Abstract
Accumulating evidence suggests that memory is impaired in posterior cortical atrophy (PCA), alongside the early and defining visual disorder. The posterior parietal cortex is a key region of pathology in PCA and memory impairment may be the result of dysfunction of parietally dependent network function rather than the medial temporal lobe dependent dysfunction that defines the storage deficits in typical Alzheimer's disease.
We assessed episodic memory performance and network function in16 PCA patients and 19 healthy controls who underwent structural and resting-state functional MRI and neuropsychological testing. Memory was assessed using the Free and Cued Selective Reminding Test (FCSRT), a sensitive test of episodic memory storage and retrieval. We examined correlations between memory performance and functional connectivity in the dorsal attention (DAN) and default mode network (DMN).
Immediate recall on the FCSRT was relatively preserved in PCA patients. Total recall performance was impaired in patients relative to healthy controls and performance benefitted from retrieval cues. In patients only, disrupted connectivity in the DAN, but not the DMN, was associated with total recall.
Memory impairment may arise from disruption to the dorsal attention network, subserved by the dorsal posterior parietal cortex, a key region of pathology in PCA, rather than classic medial temporal lobe memory circuitry.We propose that functional dysconnectivity in attentional circuits underpins memory impairment in PCA.
Keywords: Posterior cortical atrophy, Episodic memory, Default mode network, Resting-state
Highlights
-
•
Memory impairment is a feature of posterior cortical atrophy (PCA) alongside higher visual impairment.
-
•
Retrieval cues ameliorate memory recall impairments in PCA.
-
•
Memory performance in PCA is associated with dysfunction in the dorsal attention network.
-
•
There should be wider recognition of memory impairment as an early feature of PCA.
1. Introduction
The hallmark of posterior cortical atrophy (PCA) is progressive impairment in higher visual function and prominent atrophy of parieto-occipital and temporo-occipital posterior cortices that gives the syndrome its name (Crutch et al., 2017; Tang-Wai et al., 2004). The underlying pathology, determined post-mortem or via in vivo biomarkers, is most often Alzheimer's disease (AD) (Crutch et al., 2017; Teichmann et al., 2017). Though defined by the salient visual impairment, a recent consensus framework highlights the need for clarity on episodic memory impairments(Crutch et al., 2017). Such impairments are noted in the majority of cases at initial clinical assessment (Crutch et al., 2017), and we have previously provided objective evidence for the frequency of episodic memory impairments early in PCA (Ahmed et al., 2016; Ahmed et al., 2018). Our study showed deficits in encoding and retrieval of verbal information at clinical presentation, equivalent to those seen in typical amnestic AD (Ahmed et al., 2016; Ahmed et al., 2018). In support, a recent examination of verbal episodic memory function found evidence of impairment in 65% of PCA patients (Putcha et al., 2018). Impairment in encoding and delayed recall was associated with verbal executive dysfunction, suggesting impaired verbal episodic memory that is different from the storage deficits that characterize typical AD.
Early in PCA, there is typically relative preservation of the medial temporal lobes, traditionally considered the central hub for human memory processing. The neuroanatomical basis of memory impairment in PCA therefore remains to be clarified. We have previously shown that encoding and retrieval performance on the Rey auditory verbal learning task (RAVLT) is associated with volume of the posterior parietal cortex (PPC) in PCA(Ahmed et al., 2018), rather than with volume of the medial temporal lobes as is characteristically seen in typical AD (although see Wolk & Dickerson (2011) (Wolk et al., 2011) for evidence of regions extending beyond the MTL (including lateral parietal cortex) implicated in verbal episodic memory in AD). The posterior parietal cortex encompasses the superior parietal lobule (SPL), intraparietal sulcus (IPS) and the intraparietal lobule (IPL)(Davidson et al., 2008). This region can be further subdivided along a dorsal (SPL and IPS) and ventral (supramarginal and angular gyri) axis (Sestieri et al., 2017). The posterior parietal cortex is classically associated with attention, visuospatial and sensorimotor function, but there is now a substantial body of evidence associating the region with episodic memory (Cabeza et al., 2011; Sestieri et al., 2010; Sestieri et al., 2017). According to the Attention to Memory (AtoM) model (Cabeza et al., 2011), the dorsal PPC is associated with top-down attention, or voluntary orientation, guided by retrieval goals, while ventral PPC is associated with bottom up attention to memory. The dorsal PPC regions shows activation in association with verbal episodic memory tasks requiring participants to orient to internally retrieved memories (Cabeza et al., 2011). Co-localisation of memory and attention processing in the dorsal PPC, a key site of atrophy and hypometabolism in PCA, may therefore underlie the memory retrieval deficits seen in PCA. These memory retrieval deficits are unlike those associated with medial temporal lobe damage where impairment reflects pure storage failure.
