Summary
1. Environmental stressors can be key drivers of phenotypes, including reproductive strategies and morphological traits. The response to stress may be altered by the presence of microbial associates. For example, in aphids, facultative (secondary) bacterial symbionts can provide protection against natural enemies and stress induced by elevated temperatures. Furthermore, aphids exhibit phenotypic plasticity, producing winged (rather than wingless) progeny that may be better able to escape danger, and the combination of these factors improve the response to stress. How symbionts and phenotypic plasticity, both of which shape aphids’ stress response, influence one another, and together influence host fitness, remains unclear.
2. In this study, we investigate how environmental stressors drive shifts in fecundity and winged/wingless offspring production, and how secondary symbionts influence the process. We induced production of winged offspring through distinct environmental stressors, including exposure to aphid alarm pheromone and crowding, and, in one experiment we assessed whether the aphid response is influenced by host plant.
3. In the winged morph, energy needed for wing maintenance may lead to trade-offs with other traits, such as reproduction or symbiont maintenance. Potential trade-offs between symbiont maintenance and fitness have been proposed but have not been tested. Thus, beyond studying the production of offspring of alternative morphs, we also explore the influence of symbionts across wing/wingless polyphenism as well as symbiont interaction with cross-generational impacts of environmental stress on reproductive output.
4. All environmental stressors resulted in increased production of winged offspring and shifts in fecundity rates. Additionally, in some cases, aphid host-by-symbiont interactions influenced fecundity. Stress on first generation aphids had cross-generational impacts on second generation adults, and the impact on fecundity was further influenced by the presence of secondary symbionts and presence/absence of wings.
5. Our study suggests a complex interaction between beneficial symbionts and environmental stressors. Winged aphids have the advantage of being able to migrate out of danger with more ease, but energy needed for wing production and maintenance may come with reproductive costs for their mothers and for themselves, where in certain cases, these costs are altered by secondary symbionts.
Keywords: Symbiosis, Environmental Stressors, Trade-offs, Pea Aphid, Life-History, Phenotypic Plasticity
Graphical Abstract
The authors work sheds new light on the interaction between environmental stressors and beneficial symbionts, and how these factors act as drivers in pea aphid life-history characteristics. Organisms need to adapt to ever-changing environments, and this study shows how beneficial symbionts influence shifts in response to distinct types of environmental stress.
1. Introduction
Long-term interactions between two or more different species living in close association are ubiquitous in nature, and these symbioses may be mutually beneficial or may come at a cost to one or both organisms (Buchner 1965; Margulis & Fester 1991; Pineda et al. 2013). For example, virtually all animals carry within them symbiotic bacterial communities that are often referred to as the microbiome of the host (Näpflin & Schmid-Hempel 2016). Across organisms, benefits of symbioses for hosts include more rapid growth, increased reproduction (Himler et al. 2011), and/or higher survival (Rodriguez, Redman & Henson 2004). In insects, some microbial symbionts provide nutrients to their hosts (Moran 2003), while others provide protection from pathogens (Scarborough, Ferrari & Godfray 2005; Hedges et al. 2008) and parasites (Oliver et al. 2003). Although harboring microbes often provides adaptive advantages to hosts, the energy expense to sustain such microbiota is hypothesized to lead to physiological trade-offs arising from allocating resources to the maintenance of symbionts (Kitano & Oda 2006; Porter & Rice 2013; Polin, Simon & Outreman 2014; Horgan & Ferrater 2017).
Insects provide tractable systems to study symbiotic interactions due to their relatively simple microbiomes, and the pea aphid (Acyrthosiphon pisum) and its bacterial endosymbionts have emerged as an important model. All pea aphids harbor the vertically transmitted obligate symbiont Buchnera aphidicola, which provides essential amino acids missing from the aphid’s diet of plant phloem sap (Douglas 1998; Sandström & Moran 1999). In addition, pea aphids can also harbor zero to a few vertically transmitted facultative, secondary symbionts, which are not strictly required for survival or reproduction, yet provide defense against biotic and abiotic stressors, including natural enemies (Montllor, Maxmen & Purcell 2002; Scarborough, Ferrari & Godfray 2005; Russell & Moran 2006) and elevated temperatures (Harmon, Moran & Ives 2009). One common secondary symbiont, for example, is Regiella instecticola, a gammaproteobacteria that provides aphids protection against several species of aphid-specific fungal pathogens (Parker et al. 2013). Although symbionts provide a wide variety of benefits to their hosts, which may drive eco-evolutionary dynamics (Zytynska & Weisser 2016), these partnerships may be costly, leading to trade-offs between symbiont maintenance and other traits, such as reproduction and/or growth (Fautin, Guo & Hwang 1995; Frederickson et al. 2012; Polin, Simon & Outreman 2014). The cost of these trade-offs may also intensify under conditions in which resources need to be allocated to lessen the negative impacts of environmentally deteriorating conditions (e.g., resource competition/limitation, heat stress, increased predation risk, etc.).
Aphids are continuously exposed to distinct types of environmental stressors (Fig. 1a), however, aphids have the ability to respond to stressors in different ways, including wing polyphenism (Fig. 1b). Under optimal conditions, aphids asexually produce mostly wingless (apterous) offspring. Under environmental stress, such as crowding and/or predation risk, however, females plastically produce a greater proportion of winged (alate) offspring (Lees 1967; Müller, Williams & Hardie 2001; Kunert et al. 2005). Although these aphid progeny from the same mother are genetically identical, the energy requirements for adult winged and wingless aphids are different. The presence of wings and associated muscles results in trade-offs between fecundity and the ability to migrate more easily (Groeters & Dingle 1989; Brisson 2010). Furthermore, winged aphids produce fewer and smaller offspring when compared to wingless aphids (Brisson 2010), which may be due to higher reproductive costs. Although previous work has focused on aphid reproduction under stress via heat-shock (Montllor, Maxmen & Purcell 2002), only one previous study has linked plasticity in wing production with one common aphid secondary symbiont, R. insecticola: Leonardo and Mondor (2006) demonstrated, using two different aphid lines with two presumably different R. insecticola symbiont strains, that aphid mothers with R. insecticola tend to produce fewer winged offspring in response to crowding than genetically identical mothers without R. insecticola. Despite the potential importance of this result for understanding the influence of symbiosis on phenotypic plasticity and responses to stress, there has been little work done to determine whether other symbiont species influence aphid polyphenism, whether R. insecticola strains vary in their influence, and how symbiosis and polyphenism interact to influence aphid fecundity. Addressing these knowledge gaps is key to understanding how symbionts drive the evolution of aphid polyphenism.
