Abstract
Background
Titin is a giant elastic protein that spans the half-sarcomere from Z-disk to M-band. It acts as a molecular spring and mechanosensor and has been linked to striated muscle disease. The pathways that govern titin dependent cardiac growth and contribute to disease are diverse and difficult to dissect.
Methods
To study titin deficiency versus dysfunction, we generated and compared striated muscle specific knockouts with progressive postnatal loss of the complete titin protein by removing exon 2 (E2-KO) or an M-band truncation that eliminates proper sarcomeric integration but retains all other functional domains (M1/2-KO). We evaluated cardiac function, cardiomyocyte mechanics, and the molecular basis of the phenotype.
Results
Skeletal muscle atrophy with reduced strength, severe sarcomere disassembly, and lethality from 2 weeks of age were shared between the models. Cardiac phenotypes differed considerably: loss of titin leads to dilated cardiomyopathy (DCM) with combined systolic and diastolic dysfunction – the absence of M-band titin to cardiac atrophy and preserved function. The elastic properties of M-1/2-KO cardiomyocytes are maintained, while passive stiffness is reduced in the E2-KO. In both KOs, we find an increased stress response and increased expression of proteins linked to titin-based mechanotransduction (CryAB, ANKRD1, MLP, FHLs, p42, Camk2d, p62 and Nbr1). Among them, FHL2, and the M-band signaling proteins p62 and Nbr1 are exclusively upregulated in the E2-KO suggesting a role in the differential pathology of titin truncation versus deficiency of the full-length protein. The differential stress response is consistent with truncated titin contributing to the mechanical properties in M1/2-KOs, while low titin levels in E2-KOs lead to reduced titin-based stiffness and increased strain on the remaining titin molecules.
Conclusions
Progressive depletion of titin leads to sarcomere disassembly and atrophy in striated muscle. In the complete knockout, remaining titin molecules experience increased strain, resulting in mechanically induced trophic signaling and eventually DCM. The truncated titin in M1/2-KO helps maintain the passive properties and thus reduces mechanically induced signaling. Together, these findings contribute to the molecular understanding of why titin mutations differentially affect cardiac growth and have implications for genotype-phenotype relations that support a personalized medicine approach to the diverse titinopathies.
Keywords: cardiomyopathy, heart disease, biomechanics, animal models, muscle, myocardial contraction, hypertrophy signaling
Introduction
Titin mutations are the most frequent genetic cause of heart disease and lead to diverse cardiac phenotypes from DCM to altered cardiac growth.1 The broader phenotypic spectrum associated with titin mutations extends from no obvious disease to isolated cardiac or skeletal muscle disease and combined disease.2 The complex titin genotype–phenotype relations do not only cause difficulties in dissecting disease-causing mutations from normal variants but also complicate the development of efficient personalized treatment strategies.
Titin is the largest known protein and spans the half sarcomere. It is anchored in the Z-disk by T-cap3 and extends into the I-band region where the elastic N2B and PEVK segments, as well as the N2A signaling domain are located.4 In the A-band, titin connects to the thick filament and integrates into the M-band via myomesin where it overlaps with M-band titin of the adjacent sarcomere to form a continuous filament.5 Titin’s interactions with four-and-a-half lim domain proteins (FHLs) and a complex between p62, Nbr1, and the MuRF ubiquitin ligases play a role in hypertrophy signaling and the atrophy response.6,7 FHL proteins bind the heart specific titin N2B region and its deletion leads to cardiac atrophy.8 This is unlike knockouts of other titin domains such as the PEVK,9 M-Band,10 the I/A junction,11 as well as the deletion of select titin IG-domains in the I-band, which lead to hypertrophy with increased expression of FHL proteins.12 Both FHL1 and FHL2 interact with extracellular signal regulated kinase (ERK) 13,14 and regulate cardiac growth.15,16 The same applies to CamKIIδ,17,18 which phosphorylates the titin N2B domain to adapt its elastic properties.19
Recent work has shown that truncating titin mutations frequently lead to DCM with increased severity of the phenotype associated with how close the mutation is to the C-terminus i.e. with increased size of the mutant protein.20 Missense variants are more difficult to classify and to utilize for predictions of future disease. This relates in part to the high background of titin single nucleotide polymorphisms (SNPs) in the population.21,21 The limited understanding of how titin mutations cause disease extends to our inability to dissect loss of function from dominant negative effects, as a mutant titin allele leads to both reduced levels of normal titin and expression of a pathological titin protein – both potentially contributing to the pathology.
To determine the effect of reduced titin expression in the absence of pathological titin protein on striated muscle function and understand the role of titin in sarcomere biology, we generated a conditional complete knockout of titin. Here, exon 2 (which contains the translation start) is flanked with loxP sites and the MCK-Cre transgene restricts the knockout to striated muscle. We compared the phenotype of the exon 2 knockout (E2-KO) that results in a complete loss of titin with that of the M-band exon 1/2 knockout (M1/2-KO) that has an intact Z-disk, I-band, and A-band region but does not integrate into the M-band.22 With the gradual depletion of wildtype titin in striated muscle, these models allow us to compare the response of the remaining full-length titin molecules in the E2-KO to strain versus the additional contribution of the M-band detached titin molecules in the M1/2-KO. The skeletal muscle phenotype was strikingly similar between knockouts with sarcomeric disassembly, muscle weakness, and atrophy. Unexpectedly, the E2-KO results in DCM with enlarged hearts, while the M1/2-KOleads to cardiac atrophy. Expression profiling of sarcomeric and signaling proteins in the heart suggests a dominant role of FHL2, Nbr1, and p62 in communicating mechanical input in a titin depleted myofilament with few remaining titin molecules to accommodate strain versus a more robust sarcomeric backbone in the presence of M-band deficient titin.
Taken together, the development of the diverse cardiac phenotypes with DCM and combined systolic and diastolic dysfunction of the E2-KO versus isolated atrophy of the M1/2-KO relates to mechanically induced trophic signaling via FHL2, p62, and Nbr1. Interestingly, shorter titin truncations are associated with a less severe DCM phonotype in patients,20 but in our E2-KO with zero-length titin, the phenotype is much more severe than in the M1/2-KO titin, which expresses one of the largest truncated titin proteins described so far.
Methods:
The authors declare that all supporting data are available within the article and its online supplemental files. The analytic methods will be made available to other researchers for purposes of reproducing the results at the authors’ laboratories.
Generation of titin knockout mice
Titin E2-KO mice were generated by homologous recombination in ES cells. Lox sites were inserted into intron 1 and intron 2 to enable the Cre mediated excision of exon 2, which contains the translation start site (Supplemental Figure 1). Chimeric animals generated from the ES-cells were bred with FLP transgenic animals23 to remove the neomycin resistance cassette. Animals were genotyped by PCR. Additional details including information on the amplicons are provided in the online supplement (Supplemental table 1, 2).
The M1/2-KO mice with loxP sites flanking titin´s M-band exons 1 and 2 have been described previously.22 In both strains recombination was restricted to striated muscle by expression of the Cre recombinase under control of the MCK promoter.24 The cre is constitutively expressed in heart and skeletal muscle, which is unlike the published M1/2-KO with the MerCreMer transgene, which restricts the phenotype to the heart and produces a different timeline for development of the phenotype after tamoxifen injection in adult mice.22 Animals were housed in individually ventilated cages and had access to food and water ad libitum. Sex and age matched animals were followed over up to 5 weeks and functional and expression analysis were performed at 4 weeks of age.
All experiments involving animals were carried out following institutional and National Institutes of Health guidelines and follow the Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Animal protocols were approved by the local authorities.
Echocardiography
Cardiac function was determined by echocardiography using the VEVO 2100 system with the ultrasonic probe MS-400 (Visualsonics Fujifilm). Mice at 4 weeks of age were anesthetized with isoflurane. Cardiac dimensions and function were measured as described previously.25
Cardiomyocyte mechanics
Skinned myocytes were glued at one end to a force transducer (Model 406A, Aurora Scientific). The other end was bent with a pulled glass pipette and attached to micromanipulator so that the myocyte axis aligned with the microscope optical axis and cross sectional area (CSA) was measured directly. Then, the free end was glued to a servomotor (Model 315C-I, Aurora Scientific) that imposes controlled stretch-release protocols. Sarcomere length (SL) was measured using the Video sarcomere length software (VSL 900B, Aurora Scientific). Passive stress was measured in relaxing solution (pCa 9) with protease inhibitors at 15 °C. Cells were stretched from a base length at a speed of 1.0 base length/sec with different stretch amplitude based on % cell length followed by a 20 sec hold and then a release back to the original length. Data were collected using real-time data acquisition and analysis software (600A, Aurora Scientific) at a sample rate of 2 kHz. Measured forces were converted to stress (force/unit undeformed CSA).
