenberg tumor: intrahepatic Cholangiocarcinoma. Open J. Med. Imaging. 2015;5:159–164. [Google Scholar]
- Majumdar K., D S, S K, A R, R G Papillary adenocarcinoma gallbladder with simultaneously detected bilateral ovarian metastasis: a case report. Int. J. Gynecol. Obstet. 2007;9(1) [Google Scholar]
- McCluggage W.G., Young R.H. Primary ovarian mucinous tumors with signet ring cells: report of 3 cases with discussion of so-called primary Krukenberg tumor. Am. J. Surg. Pathol. 2008;32(9):1373–1379. doi: 10.1097/PAS.0b013e31816b18c1. [DOI] [PubMed] [Google Scholar]
- Meriden Z., Yemelyanova A.V., Vang R., Ronnett B.M. Ovarian metastases of pancreaticobiliary tract adenocarcinomas: analysis of 35 cases, with emphasis on the ability of metastases to simulate primary ovarian mucinous tumors. Am. J. Surg. Pathol. 2011;35(2):276–288. doi: 10.1097/PAS.0b013e31820508d0. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone A.M.H.N., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., editors. SEER Cancer Statistics Review, 1975–2015. National Cancer Institute; Bethesda, MD: 2015. [Google Scholar]
- Okamoto T., Matsumura N., Mandai M., Oura T., Yamanishi Y., Horiuchi A. Distinguishing primary from secondary mucinous ovarian tumors: an algorithm using the novel marker DPEP1. Mod. Pathol. 2011;24(2):267–276. doi: 10.1038/modpathol.2010.204. [DOI] [PubMed] [Google Scholar]
- Park C.K., Kim H.S. Clinicopathological characteristics of ovarian metastasis from colorectal and Pancreatobiliary carcinomas mimicking primary ovarian mucinous tumor. Anticancer Res. 2018;38(9):5465–5473. doi: 10.21873/anticanres.12879. [DOI] [PubMed] [Google Scholar]
- Petru E., Pickel H., Heydarfadai M., Lahousen M., Haas J., Schaider H. Nongenital cancers metastatic to the ovary. Gynecol. Oncol. 1992;44(1):83–86. doi: 10.1016/0090-8258(92)90017-d. [DOI] [PubMed] [Google Scholar]
- Pleskow D.K., Berger H.J., Gyves J., Allen E., McLean A., Podolsky D.K. Evaluation of a serologic marker, CA19-9, in the diagnosis of pancreatic cancer. Ann. Intern. Med. 1989;110(9):704–709. doi: 10.7326/0003-4819-110-9-704. [DOI] [PubMed] [Google Scholar]
- Seidman J.D., Kurman R.J., Ronnett B.M. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am. J. Surg. Pathol. 2003;27(7):985–993. doi: 10.1097/00000478-200307000-00014. [DOI] [PubMed] [Google Scholar]
- Sharma P., Jaffe P.E., Bhattacharyya A. Metastatic cholangiocarcinoma presenting as ovarian cancer: a rare Krukenberg tumor. Am. J. Gastroenterol. 1997;92(3):531–533. [PubMed] [Google Scholar]
- Simons M., Bolhuis T., De Haan A.F., Bruggink A.H., Bulten J., Massuger L.F. A novel algorithm for better distinction of primary mucinous ovarian carcinomas and mucinous carcinomas metastatic to the ovary. Virchows Arch. 2019;474(3):289–296. doi: 10.1007/s00428-018-2504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.D., Tsai C.C., Hsiao S.M., Wei M.C., Wang K.C., Wang P.H. Primary gallbladder carcinoma presenting as advanced-stage ovarian cancer. Taiwan J. Obstet. Gynecol. 2012;51(3):443–445. doi: 10.1016/j.tjog.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Taranto A.J., Lourie R., Lau W.F. Ovarian vascular pedicle sign in ovarian metastasis arising from gall bladder carcinoma. Australas. Radiol. 2006;50(5):504–506. doi: 10.1111/j.1440-1673.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Testa A.C., Ferrandina G., Timmerman D., Savelli L., Ludovisi M., Van Holsbeke C. Imaging in gynecological disease (1): ultrasound features of metastases in the ovaries differ depending on the origin of the primary tumor. Ultrasound Obstet. Gynecol. 2007;29(5):505–511. doi: 10.1002/uog.4020. [DOI] [PubMed] [Google Scholar]
- Tot T. Adenocarcinomas metastatic to the liver: the value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer. 1999;85(1):171–177. doi: 10.1002/(SICI)1097-0142(19990101)85:1<171::AID-CNCR24>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Vang R., Gown A.M., Barry T.S., Wheeler D.T., Yemelyanova A., Seidman J.D. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am. J. Surg. Pathol. 2006;30(9):1130–1139. doi: 10.1097/01.pas.0000213281.43036.bb. [DOI] [PubMed] [Google Scholar]
- Vang R., Gown A.M., Wu L.S., Barry T.S., Wheeler D.T., Yemelyanova A. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod. Pathol. 2006;19(11):1421–1428. doi: 10.1038/modpathol.3800698. [DOI] [PubMed] [Google Scholar]
- Wang J., El-Bahrawy M.A. Expression profile of mucins in ovarian mucinous tumors: distinguishing primary ovarian from metastatic tumors. Int. J. Gynecol. Pathol. 2014;33(2):166–175. doi: 10.1097/PGP.0b013e318288b384. [DOI] [PubMed] [Google Scholar]
- Yemelyanova A.V., Vang R., Judson K., Wu L.S., Ronnett B.M. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classification. Am. J. Surg. Pathol. 2008;32(1):128–138. doi: 10.1097/PAS.0b013e3180690d2d. [DOI] [PubMed] [Google Scholar]
- Yin B.W., Lloyd K.O. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J. Biol. Chem. 2001;276(29):27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- Young R.H. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary: part I. Historical perspective, general principles, mucinous tumors including the krukenberg tumor. Adv. Anat. Pathol. 2006;13(5):205–227. doi: 10.1097/01.pap.0000213038.85704.e4. [DOI] [PubMed] [Google Scholar]
- Young R.H. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part II. Adv. Anat. Pathol. 2007;14(3):149–177. doi: 10.1097/PAP.0b013e3180504abf. [DOI] [PubMed] [Google Scholar]
- Young R.H., Hart W.R. Metastases from carcinomas of the pancreas simulating primary mucinous tumors of the ovary. A report of seven cases. Am. J. Surg. Pathol. 1989;13(9):748–756. doi: 10.1097/00000478-198909000-00004. [DOI] [PubMed] [Google Scholar]
- Young R.H., Scully R.E. Ovarian metastases from carcinoma of the gallbladder and extrahepatic bile ducts simulating primary tumors of the ovary. A report of six cases. Int. J. Gynecol. Pathol. 1990;9(1):60–72. doi: 10.1097/00004347-199001000-00006. [DOI] [PubMed] [Google Scholar]