Regions of the dorsal PPC and the medial temporal lobes are prominent nodes in distinct but overlapping functional networks associated with memory and attention processing (Sestieri et al., 2017). The SPL and IPS central to the dorsal attention network (DAN), a network most closely associated with externally directed attention (Fox et al., 2006; Vossel et al., 2014). The major hubs of this bilateral frontoparietal network are the intraparietal sulcus (IPS) and the frontal eye fields (FEF) (Vossel et al., 2014), and the network extends to the midline supplementary motor area (SMA), pre-SMA and middle temporal/medial superior temporal (MT+) (Fox et al., 2006). The major hubs of the DMN are the posterior cingulate, ventral and dorsal medial prefrontal cortex and nodes include the medial precuneus and the medial temporal lobes (Raichle, 2015). The DMN has been repeatedly implicated in typical AD, because of its strong association with memory function, and because it is the key network to show hypometabolism and dysfunction in the prodromal phase and throughout the course of the disease (Jones et al., 2015; Seeley et al., 2009; Zhou and Seeley, 2014). The cascading network failure theory posits that functional network disruption interacts with local pathophysiological changes resulting in systems-level disruptions and syndrome specific cognitive impairments in the AD spectrum (Jones et al., 2015). Depending on the location of the pathophysiology, the functional networks affected, and the cognitive consequences of this, will differ (Jones et al., 2015). Pathophysiological changes, including atrophy and hypometabolism, are prominent in the parietal lobes in PCA. We would therefore expect to see altered dorsal PPC dependent DAN function and relatively preserved DMN function in PCA.
Assessing memory performance in PCA requires a sensitive test that is able to stringently examine sublevels of memory processing and distinguish between profiles of memory impairment. The Free and Cued Selective Reminding Test (FCSRT) is recognized as a highly reliable tool for such assessment and is recommended by the International Working Group for AD diagnostic criteria (Dubois et al., 2014; Dubois et al., 2007; Grober et al., 2000; Teichmann et al., 2017). The test has recently shown high sensitivity (100%) and good specificity (75%) in distinguishing AD from other neurodegenerative dementias, including PCA (Teichmann et al., 2017). The FCSRT includes semantic category cues that serve to control for attentional problems at the encoding stage and help to distinguish between retrieval and storage deficits (Teichmann et al., 2017). The FCSRT is therefore an ideal test to distinguish the medial temporal and DMN dependent storage deficits seen in typical AD, from what we hypothesise to be DAN driven attention to memory deficits underlying memory dysfunction in PCA. Memory storage problems seen in typical AD are characterized by low total recall in the FCSRT and cue insensitivity (i.e. no improvement in performance with retrieval cues). We recently showed that PCA patients performed better on immediate recall than typical AD patients but that delayed recall was impaired (Ahmed et al., 2018). Based on these findings, we expect relatively preserved encoding on the FCSRT in PCA. In addition, we predict that PCA patients will benefit from memory cues, as evidence that their memory impairment is not the result of storage failures as seen in typical AD.
The aim of this study was to investigate the neurocognitive basis of memory deficits in PCA. We predicted that patients would show reduced episodic memory performance compared to healthy controls as further evidence of memory impairements as an often overlooked feature of PCA. Specifically, we hypothesised that reduced overall recall performance would not be driven by deficits at encoding or the type of storage failures typically seen in AD, but by deficits in directing attention to memory representations. This would be evident if encoding performance was preserved and if recall cues benefited free recall performance. We further predicted that memory impairment in PCA would be associated with the DAN, whose prominent node, the IPS, is within the primary site of pathology in PCA. We hypothesised that this prominent node, the IPS, would show hypoconnectivity to the rest of the network in PCA and that hypoconnectivity would be associated with task performace. In contrast we predicted relatively preserved DMN function with no association between task performance and DMN connectivity.
2. Methods
2.1. Participants
PCA patients (n = 18) were recruited through the Oxford Cognitive Disorders Clinic, Oxford, UK. Healthy controls (n = 21) were recruited via poster advertisements in local community centres. All participants provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the National Research Ethics Service South Central - Hampshire B and Oxford C. PCA diagnoses were made by senior behavioural neurologists (CB, MH, ST) and neuropsychologists (IB and SA) and all patients fulfilled the clinical, neuropsychological and brain imaging consensus criteria for PCA (Crutch et al., 2017; Tang-Wai et al., 2004). Healthy control participants had no history of psychiatric illness, head injury or cerebrovascular disease. Control participants declared no prescribed medications affecting cognition and scored within the normal range (all control participants >88/100) on the Addenbrooke's Cognitive Examination-III (Hsieh et al., 2013). PCA patients and healthy controls were matched on age and years of education (see Table 1). PCA patients were, on average, <4 years from symptom onset.
Table 1.
Demographic and clinical characteristics of control and patient groups. Standard deviation given in brackets.