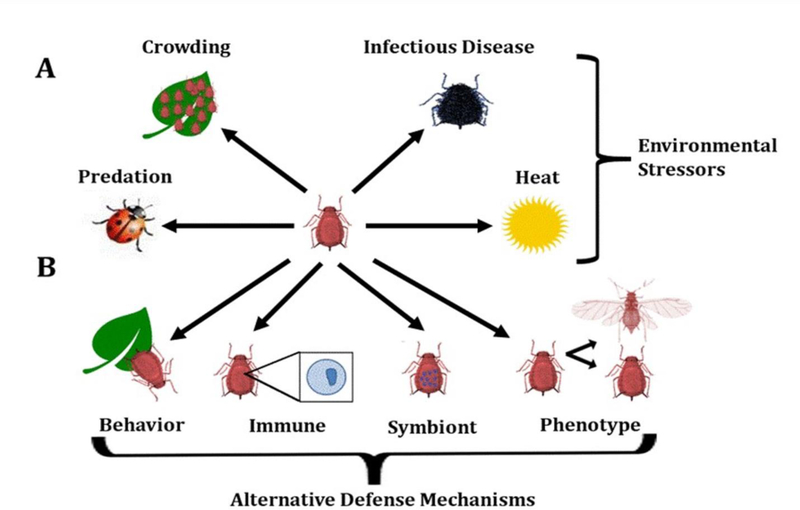
Figure 1. Pea aphid alternative defense mechanisms.
A. Upon sensing environmental stressors such as predation, crowding, disease and environmental temperatures shifts, aphids can utilize an array of protective strategies. B. Alternative defenses to these stressors include dropping behaviors, immunological defenses, symbiont-conferred protection, and polyphenism-based defense via production of winged offspring with the ability to migrate to safer habitats. Each form of defense likely has costs that shape both the ability of individuals to mount other defenses and the evolution of alternative defenses at the population level.
Here, we analyzed variation in the propensity to produce winged offspring by exposing aphids with varying secondary symbionts to biotic and abiotic stressors. Throughout our work, we tested the hypothesis that aphids harboring secondary symbionts will respond differently to stress compared to aphids without secondary symbionts. In the first experiment, we exposed aphids harboring alternative symbiont species to (E) -ß-farnesene (EBF), an alarm pheromone that is released in response to the presence of predators (Roitberg & Myers 1978; Losey & Denno 1998). Under natural conditions, exposure to the alarm pheromone results in mothers producing more winged offspring (Kunert et al. 2005), and in our work we demonstrate that exposure to the pheromone decreases fecundity but increases proportions of winged progeny, with higher fecundity and more winged offspring linked with the presence of symbionts. In the second experiment, focusing on the association with R. insecticola, we compared offspring production of crowded aphids with and without different R. insecticola strains. These aphids were reared on two distinct host plants to assess the influence of host plant context on the impacts of symbiont association, and we demonstrate an interactive effect of Regiella and host plant species on aphid responses to crowding, where aphids harboring Regiella and reared on non-native host plants produce higher proportions of winged offspring. In the third experiment, using aphids with distinct Regiella strains and without Regiella, we assessed the cross-generational impact of environmental stress by studying offspring production of second-generation offspring from crowded and optimally-reared first-generation mothers. We demonstrate that Regiella strains vary in their impact on the production of winged offspring in response to crowding, and furthermore, that there is significant cross-generational impact from maternal stress and maternal winged/wingless phenotype.
2. Methods
2.1. Aphid Rearing
Pea aphids used were clonal females produced parthenogenetically. Pea aphids are able to feed on a variety of legumes, but all pea aphids seem to be able to feed on fava bean (Vicia faba) (Storeck et al. 2000). All experimental aphids were reared on two-week fava plants watered twice a day, planted in fresh potting soil, and kept at 20oC in summer light conditions (16L: 8D), except where noted. To maximize plant health and reduce environmental variation, only 10-day old fava bean plants were used, and aphid densities were kept at a maximum of 15 aphids per plant, except where noted.
2.2. Experiment 1. Effects of alarm pheromone exposure and secondary symbionts on fecundity and winged offspring production
The first experiment was designed to determine the influence of three common species of aphid secondary symbionts on fecundity and winged offspring production in relation to exposure to alarm pheromone. We used an aphid genotype (5A0) that was collected in 1999 from Madison, WI and maintained in the laboratory asexually. Three different secondary symbiont species were originally introduced into 5A0 aphids in 2011 producing clonal lines that harbor single infections of Serratia symbiotica (5AR), Hamiltonella defensa (5AT), or Regiella insecticola (5AU) (Oliver et al. 2003). The four 5A lines were thus presumed to be genetically identical, differing only in their secondary symbiont profile.
(E)-ß-farnesene (EBF), is the principal component of the alarm pheromone released in nature by aphids when encountering environmental stressors (Pickett et al. 1992). Upon detection, it causes aphids to exhibit predator avoidance behaviours and to increase production of winged offspring in order to facilitate dispersal away from the threat (Kunert et al. 2005; Hatano, Kunert & Weisser 2010). We exposed aphids to synthetic EBF (Bedoukian Research, Inc. Danbury, CT (P3500–90)) by dissolving it in the volatile solvent Hexane. Required amounts were then pipetted onto a small disc of filter paper, which was held in place approximately 5 cm above the soil of the aphid host plant, allowing the EBF to evaporate into the surrounding environment. Reapplications of EBF were made onto the same disc of filter paper. We used three different concentrations of EBF: control (Hexane only), Low (500ng/µl EBF in Hexane), and High (1500ng/µl in Hexane) (Barribeau, Sok & Gerardo 2010).