Histology and electron microscopy
Heart and skeletal muscle tissue were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with Masson´s Trichrome staining (HT15–1KT; Sigma-Aldrich). To visualize the ultrastructure of the muscle, tissue were embedded for electron microscopy as described previously.26
Expression analysis
Heart and skeletal muscle samples were snap frozen in liquid nitrogen and homogenized. Reverse transcription of RNA and quantitative real time PCR- reactions were performed as described previously.26 Expression data were normalized to 18S (Life Technologies) in a multiplex PCR reaction. Additional details including information on the amplicons are provided in the online supplement (Supplemental Table 3, 4).
Proteins were extracted with RIPA buffer (50 mM Tris pH 8; 150 mM NaCl; 1% IGPAL; 0.1% Na DOC; 5 mM EDTA; 0.1% SDS; protease and phosphatase inhibitor complex (Invitrogen)) and separated by SDS-PAGE. Western blot was performed on PVDF membranes. Antibodies are listed in the supplement (Supplemental Table 5) the secondary HRP conjugated antibody was detected by chemiluminescence staining with ECL (Supersignal West Femto Chemiluminescent Substrate; Pierce Chemical Co.).
To separate titin isoforms we used vertical SDS agarose gel electrophoresis (VAGE) as described.27
Statistical Analysis
Graph Pad prism software was used for statistical analysis. Results are expressed as means ± SEM. Statistical tests are indicated in the figure legend. A p value < 0.05 was considered as statistically significant (*), (** p < 0.01, *** p < 0.001, **** p < 0.0001).
For additional information, please refer to the supplemental methods section.
Results:
Progressive depletion of full-length titin from striated muscle results in failure to thrive and early death.
We used the Cre/lox recombination system in a conditional knockout approach to generate mice that are completely devoid of full length titin or titin’s M-band exons M1 and M2. This was achieved by insertion of loxP sites into the introns flanking titin’s exon 2, which contains the translation start site (Supplemental Figure 1A), and transgenic expression of the Cre recombinase. Similar to the homozygous M-band exon 1 and 2 knockout (M1/2), germline deletion of the floxed Exon 2 resulted in embryonic lethality in mid gestation (data not shown and 22,26). To generate surviving titin deficient animals, we used the MCKcre transgene to express the recombinase in striated muscle. We compared the striated muscle exon 2 knockout mice (E2-KO) to the striated muscle M1/2 knockout (M1/2-KO), with exons 358 (M1) and 359 (M2) flanked by loxP sites (Figure 1A). Excision of these exons results in the expression of truncated titin proteins that properly integrates into the Z-disk, interacts with the myosin filament, but cannot integrate into the M-band.26
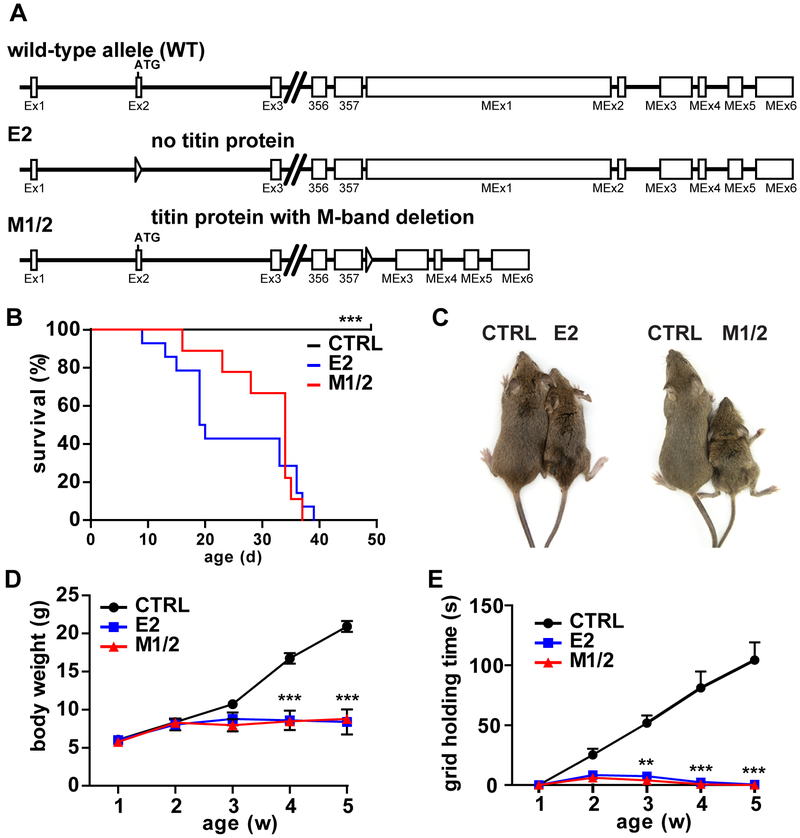
Figure 1. Failure to thrive and early lethality of titin knockouts deficient in titin (E2) versus titin’s M-band region (M1/2).
A) Schematic illustration of titin’s first and last exons of the wildtype, exon 2 (E2) and M-band knockout (M1/2). Triangles indicate lox sites (locus of X(cross)-over). B) E2-KO and M1/2-KO mice die from 10 and 15 days of life, respectively with no survivors beyond day 40, while control mice (CTRL) survive. C) Compared to the respective control without the Cre recombinase, E2-KO and M1/2-KO mice appear smaller and weaker at 35 days. Differences in posture include kyphosis and outward extended hindlimbs. D) Body weight does not increase from week two after birth and stays at ~10 g for either knockout model. E) Muscle strength as determined in an inverted screen grid holding test is severely reduced in E2 and M1/2-KO mice and precedes the reduced weight gain and muscle atrophy. Log rank (Mantel Cox) test, *** p < 0.001, n = 23 control, n = 14 for E2, n = 9 for M1/2 knockout (B). Repeated measure 2-way ANOVA with Bonferroni post-test, ** p < 0.01; *** p < 0.001, n = 12 controls, n = 6 per knockout (D, E).
The offspring of both knockout models was born at the expected Mendelian ratio as determined by PCR genotyping (Supplemental Figure 1B, C). In titin E2 and M1/2-KOs, mortality starts at 10 days of life and no knockout survived beyond the age of 40 days (Figure 1B). Both knockouts result in reduced body mass (Figure 1C) with failure to gain weight from 2 weeks of age and bodyweight remaining at ~10g (Figure 1D, Supplemental Figure 1D). Muscle strength was limited from 2 weeks of age as a sign of muscle weakness and is reflected in the grid holding time (Figure 1E). Subsequently homozygous KO skeletal muscle became atrophic in either genotype (Supplemental Figure 2A-H), while controls as well as heterozygotes developed normally and maintained sarcomere structure (Supplemental Figure 2I, J). As heterozygotes were indistinguishable from control animals, we focused our analysis on the comparison of Homozygous E2 and M1/2-KOs with controls.
Atrophy and sarcomere disassembly in skeletal muscle of titin knockout mice.
The progression of the striated muscle phenotype correlated with the loss of titin as determined by agarose protein gel electrophoresis and RT-qPCR (Figure 2A-E and Supplemental Figure 2). In quadriceps muscle, wildtype titin expression was reduced to similar amounts in the E2- and in the M1/2-KO (Figure 2C). In E2-KOs there was no truncated isoform, unlike the M1/2-KO where the mutated isoform (T1-M) amounted to 76% of total titin. The total amount of titin, relative to MHC, was unchanged in the M1/2-KO (Figure 2D, E). Progressive depletion of complete titin or titin’s M-band region both led to atrophy of the skeletal muscle with reduced quadriceps muscle weight (Figure 2F) normalized to the unchanged tibia length (Figure 2G). Atrophic fibers of the E2 and M1/2-KO were visualized by Masson trichrome staining of quadriceps muscle (Figure 2H). As the fiber cross sectional area was reduced (Figure 2I), the number of nuclei per area increased (Figure 2J). In both E2- and M1/2-KOs, electron microscopy revealed that progressive loss of titin resulted in sarcomere disassembly (Figure 2K, Supplement Figure 2I, J).