| Controls | PCA | Group comparison | |
|---|---|---|---|
| N | 21 | 18 | |
| Age (yrs) | 63 (6.1) | 64 (6.8) | 0.48 |
| Education (yrs) | 14.4 (2.1) | 13.6 (2.0) | 0.24 |
| Gender (m:f) | 9:12 | 9:9 | |
| Symptom duration (yrs) | – | 3.8 (1.9) | – |
| Addenbrooke's Cognitive Examination III (100) | 96 (4.3) | 55 (15.7) | 0.01⁎ |
| Visual Object and Space Perception Battery | |||
| Dot count (10) | 10 (0) | 3.8 (3.7) | a |
| Position discrimination (20) | 19.6 (1.1) | 13.5 (3.8) | 0.001⁎ |
| Cube analysis (10) | 9.5 (1.3) | 1.8 (2.3) | 0.001⁎ |
| Rey complex figure test | |||
| Copy (18) | 17.6 (0.7) | 1.8 (3.1) | 0.001⁎ |
| Immediate recall (18) | 10.7 (3.7) | 0.7 (1.0) | 0.001⁎ |
| Delayed Recall (18) | 10.5 (3.4) | 0.2 (0.4) | 0.001⁎ |
| FCSRT-IR | |||
| Immediate recall (16) | 14.8 (2.9) | 13.8 (2.5) | 0.29 |
| Total Recall (48) | 47.7 (0.9) | 41.6 (5.3) | 0.01⁎ |
| Free recall (48) | 32.5 (4.6) | 20.4 (8.0) | 0.01⁎ |
| Cued Recall (48) | 15.1 (4.3) | 21.2 (4.7) | 0.01⁎ |
| Cue Sensitivity (%) | 98 (4.6) | 80 (14.8) | 0.01⁎ |
| Imaging Subset and Matched controls | |||
| N | 19 | 16 | |
| Age (yrs) | 75 (8.1) | 64 (6.2) | 0.01⁎ |
| Education (yrs) | 15.1 (3.5) | 13.7 (2.1) | 0.14 |
| Gender (m:f) | 10:9 | 7:9 | |
| Symptom duration (yrs) | – | 3.8 (2.3) | |
Total scores achievable on neuropsychological tests, where applicable, in brackets in right column. Abbreviations: PCA-Posterior cortical atrophy; RAVLT-Rey auditory verbal learning task; FCSRT-IR-Free and cued selective reminding test with immediate recall.
Unable to estimate due to ceiling performance and no variance in controls.
Significant group differences estimated by Welch's t-tests at p < .01.
The imaging analysis was conducted in a subset (n = 16) of the PCA group (one patient was excluded due to a metallic artefact and one patient did not agree to be scanned) and a separate group of healthy control subjects (n = 19), who underwent scanning on the same MRI scanner with identical sequences(Zamboni et al., 2013). The imaging control group was matched for years of education but were older than the PCA patients (see Table 1). All imaging analyses have therefore been corrected for age.
2.2. Background cognitive assessment
Standardised neuropsychological tests were administered to evaluate patient and control participant function in global cognition (Addenbrooke's Cognitive Examination-III, (Hsieh et al., 2013)) and visuospatial function (Dot counting, position discrimination and cube analysis from the Visual Object and Space Perception (VOSP; (Warrington and James, 1991) and the Rey-Osterrieth Complex figure, (Corwin and Bylsma, 1993)).
2.3. Memory assessment
Patients and controls were administered the Free and Cued Selective Reminding Test with Immediate recall (FCSRT-IR)(Grober and Buschke, 1987). Participants learned a list of 16 words presented with an oral semantic category cue. To ensure retrieval deficits were not the result of deficient encoding of items into memory, the learning phase incorporated selective reminding of words not spontaneously retrieved. Recall was then assessed in 2 min of spontaneous free recall followed by selective reminding of words not retrieved using the semantic category cues. There were three recall trials in total, each preceded by backwards counting as short-term memory interference. The free recall score is the total number of items spontaneously recalled across the three trials (maximum 48). The total recall score is the total number of freely recalled items plus the number of items recalled after cueing across the three trials (maximum 48). Finally, cue sensitivity is calculated across all three trials (total recall-free recall/48-free recall; Teichmann et al., 2017). Group differences were tested with Welch's t-tests, with the significance threshold of p < .05, Bonferonni corrected for multiple comparisons.
2.4. Image acquisition
Patient and healthy control images were acquired on a Siemens 3T Tim Trio scanner (Erlangen, Germany) with a 32-channel head coil. A high-resolution structural image was acquired with 1 mm isotropic voxels, using a 2040 ms repetition time (TR), 4.7 ms echo time (TE), 8o flip angle, 100% field of view in the phase direction, and 192 × 192 acquisition matrix. Echoplanar images were acquired in the resting-state with eyes open. 180 volumes were acquired in 6 min with 3 mm isotropic voxels, 2000 ms TR, 28 ms TE, 89° flip angle and 100% field of view in the phase direction and 64 × 64 acquisition matrix.
2.5. Image preprocessing
Patient and healthy control functional resting-state images were preprocessed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Structural images were visually inspected for artefacts. One patient was excluded at this stage due to a large artefact in their structural scans. Images were slice-time corrected, before realignment, using the middle slice as the reference. Images were then realigned using least squares six parameter, rigid body spatial transformation and seventh degree B-Spline interpolation to estimate and correct subject motion. A mean image was used as the reference image. Functional images were coregistered to the structural image using rigid-body transformation and normalized mutual information cost function. Images were segmented and normalized to the MNI-152 template using the Clinical Toolbox(Rorden et al., 2012), which implements SPM's undified non-linear normalization and segmentation. Segmentations of grey matter, white matter and cerebrospinal fluid were manually inspected for quality assurance. Functional images were smoothed with an 8 mm full width-half maximum Gaussian kernel.