Ten newly sexually matured, wingless aphids per line were placed together onto a fresh plant for 24 hrs. The following day, 30 first instars from each line were collected and split across three plants (10 aphids per plant), one for each treatment group (control, high and low EBF). Five microliters of the appropriate EBF solution was applied to small discs of filter paper held above the soil, every other day, from day 1 to day 9. On either day 6 or day 8 of the experiment (based on developmental rate), the aphids were split onto individual plants to monitor the start of offspring production. Regardless of when they were transferred to new plants, EBF treatments were continued until Day 9. All offspring from each exposed aphid were collected between 0 and 96hrs of offspring production and enumerated for total fecundity over four days. Offspring were then reared on fava plants for several days, at which point wing status was recorded by assessing the presence or absence of wing buds.
2. 3. Experiment 2. Effect of Regiella symbiont, host plant and aphid genotype on winged offspring production
We next tested whether Regiella insecticola (which seemed to have the largest effect on winged offspring production in Experiment 1) increases the proportion of winged offspring. In this experiment we tested the effects of crowded conditions, which can yield high percentages of winged progeny (Sutherland 1969; Grantham et al. 2016). We used seven aphid lines that naturally harboured Regiella, and were collected from two different host plants, Trifolium pratensae (aphid host genotypes 63, 126, 313, 319) and Medicago sativa (aphid host genotypes 215, 222, 305). In a subset of aphids from each line, without harming the obligate Buchnera symbionts, secondary symbionts were cleared using an oral administration of antibiotics following the protocol of McLean et al. (2011). Following the antibiotic treatment, secondary symbiont-free and control aphids were maintained for at least five generations on Vicia faba, and lines were tested using PCR to confirm the presence/absence of secondary symbionts (Vorburger, Gehrer & Rodriguez 2010; Henry et al. 2013). We then placed two 10-day adult, wingless aphids per treatment on a plant in a cup-cage for 48 hours and collected their offspring. Half of the resulting first instars were placed on fava bean, and the other half were placed on the species of plant on which the aphid genotype was collected (Medicago or Trifolium); we refer to these at their native host plants. These wingless aphids were reared to adulthood (10 days old), and were then placed into Petri dishes in crowding conditions (5 aphids per dish) containing either fava or their native host plant. Offspring were collected from the 3rd through 5th days of reproduction, moved to fava plants (5 aphids per plant), and assigned a random number blind to treatment. We then measured winged/wingless proportions as above.
2. 4. Experiment 3. Cross-generational effects of stress and Regiella symbionts on fecundity and winged-offspring production
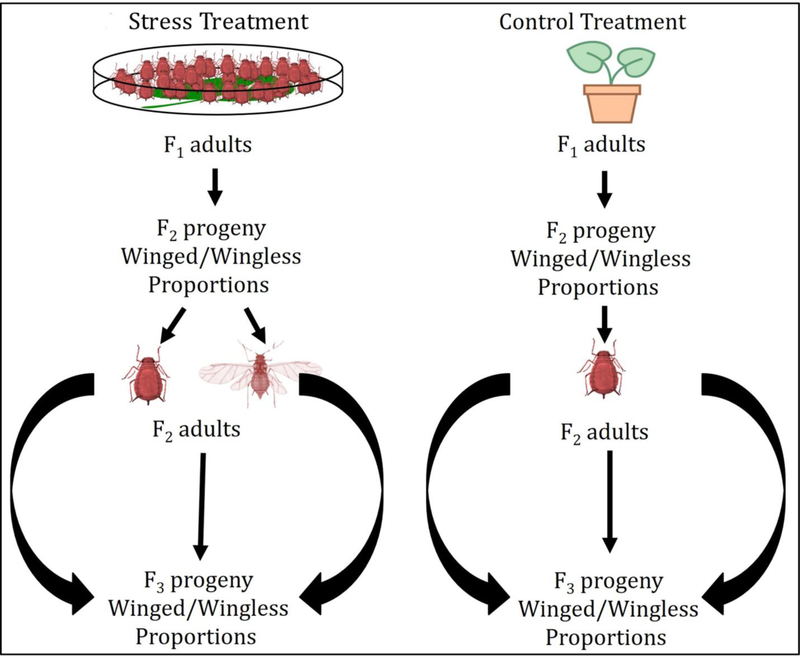
We next studied the effects of alternative Regiella symbionts on fecundity and the propensity of aphids to produce winged progeny in response to stress via crowding, assessing reproductive output of both first-generation aphids (i.e., those that were stressed) and their resulting second-generation progeny upon reaching adulthood (See Fig. 2 for general workflow).
Figure 2. Experiment 3 workflow.
Stress and control treatments were repeated for aphid groups without secondary symbionts, and aphids with distinct Regiella secondary symbionts. Adult aphids reared in control conditions rarely produce winged offspring, therefore second-generation, winged aphids from control mothers were not included for F3 progeny. Reproductive measurements included overall offspring production and winged/wingless proportions across generations.
In contrast to Experiment 2, we kept the aphid genotype constant to focus on variation caused in these traits by alternative Regiella strains. We established five Regiella strains (313, Ui, Ri, CO21, 5.15) in a common aphid genetic background (genotype LSR1–01) using established protocols (Tsuchida et al. 2011; Łukasik et al. 2013b). These Regiella lines were collected from multiple aphid species (Vorburger, Gehrer & Rodriguez 2010; Łukasik et al. 2013a), and are likely genotypically (Hansen, Vorburger & Moran 2012), and phenotypically divergent (e.g., these lines provide distinct levels of protection against the fungal pathogen Pandora neoaphidis (Parker et al. 2017)). Aphids in the experiment were used at least three generations after symbiont establishment.