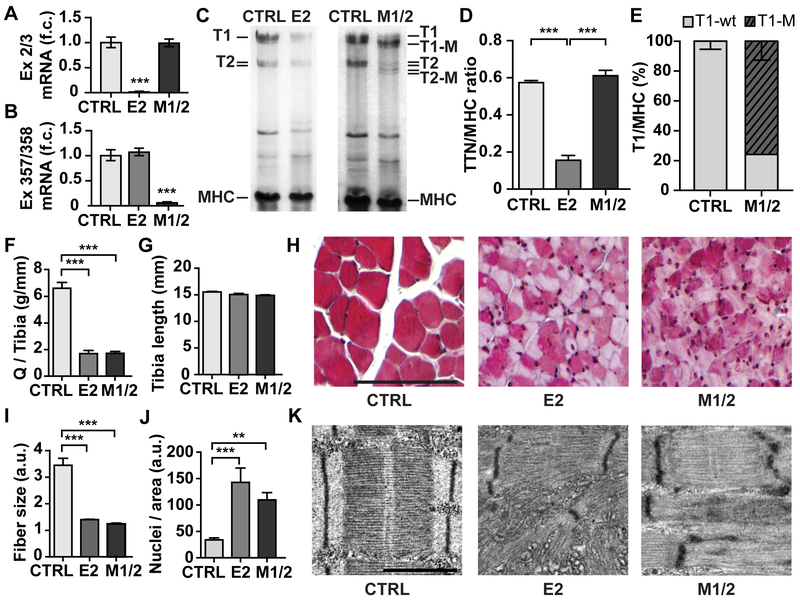
Figure 2. Progressive loss of titin causes skeletal muscle atrophy and sarcomere disassembly in E2 and M1/2 knockout mice.
A, B) Deletion of exon 2 in E2-KO (E2) and exons 358, 359 in M1/2-KO mice (M1/2) vs control (CTRL) was confirmed by real-time qPCR on cDNA derived from left ventricular mRNA of 35-day-old mice with probe-sets on exon 2 to 3 (A) and exons 357 to 358 (B). 1-way ANOVA with Tukey post-test, *** p < 0.001. n = 5 per genotype. C) Agarose gel electrophoresis of quadriceps protein from 5-week-old E2- and M1/2-KO mice. T1-M is the M-band deficient titin. T2 is the proteolytic product of T1. D) Total titin (TTN=T1+T2) protein is reduced to <20% in the E2-KO. One-way ANOVA and Tukey post-test, *** p < 0.001, n = 6 animals for control, n=3 for each knockout. E) The truncated T1-M protein in the M1/2-knockout amounts to ~80% of total T1 titin after 5 weeks. F) Reduced quadriceps (Q) muscle weight normalized to tibia length in 35-day-old animals. G) Tibia length is unchanged between control and KOs, n = 12 for controls, n = 6 per knockout. H) Masson Trichrome staining of quadriceps muscle (size bar = 100µm). I) Quantification of fiber size. J) Number of nuclei per field of view (slides from n = 6 controls and n = 3 for each knockout strain). One-Way ANOVA with Bonferroni post-test, ** p < 0.01, *** p < 0.001. K) Disassembly of the sarcomere in both knockout strains visualized by electron microscopy with partially remaining Z-discs and disorder at M- and A/I-band (size bar =1µm).
As we did not find obvious phenotypic differences in skeletal muscle between E2 and M1/2-KOs, reduced titin levels as well as expression of truncated titin protein appear to similarly affect sarcomere disassembly and result in atrophy.
Complete loss of cardiac titin causes DCM, while M-band deficient titin leads to cardiac atrophy.
In the heart, excision of titin exon 2 led to a reduced amount of titin protein as determined by the ratio of titin to MHC in hearts of 5-week-old mice (Figure 3A, B). Excision of M-band exons 1 and 2 led to the expression of truncated titin isoforms (N2BA-M; N2B-M). At 5 weeks of age only 43±7% normal wildtype titin remains in E2-KOs, as compared to 37±3% wildtype titin in the M1/2-KO. The titin isoform ratio between N2BA and N2B was unchanged in both strains (Figure 3C). The E2-KO leads to an enlarged heart with a significantly increased heart to body weight ratio. The minor increase in heart weight to tibia length was not significant (Figure 3D-G). On the cellular level, cardiomyocytes had similar cross-sectional areas between WT, E2-, and M1/2-KO hearts (Supplemental Figure 3A, B). These findings are consistent with a relative hypertrophy in the E2-KO that is mainly based on the reduced body weight and this is unlike the cardiac atrophy in the M1/2-KO with reduced heart and body weight and a significant reduction in heart weight to tibia length (Figure 3D-G). The trophic changes are reflected in altered cardiac morphology: Progressive reduction of titin exon 2 led to the development of dilated cardiomyopathy, while the deletion of titin’s M-band led to cardiac atrophy (Figure 3H). There was no fibrosis (Figure 3I) but an increased number of nuclei in both animal models (Supplemental Figure 3C).
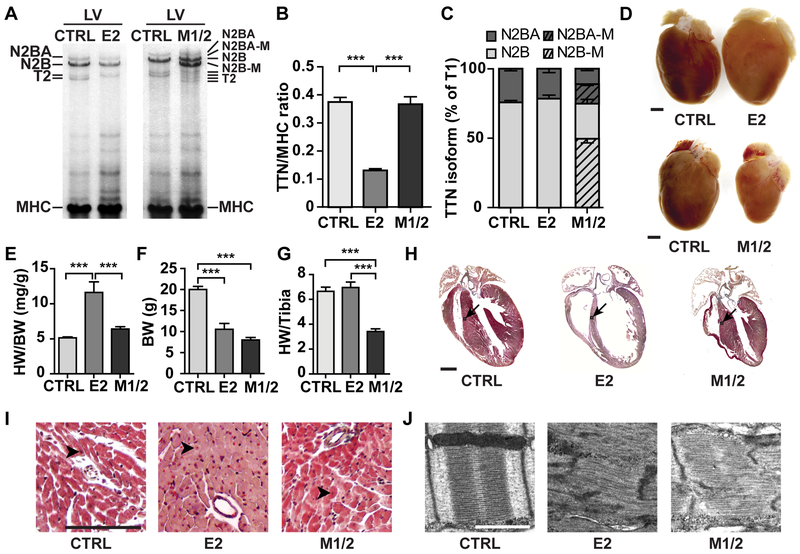
Figure 3. Complete titin loss leads to DCM while loss of M-band titin results in cardiac atrophy at 5 weeks.
A) Agarose protein gel electrophoresis of the left ventricle (LV) from control, E2- and M1/2-KO vs. control (E2, M1/2, CTRL) at 5-weeks. B) The total titin (TTN)/MHC protein ratio is reduced in the E2-KO; n = 6 control, n = 3 per KO. C) Titin N2BA and N2B isoform expression is unchanged in KO mice. In the M1/2-KO, the corresponding truncated isoforms amount to >50%; n = 6 for control n = 3 per KO. D) The heart of 5-weeks-old E2-KO mice is enlarged, while the deletion of titin’s M-band leads to cardiac atrophy (size bar = 1 mm). E, F) Heart weight/ body weight ratio (HW/BW) is increased in E2-KO, but body weight (BW) is reduced in both knockout strains. G) Normalization of the heart weight to the tibia length reflect the atrophy of the M1/2-KO; n = 13 control, n = 7 per KO. H) Masson trichrome staining of the heart from 5-weeks-old animals with DCM in the E2 knockout and atrophy of the M1/2 knockout heart (arrows: area of higher magnification in I; size bar = 1 mm). I) Higher magnification from H with increased number of nuclei (arrowheads indicate nuclei; size bar = 100 µm). J) Disassembly of the sarcomere in both strains. Electron microscopy of left ventricular tissue from 3-week-old animals (size bar = 1µm). Data in B, E, F and G were analyzed for statistical significance with one way ANOVA and Tukey post-test; *** p < 0.001
Interestingly, both deletion of complete titin or titin’s M-band resulted in upregulation of the sarcomeric protein β-MHC (~20 fold) and ANP (~20 fold), as determined by RT-qPCR (Supplemental Figure 3D-E). This regulation would be consistent with increased myocardial stretch in both KO strains. At the ultrastructural level, elimination of full-length cardiac titin or titin´s M-band both led to progressive sarcomere disassembly (Figure 3J and Supplemental Figure 3F).
Together the phenotypic changes indicate a differential trophic response of the heart to titin’s complete versus partial loss. The sarcomeric disassembly was a hallmark of both models, but the subsequent remodeling differed in mice with reduced titin levels versus expression of truncated titins with DCM in the E2-KO and cardiac atrophy in the M1/2-KO.
Combined systolic and diastolic dysfunction exclusively affects E2-KOs, while contractile and elastic properties are largely preserved in M-band deficient hearts at 4 weeks of age.