Preprocessed images were imported into the Conn Toolbox version 17.e (Whitfield-Gabrieli and Nieto-Castanon, 2012), to further clean images and reduce the influence of motion and noise from non-grey matter tissue on connectivity estimates. De-spiking was used to reduce the influence of outlier scans. Signal from non-grey matter tissue (CSF and white matter) segmentations (default 5 dimensions each) and regressors based on motion estimation (default 12 regressors) were used for Component based noise correction (Comcor) (Whitfield-Gabrieli and Nieto-Castanon, 2012; Muschelli et al., 2014; Behzadi et al., 2007). The outputs of motion scrubbing were included as regressors of no interest to further control for noise associated with head motion (Power et al., 2012). Histograms displaying voxel to voxel connectivity before and after the removal of sources of noise (motion and non-GM tissue signal) were visually inspected to check the output of the denoising pipeline and identify outlier subjects. The inbuilt Simult function was used to perform simultaneous nuisance regression and band-pass filtering, as a better means of controlling for non-neural BOLD fluctuations than band-pass filtering the signal of interest alone(Hallquist et al., 2013). Bandpass filtering retained signal between 0.01 and 0.1 Hz.
2.6. Resting-state connectivity analysis
Seed-based connectivity analysis was conducted in the Conn Toolbox version 17.e. Seeds for the functional connectivity analysis were created as 4 mm spheres around coordinates of peak activation reported in previous literature using MARSBAR toolbox (Brett et al., 2002). The dorsal intraparietal sulcus ROI represents the peak of activation from a meta-analysis of attention tasks (MNI coordinates 24.24,-16.01,54.66) and reliably produces the dorsal attention network (DAN; Fox et al., 2006). The posterior cingulate seed region was created from coordinates reported in a landmark DMN paper, which is known to reliably produce the DMN (Greicius et al., 2003; MNI coordinates −2.03 -53.9 26.6) (Fig. 1).
Fig. 1.
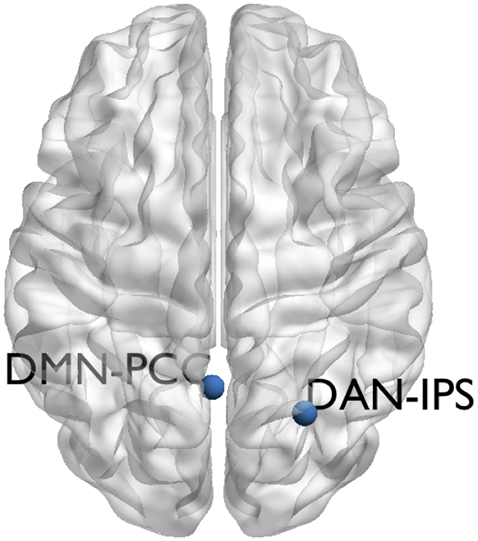
Location of seed regions of interest shown on a semi-inflated brain in axial orientation. DMN-PCC Default mode network posterior cingulate cortex seed. DAN-IPS Dorsal attention network intraparietal sulcus seed.
The average time course of signal fluctuation in seed ROIs was correlated with the time course of signal fluctuation in all other voxels within an implicit, subject-specific grey matter mask using bivariate correlation. Subject level correlation maps were Fisher normalized. Normalized age and grey matter volume were entered as covariates into a second level random effects mass univariate model for each network and for each group. Total grey matter volume was estimated using the default cortical segmentation and reconstruction pipeline in Freesurfer (http://surfer.nmr.mgh.harvard.edu/).
Group level contrasts of the PCA group compared to healthy controls were used to investigate regions of disrupted connectivity in the DAN and DMN, correcting for age and total grey matter volume. Standard parametric inference was used to obtain p-values and a cluster-level false discovery rate threshold for significance was set at p < .05 for all main effects and contrasts. Regions of disrupted connectivity were correlated with a within-group normalized FCSRT-IR total score using Pearson's correlation. We examined the regions that showed disruption in connectivity in the PCA group with the reasoning that this would be more sensitive than examining the network as a whole. We correlated connectivity with total recall sub-score (free recall + cued recall) as it has previously been shown to be most discriminative to typical AD (Teichmann et al., 2017). Normalized correlation coefficients were extracted from masks around the regions of peak connectivity for the DAN and DMN. This analysis was restricted to the PCA group who had both FCSRT-IR scores and resting-state imaging.
3. Results
3.1. Background cognitive assessment
PCA patients were impaired on the ACE-III and all visuospatial tests compared to controls, in keeping with the clinical phenotype of this syndrome(Crutch et al., 2017; Tang-Wai et al., 2004) (Table 1).