For the stress treatment, we placed 30 ten-day-old, adult, wingless aphids in a sterile Petri dish (60mm x 30mm) for 24 hours with a freshly-cut fava plant leaf to minimize starvation (Müller, Williams & Hardie 2001; Brisson, Ishikawa & Miura 2010). To assess potential stress from feeding on severed plant leaves, we ran a pilot study to compare fecundity of 10-day old aphids housed for 24 hours in two conditions-on a fresh, potted plant (max of 10 aphids per plant) and a non-crowded Petri dish (6 aphids per dish) with a freshly-cut leaf. There were no statistical differences in fecundity (p = 0.197, Fig. S1 in supporting information) and percent of winged offspring (p = 0.789, Fig. S2) between these two treatments. Thus, to minimize the need to handle control aphids, our non-crowded control group aphids were maintained entirely on non-crowded potted plants (maximum of 10 aphids per plant) without transfer to Petri dishes.
After the 24-hour stress period, a subset of 10 aphids per line from control and stressed groups were transferred from non-crowded potted plants and Petri dishes respectively and individually housed on fresh fava plants. The number of offspring and proportion of winged offspring were recorded over a period of four days. The adults were moved to a fresh plant after the first two days of reproduction to minimize crowding. After the fourth day, the adults were removed, and the offspring were grown to 4th instar to assess winged/wingless proportions. In addition, a subset of adults from each line were placed on fresh fava plants (5 aphids/plant). We gave them three days to reproduce second-generation progeny before removing them from the plant. A pilot study showed that winged offspring production is stronger several days post-stress for this aphid genotype; therefore, in order to compare reproduction of winged and wingless aphids, offspring from the first two days were discarded, leaving only a mixture of winged and non-winged offspring that were born on the third day. These second-generation aphids were reared on plants until adulthood. This generated a set of second-generation winged aphids and their wingless sisters born from crowded mothers, but almost entirely wingless aphids born from control mothers (control conditions lead to the generation of few to no winged offspring). Winged second-generation aphids from control mothers were not included in analyses due to the low number of winged offspring produced under this condition. This led to comparison across three treatment groups: second generation aphids born to winged mothers that had been stressed (Winged, Stressed), second generation aphids born to wingless mothers that had been stressed (Wingless, Stressed), and second-generation aphids born to wingless mothers that had not been stressed (Wingless, Control) (Refer to Fig. 2).
For each treatment group, we randomly chose 11 second-generation aphids per each of six lineages (with no Regiella or with one of five different Regiella) and individually reared them on fresh fava plants. When they reached adulthood, we collected their offspring for eight days. Fecundity was measured daily, and the adults were transferred to new plants after four days to avoid crowding. After the eighth reproductive day, the adults were removed. Once all third-generation offspring reached the fourth instar, the proportion of winged/wingless aphids was recorded. Thus, measurements of fecundity and the proportion of offspring that were winged were based on data from the first eight days of adulthood.
2.5. Statistics
Data from experiment 1 were analysed using linear mixed models implemented in the lme4 package (Bates et al. 2015) in R (version 3.5.1) (https://www.r-project.org/). The proportion of winged offspring produced by each aphid was modelled as a binomial trait, and the fecundity of each aphid was modelled with a Poisson distribution. Treatment (Control, Low-EBF, or High-EBF) and symbiont status (No Symbionts, Serratia, Hamiltonella, or Regiella) were treated as fixed effects. Experimental replicate was included as a random effect. Models were compared by removing the fixed effects (the interaction term, then the effect of symbiont, then treatment), followed by model comparisons using ANOVA and chi-squared statistics.
For experiment 2, data on the proportion of winged offspring were analysed using generalized linear models using R. Symbiont background (Regiella or symbiont-free), host plant (fava or the native host plant for each genotype), and genotype were modelled as fixed effects using a quasibinomial error structure. Models were derived by removing fixed effects (the three-way interaction, then host plant * genotype, symbiont background * genotype, and symbiont background * host plant, and then the main effects of genotype, host plant, and symbiont). Model comparisons were made as above using ANOVA and F-statistics.
For experiment 3, data on first-generation aphids were analysed using generalized linear models as above, using a quasipoisson distribution for fecundity data and a quasibinomial distribution for wingedness. We nested Regiella genotype within the main effect of Regiella presence, and treatment as fixed effects. We derived minimal models by first removing the interaction between Regiella presence and treatment, then Regiella genotype and treatment, and then the main effects of treatment, Regiella genotype, and Regiella presence/absence. Models were compared using ANOVA and F-statistics.
Data on second-generation aphids were divided in two different analyses using R. For the first analysis, cross-generational impact of maternal stress in second generation aphids between control wingless mothers and stressed wingless mothers were analyzed using generalized linear models, with Regiella genotype nested within Regiella presence, and maternal stress as fixed effects. Models were derived by first removing the interaction between Regiella presence and maternal treatment, then Regiella genotype and maternal treatment, and then the main effects of maternal treatment, Regiella genotype, and Regiella presence/absence. For the second analysis, impact of maternal phenotype in second generation aphids between stressed winged and stressed wingless mothers were analyzed using generalized linear models, with Regiella genotype nested within Regiella presence, and maternal phenotype as fixed effects. Minimal models were derived by first removing the interaction between Regiella presence and maternal phenotype, then Regiella genotype and maternal phenotype, and then the main effects of maternal phenotype, Regiella genotype, and Regiella presence/absence. A quasipoisson distribution for fecundity values and a quasibinomial distribution for winged offspring values were used, and models were compared using ANOVA and F-statistics. The two distinct analyses were necessary because our experimental groups did not include second generation winged aphids from non-stressed control mothers, as these mothers did not produce winged offspring.
Results
3.1. Experiment 1. Effects of alarm pheromone exposure and secondary symbionts on fecundity and winged offspring production
Presence of secondary symbionts had a significant effect on fecundity (Fig. 3a; χ2 = 22.6, 3DF, p < 0.0001). Across all aphid lines, exposure to EBF did not have a significant influence on total offspring production over four days (χ2 = 1.57, 2DF, p = 0.45).