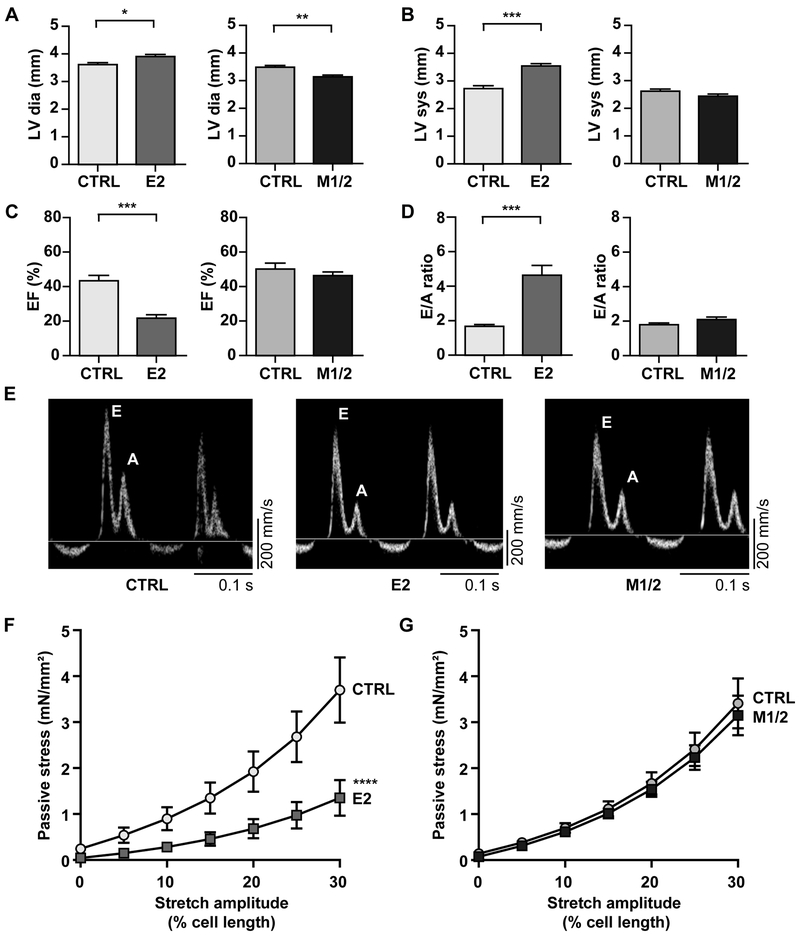
To evaluate the mechanical properties of titin deficient hearts before sarcomere disassembly dominates the underlying pathology, 4-week-old animals underwent analysis by echocardiography, mitral and tissue Doppler, as well as cardiomyocyte mechanics. Echocardiography confirmed the differences in cardiac geometry with increased LV diameter in diastole in the E2-KO vs. decreased dimensions as a sign of atrophy in M1/2-KO hearts (Table 1, Figure 4A, B, Supplemental Figure 4A). Cardiac function in M1/2-KO hearts was largely preserved with normal EF as a parameter of contractile function and normal E/A ratio indicating preserved diastolic filling. In E2-KO mice, both systolic and diastolic function were impaired with reduced EF and an increased E/A ratio (Table 1, Figure 4C-E, Supplemental Figure 4A). In several E2-KO animals, the A wave reflecting atrial contraction was reduced or not present, suggesting that the enlarged atria were unable to contribute to ventricular filling (Figure 4E, middle panel, Supplemental Figure 4B).
Table 1:
Echocardiography of E2- and M1/2-KO vs. control at 4 weeks of age
| E2-CTRL | E2-KO | M1/2-CTRL | M1/2-KO | |
|---|---|---|---|---|
| N | 8 | 8 | 7 | 9 |
| age (d) | 27 | 27 | 27 | 27 |
| body weight (g) | 13.9±0.7 | 10.7±0.6** | 15.8±0.6 | 10.2±0.5*** |
| LV mass (mg) | 66.9±3.0 | 82.4±4.8* | 53.0±3.2 | 41.9±3.3* |
| heart/body weight | 4.8±0.2 | 7.8±0.3*** | 3.4±0.1 | 4.1±0.2** |
| Echocardiography | ||||
| heart rate (bpm) | 404±18 | 417±19 | 457±11 | 362±16*** |
| stroke volume (µl) | 16.4±1.2 | 8.8±1.0*** | 17.1±1.7 | 12.0±0.8* |
| Vol dia (µl) | 38.2±1.7 | 43.0±4.0 | 33.9±1.8 | 26.1±2.3* |
| Vol sys (µl) | 22.9±2.1 | 34.0±3.2* | 16.8±1.2 | 14.6±1.9 |
| LV dia (mm) | 3.6±0.1 | 3.9±0.1* | 3.5±0.1 | 3.1±0.1** |
| LV sys (mm) | 2.7±0.1 | 3.5±0.1*** | 2.6±0.1 | 2.4±0.1 |
| LVPW dia (mm) | 0.59±0.02 | 0.63±0.03 | 0.53±0.02 | 0.50±0.02 |
| LVPW sys (mm) | 0.76±0.04 | 0.69±0.02 | 0.69±0.03 | 0.66±0.02 |
| IVS dia (mm) | 0.60±0.03 | 0.62±0.03 | 0.51±0.03 | 0.49±0.01 |
| IVS sys (mm) | 0.76±0.04 | 0.72±0.04 | 0.69±0.04 | 0.66±0.02 |
| FS (%) | 24.9±1.6 | 9.3±0.8*** | 24.8±2.2 | 22.4±1.3 |
| EF (%) | 43.4±3.1 | 21.8±1.9*** | 50.1±3.5 | 46.4±2.1 |
| Mitral, Doppler | ||||
| E (mm/s) | 502±42 | 376±38* | 552±15 | 382±46** |
| A (mm/s) | 308±25 | 72±4*** | 312±18 | 186±21*** |
| E/A ratio | 1.67±0.12 | 4.64±0.56*** | 1.80±0.09 | 2.10±0.15 |
| IVRT (ms) | 22.7±1.4 | 32.4±2.3** | 17.0±0.9 | 25.3±1.2*** |
| IVCT (ms) | 23.2±2.5 | 26.0±1.6 | 18.4±1.4 | 20.3±0.6 |
| MVDT (ms) | 24.7±2.2 | 20.2±3.2 | 23.4±1.4 | 27.6±1.5 |
| ET (ms) | 50.5±2.9 | 41.4±2.6* | 41.4±1.0 | 46.1±0.9** |
| E' (mm/s) | 16.9±1.8 | 9.4±1.1** | 26.1±4.2 | 15.0±1.8* |
| A' (mm/s) | 19.9±1.2 | 9.8±2.1*** | 27.5±2.7 | 16.6±1.4** |
| E' / A' | 0.85±0.06 | 1.12±0.18 | 0.92±0.08 | 0.90±0.05 |
| E / E' | 31.3±3.2 | 44.0±3.8* | 25.4±4.9 | 26.4±2.7 |
| Cardiac Performance | ||||
| CO (ml/min) | 6.6±0.5 | 3.8±0.5** | 7.8±0.8 | 4.4±0.5** |
| MPI | 0.92±0.07 | 1.42±0.06*** | 0.86±0.06 | 0.99±0.04 |
LV, left ventricle; Vol, volume; dia, diastole; sys, systole; LVPW, left ventricle posterior wall; IVS, interventricular septum; FS, fractional shortening; EF, ejection fraction; IVRT, isovolumetric relaxation time; IVCT, isovolumetric contraction time; MVDT, mitral valve deceleration time; ET, ejection time;
p < 0.05;
p < 0.01;
p < 0.001 versus respective control; Student’s T-test
Figure 4. Systolic and diastolic dysfunction predominantly affects the E2-KO at 4 weeks of age.
A) Left ventricular (LV) diameter in diastole (dia) is increased in E2- but decreased in M1/2-KO mice vs. control (E2, M1/2, CTRL). B, C) LV diameter in systole (sys) is increased and ejection fraction (EF) is reduced only in the E2-KO. D) E to A ratio is increased in E2-KO only. E) Doppler traces with similar E and A waves in control and M1/2 vs. E2 with a blunted A wave. F, G) Total passive stress of multicellular myocyte preparations is decreased in E2-KO, but not in M1/2-KO. The individual contribution of titin vs. collagen based stress is provided in Supplemental Figure 4B, C. Data in A-E were analyzed for statistical significance with Student’s T-test, n = 7–9. F and G were analyzed for significance with a mono-exponential curve fit and an extra sum of squares F-test, n = 7 animals per group; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
To evaluate the elastic properties of titin deficient cardiomyocytes, we recorded the passive stress with increasing cell length and determined the contribution of the myofilament vs. extracellular matrix (KCl/KI insensitive stress, which is assumed due to collagen). We found a strong reduction of passive stress only in the E2-, but not in the M1/2-KO (Figure 4F, G). This reduction was mainly titin based (Supplemental Figure 4B), but with a significant contribution of the extracellular matrix (Supplemental Figure 4C).