3.2. Memory performance
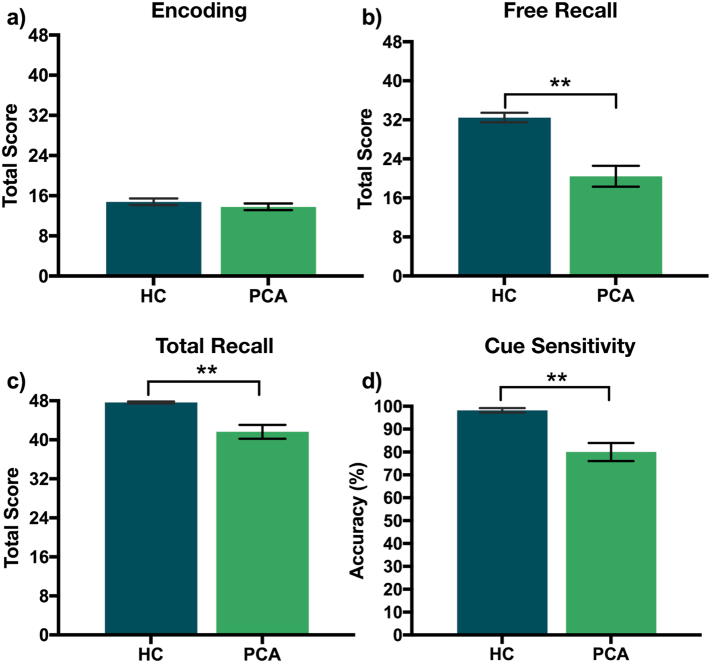
No significant difference in immediate recall (Fig. 2a) between patients and controls (t(30.20) = 1.07, p = .29, Cohen's d = 0.37) indicated encoding of memory items was equivalent between groups. PCA patients were significantly impaired in free recall compared to healthy controls (t(18.61) = 5.09, p < .001, Cohen's d = 1.95, Fig. 2b). Total recall performance was impaired in PCA patients compared to healthy controls (t(13.52) = 4.25, p < .001, Cohen's d = 1.79, Fig. 2c). Cue sensitivity was at ceiling in healthy controls and, although lower in PCA patients (t(14.65) = 4.46, p < .001, Cohen's d = 1.83, Fig. 2d), still indicated a strong sensitivity to cues: ~80% of non-recalled stimuli were remembered by PCA patients when cued. In accordance with this, healthy controls showed significantly lower mean cued recall of items as a proportion of total recall than did the PCA patients (t(19.89) = −4.63, p < .001, Cohen's d = −1.67).
Fig. 2.
Performance in the Free and Cued Selective Reminding test with immediate recall (FCSRT-IR) for healthy controls (HC) and patients with Posterior Cortical Atrophy (PCA). (a) Encoding – Total score for immediate recall. (b) Free Recall: Total score for three trials of free recall (c)Total Recall – (free recall +recall after selective reminding) (d) Cue Sensitivity – cued recall/(48-number of free recall)⁎100; ⁎⁎indicates significance to p < .001.
3.3. Functional connectivity of DMN and DAN
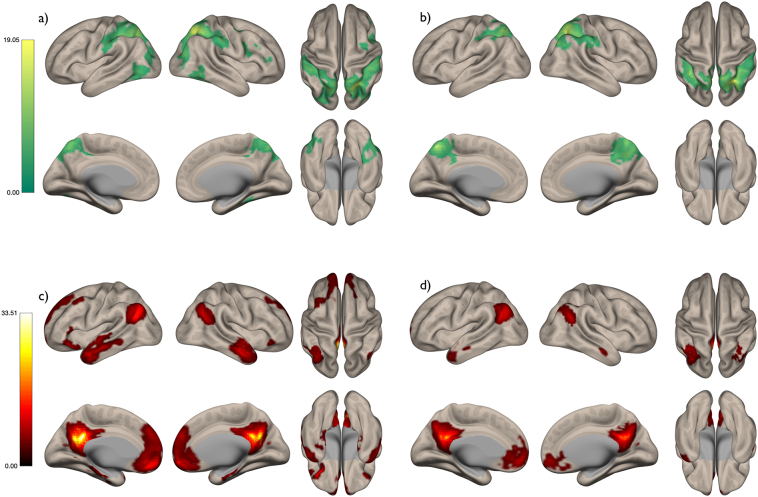
The IPS and PCC seed regions of interest produced canonical default mode and dorsal attention networks in the healthy controls (Fig. 3a and c). In PCA patients (Fig. 3b and d) both networks showed a highly similar spatial profile, with similar peak regions of connectivity to the seed regions, to the healthy controls (Fig. 3a and c). However, both networks were reduced in spatial extent in PCA patients relative to healthy controls. Direct contrasts of the HC and PCA patients provided evidence of hypo-connectivity of the DAN in PCA (Fig. 4a) in the lateral occipital cortex and the insula. In the DMN, hypo-connectivity was more restricted and only evident bilaterally in the middle temporal gyrus.
Fig. 3.
Dorsal attention network in (a) healthy controls and (b) PCA patients. Default mode network in (c) healthy controls and (d) PCA patients. FDR corrected p < .05; Colour bars represent t-values.