Figure 3. Fecundity and percent of winged offspring across aphids harboring different secondary symbiont species (Experiment 1).
A. Number of offspring produced by aphids harboring distinct secondary symbionts and exposed to various levels of EBF over a period of the first four days of reproduction. B. The percent of progeny that are winged of aphids exposed to various levels of EBF (colors are same as in A). Colored bars show mean values of measured traits, and shaded boxes represent standard error. Association with secondary symbionts led to higher fecundity, and aphids harbouring Regiella produced the highest number of offspring. Association with secondary symbionts and EBF exposure were associated with higher proportions of winged offspring, with the highest EBF level resulting in the highest proportions of winged progeny, and aphids harbouring Regiella producing the highest proportion of winged progeny.
There was a significant effect of both EBF exposure (χ2 = 1057, 2DF, p < 0.0001) and symbiont background (χ2 = 133.5, 3DF, p < 0.0001) on the proportion of winged offspring produced. More winged offspring were produced in the presence of EBF compared to the control, with elevated levels of EBF resulting in more winged offspring than low levels of EBF (Fig. 3b). Furthermore, the interaction between EBF treatment and symbiont background was significant (χ2 = 37.8, 6DF, p < 0.0001); Exposure to elevated levels of EBF results in production of higher proportions of winged offspring than in the or in the absence of secondary symbionts.
3.2. Experiment 2. Effect of Regiella symbiont, host plant and aphid genotype on winged offspring production
Curing aphid lines of Regiella significantly reduced the proportion of winged offspring born to aphids after stress (Fig. 4), (F = 5.41, 1DF, p = 0.021). Aphids on their native host plants produced significantly fewer winged offspring than on fava (F = 10.2, 1DF, p = 0.0016), but the effect of Regiella on winged offspring production was consistent (Fig. 4) between V. faba versus a genotype’s “native host plant (Trifolium or Medicago) (F = 1.54, 1DF, p = 0.22). Aphid genotype was not a significant factor in our model (F = 3.39, 1DF, p = 0.067), though we note that recent work has found that aphid genotypes significantly differ in the extent to which they produce winged offspring in response to crowding independently of symbiont background (Grantham et al. 2016). Genotypes differed in the extent to which host plant species influenced winged offspring production (F = 10.3, 1DF, p = 0.0015).
Figure 4. Percent winged offspring produced by distinct aphid genotypes across different host plants (Experiment 2).
A. Percent winged offspring was measured for multiple aphid lineages without secondary symbionts (light orange bars) and with their native strain of Regiella (dark orange bars). Each aphid cage (offspring produced by 5 adult aphids) is shown by a grey dot, with colored bars indicating mean values and shaded boxes representing standard error. The aphid genotypes are listed along the bottom of the figure; the host plant on which each aphid was collected is also shown, which indicates which “native” host plant was used in the experiment. B. Data compiled across aphid lineages to illustrate influence of host plant, with native host plant indicating when aphids are reared on what they were collected on in nature (Medicago or Trifolium). Colored bars show mean values of measured traits and shaded boxes represent standard error. Aphids cleared of Regiella and reared on native host plants produced lower proportions of winged offspring.
3.3. Experiment 3. Cross-generational effects of stress and Regiella symbionts on fecundity and winged-offspring production
Across aphid lineages, first-generation, stressed aphids had lower fecundity over four days in comparison to control aphids (Fig. 5a; F=50.9, 1DF, p < 0.0001), with no differences due to Regiella presence (F=0.047, 1DF, p = 0.83) or Regiella genotype (F=0.91, 4DF, p=0.46). Stress also elevated winged offspring production (Fig. 5b; F=250.3, 1DF, p < 0.0001). Overall, Regiella presence had no effect on winged offspring production (F=2.32, 1DF, p = 0.13), but there was a significant effect of Regiella genotype on wingedness (F=4.15, 4DF, p = 0.0036).
Figure 5. Fecundity and percent of winged offspring across aphids harboring different Regiella strains (Experiment 3).
A. Fecundity, and B. percent winged offspring over the first four days of reproduction measured in aphid groups harboring different Regiella strains and exposed to stress via crowding (Figure 2). Control aphids (not stressed) are indicated in light green, and aphids exposed to stress are indicated in dark green. Colored bars show mean values of measured traits, and shaded boxes represent standard error. Stress resulted in lower reproduction and higher proportion of winged offspring across all aphid groups, with minor difference across Regiella strains.