Protein expression reflects increased strain without major structural changes at 4 weeks of age.
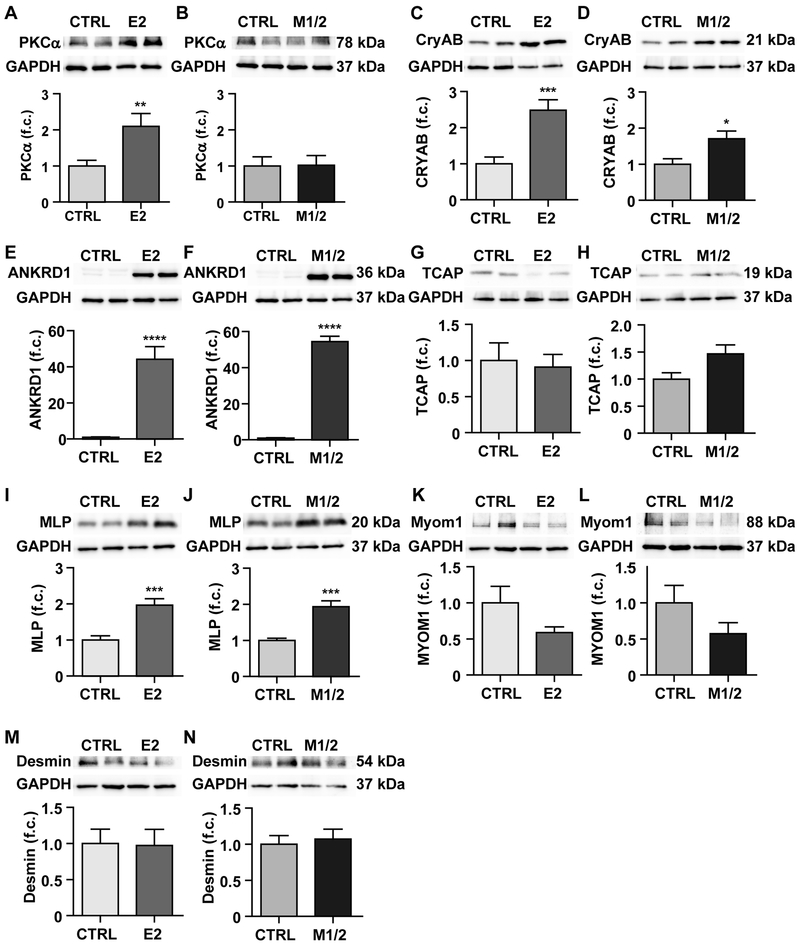
The terminal regions of titin molecules overlap at the Z-disc and M-band, where they interact with diverse proteins and anchor titin in the sarcomere. In animals lacking all domains from Z-disc to M-band, we found increased expression of PKCα in E2-KO only (Figure 5A, B) as well as a 2.5-fold increase in the chaperone alpha-B-crystalline vs a < 2-fold increase in the M1/2-KO (Figure 5C, D), both consistent with increased strain in the E2-KO. PKCα phosphorylation levels were unchanged (Supplemental Figure 5A, B). Ankrd1 expression is increased with pathological remodeling28 and we found a strong > 40-fold upregulation in KO hearts of either genotype (Figure 5E, F).
Figure 5. Expression analysis of titin related kinases, chaperone, and anchoring proteins.
Western blot analysis of heart lysate from control (CTRL) and E2-KO (left) and control and M1/2-KO (right) at 4 weeks of age. A, B) The tinin kinase PKCα is upregulated only in the E2-KO. C-F) The stress response proteins CryAB and ANKRD1 are upregulated in both KO strains, but CryAB to a lesser degree in the M1/2-KO. G-L) At 4 weeks of age, there is no significant deregulation of proteins that anchor titin at the Z-disc (T-cap) or the M-band (myomesin 1), but the Z-disc signaling protein MLP is upregulated in both KO strains. M, N). Expression of the extra-sarcomeric protein desmin is not affected in either KO. Western blot of left ventricular tissue lysate was quantified by densitometry; n = 8-11 animals per group. Statistical significance was calculated using Student’s T-test, * p < 0.05; ** p < 0.01; *** p < 0.001;**** p < 0.0001. f.c. fold change
To confirm that secondary changes resulting from sarcomeric disassembly did not obscure the primary molecular changes, we compared expression of Z-disc, M-band, and non-sarcomeric structural proteins between KOs and WT. Tcap, myomesin-1, and desmin were not significantly deregulated on the protein level (Figure 5G-N). The only change was in MLP expression with elevated levels in both KO strains (Figure 5I, J). On the RNA level we found early changes in myomesin expression with upregulation of myomesin-1, −2 and −3 predominantly in the M1/2-KO with intermediate levels in the E2-KO (Supplemental Figure 5C-H). In the E2-KO only, we found increased RNA levels of the embryonic myomesin EH-isoform (Supplemental Figure 5E, F). This isoform - which was only present in our E2-KO - has been described as a marker for DCM.29
FHL2 and atrophy/hypertrophy signaling is affected in complete and M-band knockout
To dissect the molecular basis of the trophic phenotype, we evaluated the regulation of several titin dependent proteins that relate to atrophy/hypertrophy signaling and protein degradation.
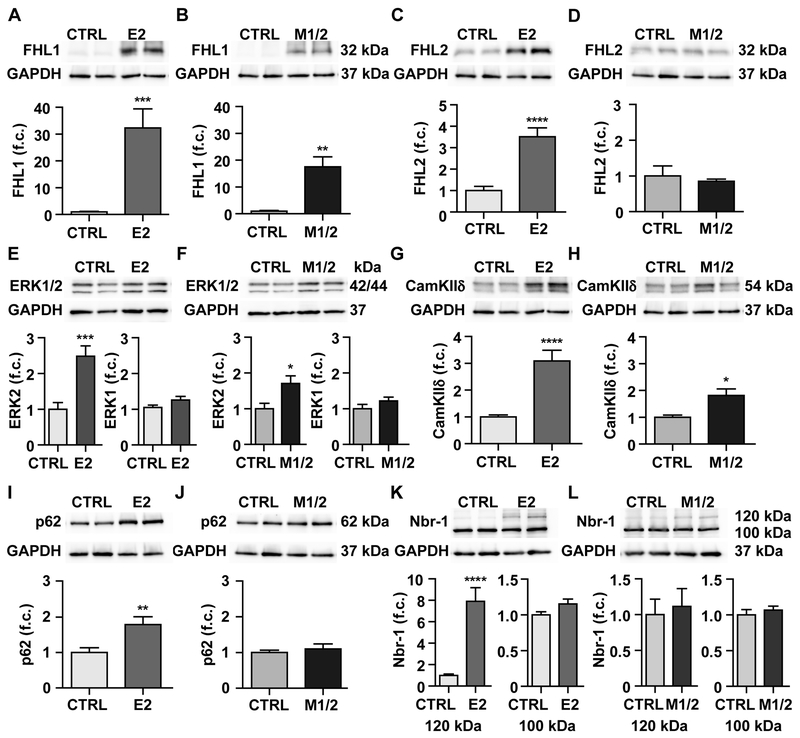
FHL1 binds titin’s N2B region, alters titin’s elasticity via phosphorylation by ERK30 and induces cardiac hypertrophy.13 In both knockouts, FHL1 was upregulated by more than an order of magnitude (> 30-fold E2, > 15-fold M1/2) (Figure 6A). On the RNA level, all isoforms – FHL1-A through –C were affected, but the increase between 2.5 and 7.5 fold suggests an additional regulation at the posttranscriptional level (Supplemental Figure 6A-D). FHL1’s homolog FHL2 also interacts with titin’s N2B region.31 Interestingly, it was differentially regulated exclusively at the protein level and increased only in the E2-KO by > 3-fold (Figure 6C, D; Supplemental Figure 6E). FHL2 RNA levels were unchanged between genotypes (Supplemental Figure 6E). ERK1/2 expression was evaluated as they bind both FHL1 and FHL214 and link to hypertrophy signaling. Only ERK2 was increased in the E2-KO > 2.5-fold and in the M1/2-KO > 1.5-fold (Figure 6E-F) with a corresponding increase in phospho-ERK that reflects the elevated protein levels (Supplemental Figure 6F-G).
Figure 6. Expression of titin binding and stretch signaling proteins.