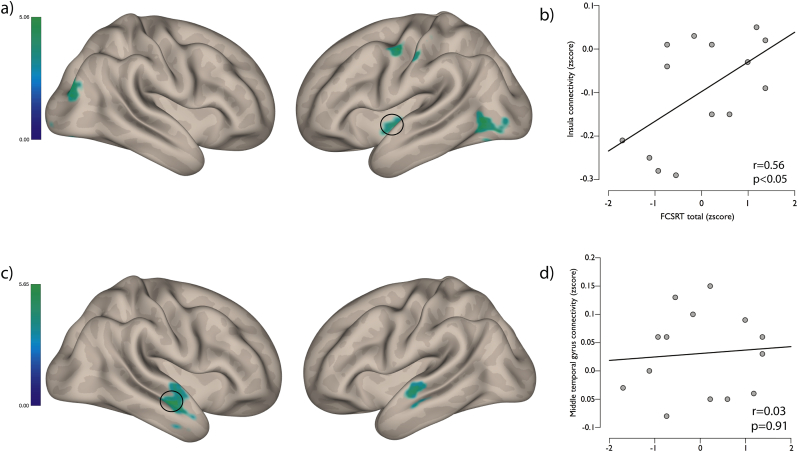
Fig. 4.
(a) Regions of significant hypo-connectivity in the dorsal attention network; FDR corrected p < .05. (b) Correlation between connectivity in the insula and FCSRT total score in PCA patients. (c) Regions of significant hypo-connectivity in the default mode network; FDR corrected p < .05. (d) Correlation between connectivity in the middle temporal gyrus and FCSRT total score in PCA patients.
The peak region of hypo-connectivity of the DAN in PCA patients correlated with free recall and total recall performace on the FCSRT-IR (see Table 2). In contrast, the peak region of the hypo-connectivity in the default mode network in the PCA group showed no significant correlation with any stages of the FCSRT-IR (see Table 2).
Table 2.
Pearson's correlations and pvalues between peak regions of hypoconnectivity in PCA patients and healthy controls in the dorsal attention network and default mode network.
| Dorsal attention network peak (insula) |
Default mode network peak (MTG) |
|||
|---|---|---|---|---|
| Pearson's r | p-value | Pearson's r | p-value | |
| Encoding | 0.48 | .10 | 0.29 | .34 |
| Free recall | 0.56 | .05⁎ | 0.11 | .71 |
| Total recall | 0.58 | .04⁎ | 0.11 | .71 |
| Cue sensitivity | 0.50 | .09 | 0.04 | .89 |
Significant to p < .0.
4. Discussion
We have previously reported memory impairments early in PCA, that are associated with posterior parietal, but not medial temporal lobe atrophy(Ahmed et al., 2018). Here, we extend this finding to show retrieval deficits are also associated with function in the dorsal PPC dependent dorsal attention network. This lends further weight to our hypothesis that deficits in attention to memory, coordinated by the dorsal PPC, may underpin retrieval deficits in PCA.
Using the FCSRT, we showed a memory performance profile for PCA that is different from the profile of typical AD (Auriacombe et al., 2010; Reitz et al., 2011; Sarazin et al., 2007; Teichmann et al., 2017). We observed equivalent performance in encoding between healthy controls and PCA patients, with no deficit in immediate recall of items. Cue sensitivity as well as the proportion of recalled trials that required a cue, indicated a benefit to memory performance from retrieval cues in PCA, boosting overall recall. The profile of memory performance in typical AD is characterized by low total recall score and insensitivity to cues in the FCSRT, suggestive of pure storage failures as a result of medial temporal lobe dysfunction. In a sample of 200 CE patients, Teichmann et al., 2017) report a mean total recall score of 27.4 (SD 1.1) out of a maximum 48, and a mean cue sensitivity of 46.4% (SD 2.2). By comparison, their PCA group had a mean total recall of 41.3 (SD 3) and a cue sensitivity of 78.3% (SD 6.5). Our results are strikingly similar to those reported by Teichmann et al. (2017). This replication of the Teichmann et al. (2017) data confirms the utility of the FCSRT in detecting a distinct profile of retrieval deficits in PCA, one in which cueing leads to a marked improvement in performance and which is consistent with deficits being underpinned by attentional rather than memory storage problems.
Seeking the neurocognitive mechanisms underlying the profile of memory retrieval in PCA, we examined default mode and dorsal attention network connectivity. In PCA patients, both the DAN and DMN showed a highly similar spatial profile to healthy controls, with similar peak regions of connectivity. However, both networks were reduced in the spatial extent in PCA. This may reflect underlying atrophy, especially in the DAN, whose anatomical profile overlaps with key regions of atrophy in PCA(Ahmed et al., 2018).
We hypothesised altered DAN function in PCA because the most prominent node of the DAN is situated in the dorsal PPC, where memory and attention co-localise(Cabeza et al., 2011) and where atrophy in PCA peaks(Ahmed et al., 2018). Rather than being specific to the DAN, we found hypoconnectivity of both the DAN and DMN in PCA. Hypoconnectivity is a common finding in typical AD (Jones et al., 2015; Greicius et al., 2003; Zhou et al., 2010) and is thought to reflect the spread of Alzheimer's pathology within interconnected and highly metabolically active hubs of the DAN and DMN, which are disproportionately vulnerable to neurodegenerative pathology (Fornito et al., 2015; Seeley et al., 2009; Zhou et al., 2012). There is mounting evidence that AD should be conceptualized as being on a temporal and phenotypic spectrum, and the network disruptions that result depend on the specific presentation of the disease. Jones et al. (2015) suggest that the variable clinical syndromes of AD, including PCA, are the result of an interaction between brain network failures and pre-existing molecular pathology. Dysfunction in one region leads to network failure that interacts with and likely initiates, or at least aggravates, pathophysiological changes. Viewed from this perspective, it is not surprising that hypoconnectivity was evident in both the DAN and DMN in PCA. As a syndrome on the AD spectrum, PCA is likely to have a different profile of DAN and DMN network dysfunction compared to typical AD, given a different pattern of pathological atrophy and memory impairment. To investigate the relationship between DAN and DMN hypoconnectivity and memory performance, we correlated peak regions of hypoconnectivity with FCSRT performance.