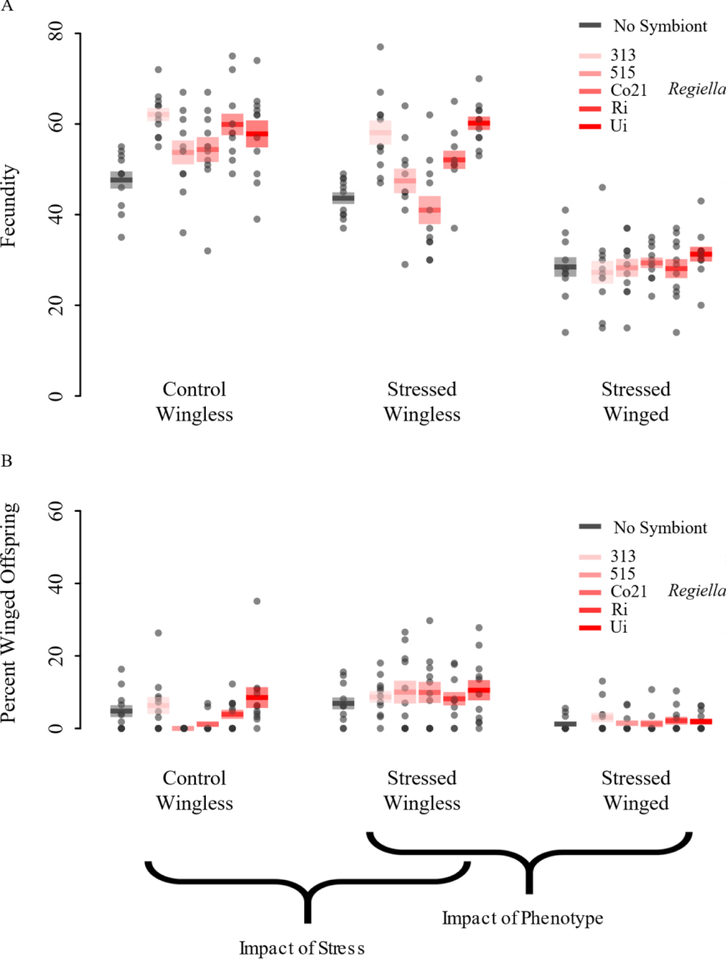
In order to assess the transgenerational impacts of maternal stress, in experiment 3, we first compared fecundity and proportion of winged offspring that were born from wingless aphids which came from stressed and control mothers. Stress on mothers influenced fecundity of these second-generation, wingless aphids who were not directly exposed to the stress (Fig. 6a; F=10.199, 2DF, p<0.0001), with aphids harboring Regiella producing more offspring (F=24.718, 1DF, p<0.0001). Regiella genotype, notably within aphids from stressed mothers, had a significant effect on fecundity (F=9.985, 4DF, p<0.0001). In addition, stress on mothers influenced second-generation winged offspring production, with aphids from stressed mothers producing more winged progeny (Fig. 6b; F=15.461, 2DF, p<0.0001), with no differences due to Regiella presence (F=0.261, 1DF, p=0.6) or Regiella genotype (F=1.877, 4DF, p=0.1), but with a significant interaction between Regiella presence and maternal phenotype (F=12.348, 1DF, p<0.0001).
Figure 6. Fecundity and percent winged offspring of second-generation aphids of different phenotypes from stressed and control mothers and harboring different strains of Regiella (Experiment 3).
A. Fecundity, and B. percent winged offspring produced by wingless offspring of control (non-stressed) wingless mothers, wingless offspring of stressed wingless mothers, and winged offspring of stressed mothers over the first eight days of reproduction. On the x-axes, ‘control’ and ‘stressed’ represent the treatment to which the previous generation was exposed, while ‘wingless’ and ‘winged’ refers to the phenotype of the current generation whose fecundity and offspring production is being measured. Aphids without secondary symbionts are shown in black, and aphid lines with distinct Regiella strains are shown by shades of red. Solid bars show mean values of measured traits, and shaded boxes represent standard error. Exposure to stress in the preceding generation and maternal phenotype influenced reproduction of these second generation aphids. Regiella strain influenced fecundity levels, but not production of winged progeny.
Then, in order to assess the impact of maternal phenotype, in experiment 3, we also compared fecundity and proportion of winged offspring that were born from winged and wingless aphids from stressed mothers. Maternal phenotype influenced fecundity of these aphids (Fig. 6a; F=289.562, 1DF, p<0.0001), with wingless mothers having fewer offspring than winged mothers. Aphids harboring Regiella producing more offspring (F=6.332, 1DF, p=0.013), and Regiella genotype (notably across aphids from wingless mothers) influenced fecundity (F=6.737, 4DF, p<0.0001). We also noted an interaction between maternal phenotype and Regiella genotype (F=3.664, 4DF, p=0.007). Furthermore, as expected, maternal phenotype influenced winged offspring production, with wingless mothers producing more winged progeny (F=20.807, 1DF, p<0.0001). In this case, there was no significant influence from presence of Regiella (F=0.524, 1DF, p=0.4) or Regiella genotype (F=1.94, 4DF, p=0.1).
5. DISCUSSION
Symbiosis and phenotypic plasticity are two important biological phenomena influencing aphid interactions with the environment, yet little is known about how these two factors influence one another. In this study, we exploited the pea aphid system to assess how secondary symbionts impact host responses to stress. Furthermore, we estimated the impacts of exposure to environmental stress across generations.
Previous work has established that Regiella provides protection from the fungus Pandora neoaphidis and other aphid-specific fungal pathogens (Scarborough, Ferrari & Godfray 2005; Parker et al. 2013). Through the performance of three independent studies, our work further suggests symbionts can alter reproductive strategies upon exposure to environmental stressors (Fig. 3a, 3b, 4), with the level of influence varying with symbiont genotype (Fig. 5a, 5b), and with significant cross-generational influence (Fig. 6a, 6b). Interestingly, our findings in experiment 2 that Regiella-association increased the production of winged offspring contradicts a previous finding, where aphids with Regiella produce significantly less winged progeny when responding to stress in contrast to aphids without the symbiont (Leonardo & Mondor 2006), however our third experiment, in which we demonstrate that Regiella genotypes vary in their effects on winged offspring production may explain why. In terms of fecundity, our results parallel variation in previous studies, where secondary symbionts have been associated with both an increase (Tsuchida et al. 2011) and decrease in reproduction (McLean et al. 2017). Here, we see that Regiella increases fecundity under some conditions (Fig. 6a) but not others (Fig. 5a).
Recent work has shown that distinct Regiella genotypes provide variable levels of protection against pathogens, with this variation being dependent on the interaction between the microbe and both host and pathogen genotypes (Parker et al. 2017). Similarly, our current study suggests the influence of Regiella on the aphid’s response to stress varies with the symbiont genotype. (Fig. 5a), and aphid host genotypes appear to vary in their responses to stress in the absence of Regiella (Fig 4a, light bars only). This is in agreement with recent work showing that aphid genotypes vary in the extent to which they produce winged offspring, independently of presence or absence of secondary symbionts (Grantham et al. 2016). What is less clear is whether symbiont-by-host genotype interactions may also influence winged offspring production or fecundity upon exposure to stressors.
A number of environmental factors could also shape symbionts’ impacts on host traits, and for aphids, as for many insects, the host plants on which they feed are a critical environmental variable. Our second experiment not only confirms that Regiella-association generally increases production of winged offspring but also demonstrates that this effect is robust to changes in host plant (Fig 4). Interestingly, while host plant context did not influence the impacts of Regiella on polyphenism, it did influence winged offspring production in its own right, suggesting a role for nutrition in altering this important response to environmental stressors.