Western blot analysis of heart lysate from control (CTRL) and E2-KO (left) and control and M1/2-KO (right) at 4 weeks of age. A-D) FHL1 is upregulated in both strains, while FHL2 is only upregulated in the E2-KO. E, F) ERK2 is upregulated in the E2 and to a lesser degree in the M1/2-KO, while ERK1 is not changed. G, H) CamKIIδ is significant upregulated in the E2 and to a lesser degree in the M1/2-KO. I, J) The titin binding protein p62 is upregulated in the E2-, but unchanged in the M1/2-KO. K, L) Nbr1 is unchanged between genotypes, except for the 120 kDa isoform, which is induced almost 8-fold in the E2-KO only. Student’s T-test, * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; n = 8-11 animals per group. f.c. fold change
Additional metabolic and signaling proteins were upregulated in knockout hearts with stronger deregulation in the E2-KO reflecting the increased severity of the phenotype. This includes CamKIIδ,32 which adapts titin’s elastic properties33 and affects striated muscle hypertrophy and remodeling.18 It was upregulated > 3-fold in the E2 and > 2-fold in the M1/2-KO with phosphorylation below background (Figure 6G, H; Supplemental Figure 6H, I). The metabolic enzyme Eno1 as an early marker of myogenesis34 was only increased significantly in E2-KO hearts (Supplemental Figure 6J, K).
MuRF1 is a ubiquitin ligase, which interacts with the titin kinase region35 and is involved in cardiac atrophy/hypertrophy signaling in a complex with Nbr1 and p6236,37 as well as protein degradation.6 While MuRF1 and MuRF2 protein expression was not significantly regulated in either strain at 4 weeks (Supplemental Figure 6L-O), we found both Nbr1 and p62 upregulated exclusively in the E2-KO heart by 8 and 2-fold, respectively (Figure 6I-L). Isoform expression of Nbr1 was not significantly different on the RNA level (Supplemental Figure 6P).
To distinguish a direct effect on protein expression and stability from mislocalization, we compared the subcellular localization of the different classes of deregulated proteins - the chaperone CryAB, the kinase CamKIIδ, and FHL2 as a stretch response protein. All proteins investigated localized properly at the I-band, with no difference of CryAB localization as compared to partially dissolved I-band staining of CamKIIδ in the M1/2-KO and more prominently in the E2-KO. Staining of FHL2 was stronger in the E2-KO, but none of the knockouts resulted in nuclear localization of the protein (Supplemental Figure 7).
Together these changes in protein expression are consistent with a model where the truncated titin in the M1/2-KO contributes to the mechanical properties of the sarcomere, which is less affected than the E2-KO, with no residual contribution from the deficient allele. Thus, deregulation of trophic signaling and increased expression of chaperones are less severe in the M1/2- vs. E2-KO. The mechanical differences of the E2- and M1/2-KO sarcomere translate to altered systolic and diastolic dysfunction only in E2-KO hearts with increased stress signaling via the FHL2/ERK2 axis and the p62/Nbr1 M-band mechanosignaling complex that leads to DCM vs. atrophy in the E2 vs. M1/2-KO.
Discussion:
Titin mutations are a frequent cause of heart disease, and one in four DCM patients carries a truncating mutation of titin.1 It has been suggested that shorter titins cause less severe disease, although the truncated titin proteins are expressed below the detection limit of agarose electrophoresis and coomassie staining.20 For other human titin mutations that do not cause truncations but change aminoacids, the genotype-phenotype relation is often less obvious. Despite the increasing number of titin deficient animal models,8,9,11,12 it is still unclear how these mutations cause hypertrophy or DCM and which associated signaling pathways contribute to the underlying pathology.
Here we dissected the phenotypic contribution of titin protein expressed from the diseased allele from the loss of the functional allele. We generated a titin mutant allele that does not express protein as it lacks the Z-disc exon containing the translation start (E2) as compared to the M1/2-KO, which expresses a truncated titin protein that could exert a dominant negative effect or contribute to the mechanical properties of the myofilament. We generated conditional knockout mice using the MCK-Cre transgene that either eliminate the complete titin protein in striated muscle or deplete the titin M-band resulting in a truncated titin, which does not form a continuous titin filament system but retains an intact Z-disk, I-band and A-band region. The MCK-Cre transgene is active from E1724 and together with titin’s half-life of ~ 2.9d38 leads to a progressive loss of titin. After 35 days of age < 1/4 of wildtype titin protein remains in skeletal muscle and ~1/3 in the heart. This suggests that MCK-Cre induced loss of titin proceeds more efficiently in skeletal muscle than in the heart and that titin levels of 25% in skeletal muscle are compatible with life.
With the E2-KO we show that the reduction of titin protein in the absence of a mutant titin allele leads to DCM. Compared to human truncating titin mutations that lead to nonsense mediated decay,39 the M-band deletion preserves the C-terminus and is not degraded. This leads to the titin M1/2-KO phenotype with cardiac atrophy, which is a unique feature of our homozygous M1/2 deficient mouse and the N2B knockout.8 Cardiac atrophy is not known to occur in human titinopathies, where heterozygous deficient patients gradually develop cardiac hypertrophy or dilation.40
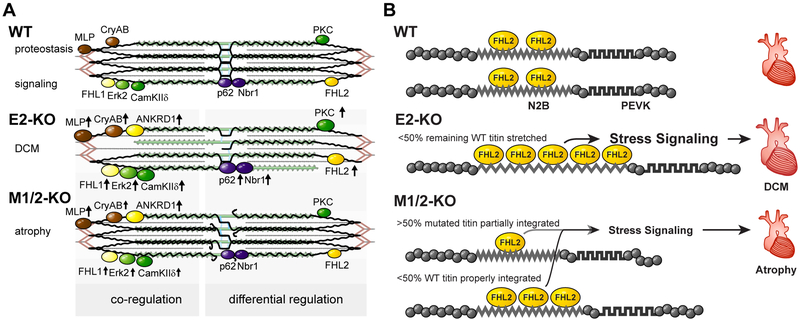
Although both the M1/2- and E2-KO result in sarcomere disassembly in cardiac and skeletal muscle, the molecular pathology is quite distinct. A summary of the molecular changes that relate to the titin filament and its integration into the Z-disc and M-band lattice of the sarcomere as well as the regulation of its binding proteins is provided in Figure 7A.
Figure 7. Titin based mechanotransduction.
A) Schematic representation of the sarcomeric and molecular phenotypes of wildtype (WT), E2-KO, and M1/2-KO mice with titin in black, actin in red, and myosin in green. There are fewer titin molecules in the E2 knockout, while truncated titin does not properly integrate into the M1/2 knockout sarcomere. This leads to destabilization of the sarcomere and altered expression of titin binding proteins. Proteins which are co-regulated in both knockouts compared to the wildtype are depicted on the left, differentially regulated proteins on the right. Arrows indicate regulation of expression. B) Model of FHL2 regulation dependent on mechanical strain. Wildtype titin (WT) binds FHL2 and stabilizes the protein. Upon loss of titin in the E2-KO, we suggest that the remaining titin molecules are stretched extensively providing additional FHL2 binding sites to translate strain into a trophic stress signal. E2-KO hearts thus develop dilated cardiomyopathy (DCM). In the M-band KO, the truncated titin protein is less well integrated and should thus experience less strain resulting in reduced binding of FHL2. The remaining titin is stretched less than the remaining titin in E2 knockouts based on the mechanical contribution of truncated titin. The resulting reduction in stress signaling causes atrophy in M1/2-KO hearts.
Expression of the M-band truncated titin, which is integrated into the Z-disc, but not the M-band results in a stable Z-disc protein network versus a titin deficient M-band. The complete lack of titin results in unoccupied integration sites at both the Z-disc and M-band. Here, we evaluated 4-week-old animal to dissect primary effects on mechanotransduction from secondary changes resulting from progressive sarcomere disassembly. At this early time point, we found no compensatory deregulation of T-cap and myomesin, which integrate titin at the Z-disc and M-band, respectively. Interestingly, myomesin isoform expression is altered at this early stage of the disease towards increased expression of the embryonic EH-isoform in the E2-KO, consistent with its association to DCM.29
To test if the decrease in functional titin molecules leads to changes in systolic or diastolic function and altered titin based stiffness, we used echocardiography and cardiomyocyte mechanics. Indeed we found systolic and diastolic dysfunction only in E2-KO hearts. On the level of the cardiomyocyte, loss of titin resulted in > 70% reduction in passive stress in the E2-KO, while M1/2 cardiomyocytes were largely normal. This is consistent with increased stretch of the remaining healthy wildtype titin and subsequent titin based atrophy/ hypertrophy signaling.