We showed hypoconnectivity between the insula and the DAN that is associated with free and total recall performance. Hypoconnectivity was not associated with immediate recall performance, in line with our finding that encoding in PCA is relatively spared. Our findings fit well with the AtoM model of parietal contributions to episodic memory(Cabeza et al., 2011) in which dorsal PPC mediates top-down attention to memory representations. Indeed, in highly similar verbal episodic cueing tasks, dorsal PPC activation appears to be specific to the orientation of memory to stored representations(Cabeza et al., 2011; Sestieri et al., 2017).In contrast, the peak of hypoconnectivity of the DMN did not show a relationship with task performance. In a meta-analysis of episodic memory tasks, Kim (2010) showed dissociable roles for the DMN and DAN, with the latter implicated in the attentional and executive components of episodic retrieval. This underscores our finding that, although both networks are involved in episodic memory, it is the attentional component coordinated by the DAN that is impacted upon in PCA.
Although not considered part of the canonical DAN, meta-analysis of visuo-spatial tasks has shown the insula as a key region to co-activate with the dorsal attention network (Vossel et al., 2014). This is likely because the insula is the major hub of the fronto-parietal network which mediates goal-directed attention (Spreng et al., 2013). The DAN and DMN are functionally competitive and independent networks and their interaction is mediated by fronto-parietal network hubs such as the insula (Spreng et al., 2013). These hubs flexibly couple to the DAN or DMN, depending on task context. The FCSRT engages directed attention as well as episodic memory and should therefore engage all three networks. Altered function in nodes of these networks therefore not only affects functioning of the network itself, but also the interaction with the other networks. Future work should more closely examine the DAN and DMN and their interaction with the fronto-parietal network which will likely show dysfunction in PCA.
Our findings have a number of important clinical implications. Memory function is often overlooked in the clinical assessment of PCA. While higher visual deficits remain the dominant feature in PCA, our findings support the presence of retrieval deficits in the majority of cases, and should be reflected in descriptions of the clinical phenotype of this syndrome. Identification of additional decline in memory in PCA may assist in differential diagnosis with other disorders that mimic the early visual impairments, such as ophthalmologic conditions. Our findings support other studies purporting the merits of the FCSRT as a tool for the investigation of memory in the Alzheimer's disease spectrum (Teichmann et al., 2017).
Secondly, our findings suggest that clinical interventions to enhance memory performance in PCA should be directed at attention to memory, rather than storage failures as in typical AD. For example, cue-based memory strategies are likely to be effective, given the cue-sensitivity shown in the FCSRT. Finally, drugs that are known to modulate attentional function, and already commonly used in typical AD, such as acetylcholinesterase inhibitors (AChEI), may also prove effective in ameliorating retrieval deficits in PCA. Although a recent clinical trial of the AchEI Donepezil showed no significant treatment effects on standard tests of higher visual and attention function (Ridha et al., 2018), sensitive tests of memory function were neglected.
Some limitations to this study should be addressed in future experiments. The healthy control and PCA groups had identical imaging on the same scanner, allowing us to directly compare network function. Unfortunately, our healthy control cohort was not tested on the FCSRT-IR meaning that we could not associate memory performance with network function as we did in the PCA cohort. Given that FCSRT performance is near ceiling in the healthy control group, it is unlikely that we would have been able to detect a correlation between memory performance and network function in this group anyway. The absence of an AD group, meant that we could not do a double dissociation, and future work would benefit from examining memory performance and attention and network function directly between these groups. Additional tests of attention and working memory could also be employed to understand more completely the contribution of these systems to memory impairment in PCA. Further, while the sample size of PCA patients included in this study is similar to cohorts reported in other empirical studies (e.g. Putcha et al., 2018), replication of these findings is imperative in a larger sample of patients.
In summary, we demonstrate a profile of memory impairment early in the course of PCA that is qualitatively different to that observed in typical AD. The FCSRT detected retrieval deficits not based on storage failures since they could be ameliorated by retrieval cues. Two key attention and memory related networks, the DAN and the DMN showed hypoconnectivity in PCA. DAN function was associated with memory performance, demonstrating the cognitive consequences of network dysfunction and supporting our hypothesis that impaired attention underlies memory deficits in PCA.
Acknowledgments
We are indebted to the patients and families for their kind participation in our research, and thank Cristina Blanco-Duque and Sara Bartels for help with data collection.
SA is supported by Alzheimer's Research UK. CRB is supported by a Medical Research Council Clinician Scientist Fellowship (MR/K010395/1).