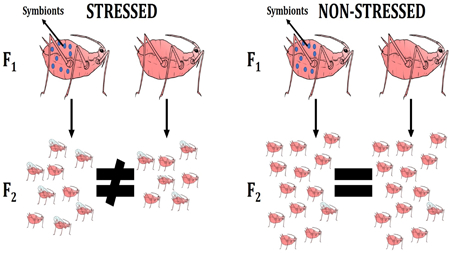
Recent work has aimed to understand the cross-generational impacts of stress on insects (Thesing et al. 2015). We focused on whether maternal stress and symbiosis interact to influence offspring production of subsequent generations. Maternal stress and maternal phenotype, in conjunction with presence of secondary symbionts and specific symbiont genotypes had cross-generational influence on fecundity (Fig 6a). This provides further evidence that the costs of being winged and exposure to stress have important carry-over effects to new generations, and such costs are under certain influence of symbionts. Interestingly, cross-generation impact on production of winged offspring was limited to maternal stress, with a limited interaction with presence of Regiella, where second-generation adults from stressed wingless mothers produced higher numbers of winged offspring, but variation across Regiella genotypes was only noted on aphid adults from non-stressed mothers. Moreover, the influence of maternal phenotype on winged offspring production was limited to second-generation adults from wingless mothers, and in contrast to our expectations, Regiella presence or genotype did not interact with maternal phenotype (Fig. 6b).
Our study provides new insight into how distinct environmental stressors, maternal stress, body morphology, the presence of secondary symbionts, and their interactions, act as drivers for variation in life-history characteristics. Organisms across nature are constantly adapting to ever-changing environments. In insects, especially aphids, adaptive traits via phenotypic plasticity and intimate relationships with secondary symbionts have been well characterized (Buchner 1965; Douglas 1998; Leclair et al. 2017). We provide new evidence that symbiosis can influences both phenotypic plasticity and fecundity in the face of stress, suggesting that symbiosis could be a principal factor shaping the evolution of aphids’ responses to signals of deteriorating conditions.
Supplementary Material
ACKNOWLEDGEMENTS
We want to thank other members of the Gerardo lab at Emory University, Department of Biology for their assistance and guidance. The research was funded by the National Science Foundation (NSF) IOS-1025853, NIH/NIGMS Institutional Research and Academic Career Development Award 5K12-GM000680–17, and NSF grant DBI1306387. The authors do not have any conflict of interests.
Footnotes
DATA ACCESIBILITY
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.pv952ps (Reyes et al. 2018)
REFERENCES
- Barribeau SM, Sok D & Gerardo NM (2010) Aphid reproductive investment in response to mortality risks. BMC Evolutionary Biology, 10, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M, Maechler M, Bolker B & Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Brisson JA (2010) Aphid wing dimorphisms: linking environmental and genetic control of trait variation. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 365, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson JA, Ishikawa A & Miura T (2010) Wing development genes of the pea aphid and differential gene expression between winged and unwinged morphs. Insect Molecular Biology, 19, 63–73. [DOI] [PubMed] [Google Scholar]
- Buchner P (1965) Endosymbiosis of Animal with Plant Microorganisms John Wiley & Sons, New York. [Google Scholar]
- Douglas A (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annual Review of Entomology, 43, 17–37. [DOI] [PubMed] [Google Scholar]
- Fautin DG, Guo C-C & Hwang J-S (1995) Costs and benefits of the symbiosis between the anemoneshrimp Periclimenes brevicarpalis and its host Entacmaea quadricolor. Marine Ecology Progress Series, 59, 77–84. [Google Scholar]
- Frederickson ME, Ravenscraft A, Miller GA, Arcila Hernández LM, Booth G & Pierce NE (2012) The direct and ecological costs of an ant-plant symbiosis. The American Naturalist, 179, 768–778. [DOI] [PubMed] [Google Scholar]
- Grantham ME, Antonio CJ, O’Neil BR, Zhan YX & Brisson JA (2016) A case for a joint strategy of diversified bet hedging and plasticity in the pea aphid wing polyphenism. Biology Letters, 12, 20160654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeters FR & Dingle H (1989) The cost of being able to fly in the milkweed-oleander aphid, Aphis nerii (Homoptera: Aphididae). Evolutionary Ecology, 3, 313–326. [Google Scholar]
- Hansen AK, Vorburger C & Moran NA (2012) Genomic basis of endosymbiont-conferred protection against an insect parasitoid. Genome Research, 22, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon JP, Moran NA & Ives AR (2009) Species response to environmental change: impacts of food web interactions and evolution. Science, 323, 1347–1350. [DOI] [PubMed] [Google Scholar]
- Hatano E, Kunert G & Weisser WW (2010) Aphid wing induction and ecological costs of alarm pheromone emission under field conditions. PLoS ONE, 5, e11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O’neill SL & Johnson KN (2008) Wolbachia and virus protection in insects. Science, 322, 702–702. [DOI] [PubMed] [Google Scholar]
- Henry LM, Peccoud J, Simon J-C, Hadfield JD, Maiden MJ, Ferrari J & Godfray HCJ (2013) Horizontally transmitted symbionts and host colonization of ecological niches. Current Biology, 23, 1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ & Zchori-Fein E (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science, 332, 254–256. [DOI] [PubMed] [Google Scholar]
- Horgan FG & Ferrater JB (2017) Benefits and potential trade‐offs associated with yeast‐like symbionts during virulence adaptation in a phloem‐feeding planthopper. Entomologia experimentalis et applicata, 163, 112–125. [Google Scholar]
- Kitano H & Oda K (2006) Robustness trade‐offs and host–microbial symbiosis in the immune system. Molecular systems biology, 2, 10.1038/msb4100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert G, Otto S, Röse US, Gershenzon J & Weisser WW (2005) Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecology Letters, 8, 596–603. [Google Scholar]