With sarcomere disassembly in both knockouts and a mechanical phenotype in the E2-KO only, we hypothesize that proteins deregulated in both knockouts would mainly relate to sarcomere disassembly while exclusive deregulation in the E2-KO would suggest a role in titin dependent mechanotransduction. Thus, the increased expression of PKCα in E2-KO only might indicate compensatory upregulation to adapt passive properties. We find the stress responsive proteins alpha B crystalline and ANKRD1, as well as MLP, FHL1, ERK2, and CamKIIδ upregulated in both knockouts. For all except MLP and ANKRD1, expression levels are consistently higher in E2- as compared to M1/2-KO hearts, consistent with the severity of the phenotype. The p62/Nbr1 signaling complex links to protein degradation7 and the stress responsive FHL2 are only upregulated in the E2-KO. Their differential regulation can help explain the phenotypic differences of animals with truncated titin vs. loss of titin (Figure 7A): In the M1/2-KO reduced hypertrophy- and increased atrophy signaling lead to reduced heart weight, while balanced hypertrophy and atrophy signaling would preserve myocyte mass of the DCM heart of the E2-KO.
The induction of the chaperone CryAB in response to the partial disintegration of the titin filament is likely related to a compensatory upregulation of protein expression and would help maintain folding and thus function of the remaining titin molecules. PKCα phosphorylates titins PEVK region and increases passive stiffness of the titin filament41, while CamKIIδ phosphorylates titin’s N2B and PEVK region and decreases titin based stiffness.19,32,33 It also regulates hypertrophy signaling and thus relates to cardiac disease.18 The predominant induction after initiation of sarcomere disassembly in the E2-KO, is expected to reduce titin based stiffness consistent with the results of the cardiomyocyte experiments. FHL1 binds to titin’s N2B-domain and is also involved in the hypertrophy signaling.13 Mutations in FHL1 lead to hypertrophic cardiomyopathy (HCM).42,43 We find FHL1 upregulated and differential isoform expression with a shift to FHL1b in the homozygous knockouts. A similar upregulation of FHL1 (independent of the type of the disease) has been reported previously in other titin mutants and animal models deficient in titin splicing.12,25,44
Unlike FHL1, the titin N2B binding protein FHL2 was only upregulated in our E2-KO, implying a role in the differential cardiac phenotypes. Human mutations in FHL2 or the titin N2B domain, which disrupt their interaction, result in DCM.45,46 On the other hand, mutations in titin’s N2B region which lead to increased binding of FHL2 result in hypertrophy.45 In the titin N2B knockout with cardiac atrophy, which lacks the FHL2 binding site in titin, FHL2 is downregulated.8 In the hypertrophic titin PEVK knockout, the knockout of titin’s IG-domains or the A/I junction, which all lead to increased stretch of the remaining N2B domain, result in increased FHL2 levels.9,11,12 Conversely, reduced strain on the titin filament secondary to the deletion of the titin splice factor RBM20 decreased FHL2 protein levels.25 In the M-band KO, this would argue for an effect of the remaining unstretched M-band deficient titin-filament that results in reduced FHL2 levels while the overstretched remaining wildtype titin leads to an increase in FHL2. The net effect in the M-band KO would be normal FHL2 levels as compared to the E2-KO with only overstretched titin and thus increased FHL2. The signaling pathway downstream of FHL2 involves ERK, which binds to FHL2. Activated ERK is thus retained in the cytosol preventing a hypertrophic gene response.14 Indeed ERK2 is differentially regulated in the E2 versus M1/2-KO mice. A model on the stretch depending regulation of FHL2 is provided in Figure 7B.
Taken together, we found that deletion of complete titin or titin’s M-band leads to similar phenotypes in the skeletal muscle but opposing phenotypes in the heart. The major difference between cardiac and skeletal muscle titin is the presence of the N2B domain. Both FHL1 and FHL2 bind to the N2B region, but while FHL2 is exclusively upregulated in the E2-KO, FHL1 is upregulated in both strains. This supports a dominant role of FHL2 in atrophy / hypertrophy signaling initiated by mechanical input acting on titin’s N2B domain. Additional trophic signaling originates from the M-band, as Nbr1 and p62, which bind proximal to the titin kinase domain, are upregulated only in the E2-KO. Progressive depletion of titin leads to increased mechanical strain on the remaining titin molecules that translates to compensatory hypertrophy signaling via FHL2, Nbr1, and p62 and no atrophy but DCM in the E2-KO heart. The additional presence of titin´s I-band in the M1/2-KO causes an opposite effect on FHL2 and leads to cardiac atrophy.
The increased severity of the functional impairment and molecular phenotype in E2- vs. M1/2-KO animals suggests a contribution of the truncated titin protein to the mechanical properties of the sarcomere and argues against a dominant negative effect from the M-band deficient titin. Similarly, other titin mutations that cause expression of truncated titin isoforms which help stabilize sarcomere structure or impair mechanotransduction can lead to diverse cardiac hypertrophy or atrophy phenotypes and support a personalized medicine approach to the future treatment of the diverse titinopathies.
Supplementary Material
Clinical Perspective:
What is new?
Truncations versus loss of titin differentially affect cardiac pathology with atrophy vs DCM, respectively.
The differential trophic phenotypes relate to FHL2 and the M-band signaling complex with Nbr1 and p62 as key players in sensing cardiac strain.
Skeletal muscle atrophy and sarcomere disassembly are identical in knockout mice with titin truncation or depletion.
What are the clinical implications?
Our findings are clinically relevant for understanding genotype-phenotype relations of titin mutations, the most common genetic basis of heart disease.
Understanding the integration of titin based signaling and sarcomere biology could help personalize diagnostics for improved clinical decisions and identify suitable therapeutic targets for titinopathies.
Acknowledgments:
We are grateful to Carmen Judis for expert technical assistance, Nora Bergman for support with constructing the E2-targeting vector, the transgenic core facility for the ES-cell injection and Bettina Purfürst for support with electron microscopy (all MDC). We thank Arnd Heuser and Martin Taube for support with echocardiography analysis.
Funding Sources:
This work was funded by the German research Foundation (DFG), the Alexander von Humboldt foundation (to M.G.), and R01HL115988 (to H.G.); C.P. was supported by a MyoGrad fellowship from the DFG.