References
- Ahmed S., Baker I., Husain M., Thompson S., Kipps C., Hornberger M. Memory impairment at initial clinical presentation in posterior cortical atrophy. J. Alzheimers Dis. 2016;52:1245–1250. doi: 10.3233/JAD-160018. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Loane C., Bartels S., Zamboni G., Mackay C., Baker I. Lateral parietal contributions to memory impairment in posterior cortical atrophy. Neuroimage Clin. 2018 doi: 10.1016/j.nicl.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriacombe S., Helmer C., Amieva H., Berr C., Dubois B., Dartigues J.F. Validity of the free and cued selective reminding test in predicting dementia: the 3C study. Neurology. 2010;74:1760–1767. doi: 10.1212/WNL.0b013e3181df0959. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett Matthew, Anton Jean-Luc, Valabregue Romain, Poline Jean-Baptiste. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16 [Google Scholar]
- Cabeza R., Mazuz Y.S., Stokes J., Kragel J.E., Woldorff M.G., Ciaramelli E. Overlapping parietal activity in memory and perception: evidence for the attention to memory model. J. Cogn. Neurosci. 2011;23:3209–3217. doi: 10.1162/jocn_a_00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J., Bylsma F.W. Psychological examination of traumatic encephalopathy. Clin. Neuropsychol. 1993;7:3–21. [Google Scholar]
- Crutch S.J., Schott J.M., Rabinovici G.D., Murray M., Snowden J.S., van der Flier W.M. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017 doi: 10.1016/j.jalz.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P.S.R., Anaki D., Ciaramelli E., Cohn M., Kim A.S.N., Murphy K.J. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Feldman H.H., Jacova C., DeKosky S.T., Barberger-Gateau P., Cummings J. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Breakspear M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E., Buschke H. Genuine memory deficits in dementia. Dev. Neuropsychol. 1987;3:13–36. [Google Scholar]
- Grober E., Lipton R.B., Hall C., Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S., Schubert S., Hoon C., Mioshi E., Hodges J.R. Validation of the Addenbrooke's cognitive examination III in Frontotemporal dementia and Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2013;36:242–250. doi: 10.1159/000351671. [DOI] [PubMed] [Google Scholar]
- Wolk D.A., Dickerson B.C. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage. 2011;54(2):1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Knopman D.S., Gunter J.L., Graff-Radford J., Vemuri P., Boeve B.F. Cascading network failure across the Alzheimer's disease spectrum. Brain. 2015;139:547–562. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50:1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Muschelli J., Nebel M.B., Caffo B.S., Barber A.D., Pekar J.J., Mostofsky S.H. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage. 2014;96:22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D., McGinnis S.M., Brickhouse M., Wong B., Sherman J.C., Dickerson B.C. Executive dysfunction contributes to verbal encoding and retrieval deficits in posterior cortical atrophy. Cortex. 2018;106:36–46. doi: 10.1016/j.cortex.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The Brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridha B.H., Crutch S., Cutler D., Frost C., Knight W., Barker S. A double-blind placebo-controlled cross-over clinical trial of DONepezil in posterior cortical atrophy due to underlying Alzheimer's disease: DONIPAD study. Alzheimers Res. Ther. 2018;10 doi: 10.1186/s13195-018-0363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C., Bonilha L., Fridriksson J., Bender B., Karnath H.-O. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarazin M., Berr C., De Rotrou J., Fabrigoule C., Pasquier F., Legrain S. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C., Shulman G.L., Corbetta M. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J. Neurosci. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C., Shulman G.L., Corbetta M. The contribution of the human posterior parietal cortex to episodic memory. Nat. Rev. Neurosci. 2017;18:183–192. doi: 10.1038/nrn.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Sepulcre J., Turner G.R., Stevens W.D., Schacter D.L. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J. Cogn. Neurosci. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Wai D.F., Graff-Radford N.R., Boeve B.F., Dickson D.W., Parisi J.E., Crook R. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- Teichmann M., Epelbaum S., Samri D., Levy Nogueira M., Michon A., Hampel H. Free and cued selective reminding test—accuracy for Alzheimer's and neurodegenerative disease differential diagnosis: a large-scale biomarker-characterized monocenter cohort study (ClinAD) Alzheimers Dement. 2017;9 doi: 10.1016/j.jalz.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E.K., James M. 1991. The Visual Object and Space Perception Battery. (Thesis_references-Converted #318) [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wolk D.A., Dickerson B.C., Initiative Alzheimer's Disease Neuroimaging. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage. 2011;54:1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G., Wilcock G.K., Douaud G., Drazich E., McCulloch E., Filippini N. Resting functional connectivity reveals residual functional activity in Alzheimer's disease. Biol. Psychiatry. 2013;74:375–383. doi: 10.1016/j.biopsych.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Zhou J., Seeley W.W. Network dysfunction in Alzheimer's disease and frontotemporal dementia: implications for psychiatry. Biol. Psychiatry. 2014;75:565–573. doi: 10.1016/j.biopsych.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Zhou J., Greicius M.D., Gennatas E.D., Growdon M.E., Jang J.Y., Rabinovici G.D. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Gennatas E.D., Kramer J.H., Miller B.L., Seeley W.W. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]