- Leclair M, Polin S, Jousseaume T, Simon JC, Sugio A, Morlière S, Fukatsu T, Tsuchida T & Outreman Y (2017) Consequences of coinfection with protective symbionts on the host phenotype and symbiont titres in the pea aphid system. Insect Science, 24, 798–808. [DOI] [PubMed] [Google Scholar]
- Lees A (1967) The production of the apterous and alate forms in the aphid Megoura viciae Buckton, with special reference to the role of crowding. Journal of Insect Physiology, 13, 289–318. [Google Scholar]
- Leonardo TE & Mondor EB (2006) Symbiont modifies host life-history traits that affect gene flow. Proceedings of the Royal Society of London B: Biological Sciences, 273, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losey JE & Denno RF (1998) The escape response of pea aphids to foliar‐foraging predators: factors affecting dropping behaviour. Ecological Entomology, 23, 53–61. [Google Scholar]
- Łukasik P, Dawid MA, Ferrari J & Godfray HCJ (2013a) The diversity and fitness effects of infection with facultative endosymbionts in the grain aphid, Sitobion avenae. Oecologia, 173, 985–996. [DOI] [PubMed] [Google Scholar]
- Łukasik P, van Asch M, Guo H, Ferrari J & Godfray CJ (2013b) Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecology Letters, 16, 214–218. [DOI] [PubMed] [Google Scholar]
- Margulis L & Fester R (1991) Symbiosis as a source of evolutionary innovation: speciation and morphogenesis MIT Press. [PubMed] [Google Scholar]
- McLean A, Van Asch M, Ferrari J & Godfray H (2011) Effects of bacterial secondary symbionts on host plant use in pea aphids. Proceedings of the Royal Society of London B: Biological Sciences, 278, 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AH, Parker BJ, Hrček J, Kavanagh J, Wellham PA & Godfray HCJ (2017) Consequences of symbiont co‐infections for insect host phenotypes. Journal of Animal Ecology, 10.1111/1365-2656.12705. [DOI] [PubMed]
- Montllor CB, Maxmen A & Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecological Entomology, 27, 189–195. [Google Scholar]
- Müller CB, Williams IS & Hardie J (2001) The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecological Entomology, 26, 330–340. [Google Scholar]
- Näpflin K & Schmid-Hempel P (2016) Immune response and gut microbial community structure in bumblebees after microbiota transplants. Proceedings of the National Academy of Sciences, 283, 20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Russell JA, Moran NA & Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proceedings of the National Academy of Sciences, 100, 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker BJ, Hrček J, McLean AH & Godfray HCJ (2017) Genotype specificity among hosts, pathogens, and beneficial microbes influences the strength of symbiont‐mediated protection. Evolution, 71, 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker BJ, Spragg CJ, Altincicek B & Gerardo NM (2013) Symbiont-mediated protection against fungal pathogens in pea aphids: a role for pathogen specificity? Applied and environmental microbiology, 79, 2455–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett J, Wadhams L, Woodcock C & Hardie J (1992) The chemical ecology of aphids. Annual Review of Entomology, 37, 67–90. [Google Scholar]
- Pineda A, Dicke M, Pieterse CM & Pozo MJ (2013) Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Functional Ecology, 27, 574–586. [Google Scholar]
- Polin S, Simon JC & Outreman Y (2014) An ecological cost associated with protective symbionts of aphids. Ecology and Evolution, 4, 836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SS & Rice KJ (2013) Trade-offs, spatial heterogeneity, and the maintenance of microbial diversity. Evolution, 67, 599–608. [DOI] [PubMed] [Google Scholar]
- Reyes ML, Laughton AM, Parker BJ, Wichmann H, Fan M, Sok D, Hrček J, Acevedo T & Gerardo NM (2018) Data from: The influence of symbiotic bacteria on reproductive strategies and wing polyphenism in pea aphids responding to stress. Dryad Digital Repository 10.5061/dryad.pv952ps. [DOI] [PMC free article] [PubMed]
- Rodriguez RJ, Redman RS & Henson JM (2004) The role of fungal symbioses in the adaptation of plants to high stress environments. Mitigation and adaptation strategies for global change, 9, 261–272. [Google Scholar]
- Roitberg BD & Myers JH (1978) Adaptation of alarm pheromone responses of the pea aphid Acyrthosiphon pisum (Harris). Canadian Journal of Zoology, 56, 103–108. [Google Scholar]
- Russell JA & Moran NA (2006) Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proceedings of the Royal Society of London B: Biological Sciences, 273, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström J & Moran N (1999) How nutritionally imbalanced is phloem sap for aphids? Proceedings of the 10th International Symposium on Insect-Plant Relationships, pp. 203–210. Springer. [Google Scholar]
- Scarborough CL, Ferrari J & Godfray H (2005) Aphid protected from pathogen by endosymbiont. Science, 310, 1781–1781. [DOI] [PubMed] [Google Scholar]
- Storeck A, Poppy GM, Emden H.F.v. & Powell W (2000) The role of plant chemical cues in determining host preference in the generalist aphid parasitoid Aphidius colemani. Entomologica Experimentalis et Applicata, 97, 41–46. [Google Scholar]
- Sutherland O (1969) The role of crowding in the production of winged forms by two strains of the pea aphid, Acyrthosiphon pisum. Journal of Insect Physiology, 15, 1385–1410. [Google Scholar]
- Thesing J, Kramer J, Koch LK & Meunier J (2015) Short-term benefits, but transgenerational costs of maternal loss in an insect with facultative maternal care. Proceedings of the Royal Society, 282, 20151617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Koga R, Matsumoto S & Fukatsu T (2011) Interspecific symbiont transfection confers a novel ecological trait to the recipient insect. Biology Letters, 7, 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorburger C, Gehrer L & Rodriguez P (2010) A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biology Letters, 6, 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zytynska SE & Weisser WW (2016) The natural occurrence of secondary bacterial symbionts in aphids. Ecological Entomology, 41, 13–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.