Footnotes
Disclosures:
None
References:
- 1.Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJR, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackman P, Marchand S, Sarparanta J, Vihola A, Pénisson-Besnier I, Eymard B, Pardal-Fernández JM, Hammouda E-H, Richard I, Illa I, Udd B. Truncating mutations in C-terminal titin may cause more severe tibial muscular dystrophy (TMD). Neuromuscul Disord NMD. 2008;18:922–928. [DOI] [PubMed] [Google Scholar]
- 3.Mues A, van der Ven PF, Young P, Fürst DO, Gautel M. Two immunoglobulin-like domains of the Z-disc portion of titin interact in a conformation-dependent way with telethonin. FEBS Lett. 1998;428:111–114. [DOI] [PubMed] [Google Scholar]
- 4.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. [DOI] [PubMed] [Google Scholar]
- 5.Obermann WM, Gautel M, Weber K, Furst DO. Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 1997;16:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. [DOI] [PubMed] [Google Scholar]
- 7.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The Kinase Domain of Titin Controls Muscle Gene Expression and Protein Turnover. Science. 2005;308:1599–1603. [DOI] [PubMed] [Google Scholar]
- 8.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. ProcNatlAcadSciUSA. 2007;104:3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granzier HL, Radke MH, Peng J, Westermann D, Nelson OL, Rost K, King NMP, Yu Q, Tschöpe C, McNabb M, Larson DF, Labeit S, Gotthardt M. Truncation of titin’s elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng J, Raddatz K, Molkentin JD, Wu Y, Labeit S, Granzier H, Gotthardt M. Cardiac hypertrophy and reduced contractility in hearts deficient in the titin kinase region. Circulation. 2007;115:743–751. [DOI] [PubMed] [Google Scholar]
- 11.Granzier HL, Hutchinson KR, Tonino P, Methawasin M, Li FW, Slater RE, Bull MM, Saripalli C, Pappas CT, Gregorio CC, Smith JE. Deleting titin’s I-band/A-band junction reveals critical roles for titin in biomechanical sensing and cardiac function. Proc Natl Acad Sci U S A. 2014;111:14589–14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CS, Hutchinson KR, Methawasin M, Saripalli C, Smith JE, Hidalgo CG, Luo X, Labeit S, Guo C, Granzier HL. Shortening of the elastic tandem immunoglobulin segment of titin leads to diastolic dysfunction. Circulation. 2013;128:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheikh F, Raskin A, Chu P-H, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118:3870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell NH, Darwis D, Bueno OF, Muller JM, Schule R, Molkentin JD. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. MolCellBiol. 2004;24:1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol. 2009;41:2351–2355. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. [DOI] [PubMed] [Google Scholar]
- 18.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkin J, Slater R, Del Favero G, Lanzicher T, Hidalgo C, Anderson B, Smith JE, Sbaizero O, Labeit S, Granzier H. Phosphorylating Titin’s Cardiac N2B Element by ERK2 or CaMKIIδ Lowers the Single Molecule and Cardiac Muscle Force. Biophys J. 2015;109:2592–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts AM, Ware JS, Herman DS, Schafer S, Baksi J, Bick AG, Buchan RJ, Walsh R, John S, Wilkinson S, Mazzarotto F, Felkin LE, Gong S, L MacArthur JA, Cunningham F, Flannick J, Gabriel SB, Altshuler DM, Macdonald PS, Heinig M, Keogh AM, Hayward CS, Banner NR, Pennell DJ, O’Regan DP, San TR, de Marvao A, W Dawes TJ, Gulati A, Birks EJ, Yacoub MH, Radke M, Gotthardt M, Wilson JG, O’Donnell CJ, Prasad SK, R Barton PJ, Fatkin D, Hubner N, Seidman JG, Seidman CE, Cook SA. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci Transl Med. 2015;7:270ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begay RL, Graw S, Sinagra G, Merlo M, Slavov D, Gowan K, Jones KL, Barbati G, Spezzacatene A, Brun F, Di Lenarda A, Smith JE, Granzier HL, Mestroni L, Taylor M, Familial Cardiomyopathy Registry. Role of Titin Missense Variants in Dilated Cardiomyopathy. J Am Heart Assoc. 2015;4:e002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotthardt M, Hammer RE, Hubner N, Monti J, Witt CC, McNabb M, Richardson JA, Granzier H, Labeit S, Herz J. Conditional expression of mutant M-line titins results in cardiomyopathy with altered sarcomere structure. J Biol Chem. 2003;278:6059–6065. [DOI] [PubMed] [Google Scholar]
- 23.Dymecki SM. Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. ProcNatlAcadSciUSA. 1996;93:6191–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–69. [DOI] [PubMed] [Google Scholar]
- 25.Hinze F, Dieterich C, Radke MH, Granzier H, Gotthardt M. Reducing RBM20 activity improves diastolic dysfunction and cardiac atrophy. J Mol Med Berl Ger. 2016;94:1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinert S, Bergmann N, Luo X, Erdmann B, Gotthardt M. M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere. JCell Biol. 2006;173:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. [DOI] [PubMed] [Google Scholar]
- 28.Laure L, Suel L, Roudaut C, Bourg N, Ouali A, Bartoli M, Richard I, Danièle N. Cardiac ankyrin repeat protein is a marker of skeletal muscle pathological remodelling. FEBS J. 2009;276:669–684. [DOI] [PubMed] [Google Scholar]
- 29.Schoenauer R, Emmert MY, Felley A, Ehler E, Brokopp C, Weber B, Nemir M, Faggian GG, Pedrazzini T, Falk V, Hoerstrup SP, Agarkova I. EH-myomesin splice isoform is a novel marker for dilated cardiomyopathy. Basic Res Cardiol. 2011;106:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raskin A, Lange S, Banares K, Lyon RC, Zieseniss A, Lee LK, Yamazaki KG, Granzier HL, Gregorio CC, McCulloch AD, Omens JH, Sheikh F. A novel mechanism involving four-and-a-half LIM domain protein-1 and extracellular signal-regulated kinase-2 regulates titin phosphorylation and mechanics. J Biol Chem. 2012;287:29273–29284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lange S, Auerbach D, McLoughlin P, Perriard E, Schäfer BW, Perriard J-C, Ehler E. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115:4925–4936. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo CG, Chung CS, Saripalli C, Methawasin M, Hutchinson KR, Tsaprailis G, Labeit S, Mattiazzi A, Granzier HL. The multifunctional Ca(2+)/calmodulin-dependent protein kinase II delta (CaMKIIδ) phosphorylates cardiac titin’s spring elements. J Mol Cell Cardiol. 2013;54:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamdani N, Krysiak J, Kreusser MM, Neef S, Dos Remedios CG, Maier LS, Krüger M, Backs J, Linke WA. Crucial role for Ca2(+)/calmodulin-dependent protein kinase-II in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ Res. 2013;112:664–674. [DOI] [PubMed] [Google Scholar]
- 34.Fougerousse F, Edom-Vovard F, Merkulova T, Ott MO, Durand M, Butler-Browne G, Keller A. The muscle-specific enolase is an early marker of human myogenesis. J Muscle Res Cell Motil. 2001;22:535–544. [DOI] [PubMed] [Google Scholar]
- 35.Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of Muscle Specific Ring Finger Proteins as Potential Regulators of the Titin Kinase Domain. JMolBiol. 2001;306:717–726. [DOI] [PubMed] [Google Scholar]
- 36.Willis MS, Rojas M, Li L, Selzman CH, Tang R-H, Stansfield WE, Rodriguez JE, Glass DJ, Patterson C. Muscle ring finger 1 mediates cardiac atrophy in vivo. Am J Physiol Heart Circ Physiol. 2009;296:H997–H1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willis MS, Ike C, Li L, Wang D-Z, Glass DJ, Patterson C. Muscle ring finger 1, but not muscle ring finger 2, regulates cardiac hypertrophy in vivo. Circ Res. 2007;100:456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isaacs WB, Kim IS, Struve A, Fulton AB. Biosynthesis of titin in cultured skeletal muscle cells. J Cell Biol. 1989;109:2189–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schafer S, de Marvao A, Adami E, Fiedler LR, Ng B, Khin E, Rackham OJL, van Heesch S, Pua CJ, Kui M, Walsh R, Tayal U, Prasad SK, Dawes TJW, Ko NSJ, Sim D, Chan LLH, Chin CWL, Mazzarotto F, Barton PJ, Kreuchwig F, de Kleijn DPV, Totman T, Biffi C, Tee N, Rueckert D, Schneider V, Faber A, Regitz-Zagrosek V, Seidman JG, Seidman CE, Linke WA, Kovalik J-P, O’Regan D, Ware JS, Hubner N, Cook SA. Titin-truncating variants affect heart function in disease cohorts and the general population. Nat Genet. 2017;49:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victor A McKusick AH. OMIM Entry - TITIN [Internet]. OMIM Entry - 188840 - TITIN TTN. 2017. [cited 2018 Sep 25];Available from: https://www.omim.org/entry/188840
- 41.Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–638, 17 p following 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geier C, Perrot A, Ozcelik C, Binner P, Counsell D, Hoffmann K, Pilz B, Martiniak Y, Gehmlich K, van D, Furst DO, Vornwald A, von HE, Nurnberg P, Scheffold T, Dietz R, Osterziel KJ. Mutations in the human muscle LIM protein gene in families with hypertrophic cardiomyopathy. Circulation. 2003;107:1390–1395. [DOI] [PubMed] [Google Scholar]
- 43.Friedrich FW, Wilding BR, Reischmann S, Crocini C, Lang P, Charron P, Müller OJ, McGrath MJ, Vollert I, Hansen A, Linke WA, Hengstenberg C, Bonne G, Morner S, Wichter T, Madeira H, Arbustini E, Eschenhagen T, Mitchell CA, Isnard R, Carrier L. Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy. Hum Mol Genet. 2012;21:3237–3254. [DOI] [PubMed] [Google Scholar]
- 44.Christodoulou DC, Wakimoto H, Onoue K, Eminaga S, Gorham JM, DePalma SR, Herman DS, Teekakirikul P, Conner DA, McKean DM, Domenighetti AA, Aboukhalil A, Chang S, Srivastava G, McDonough B, De Jager PL, Chen J, Bulyk ML, Muehlschlegel JD, Seidman CE, Seidman JG. 5’RNA-Seq identifies Fhl1 as a genetic modifier in cardiomyopathy. J Clin Invest. 2014;124:1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto Y, Hayashi T, Inagaki N, Takahashi M, Hiroi S, Nakamura T, Arimura T, Nakamura K, Ashizawa N, Yasunami M, Ohe T, Yano K, Kimura A. Functional analysis of titin/connectin N2-B mutations found in cardiomyopathy. JMuscle ResCell Motil. 2005;26:367–374. [DOI] [PubMed] [Google Scholar]
- 46.Arimura T, Hayashi T, Matsumoto Y, Shibata H, Hiroi S, Nakamura T, Inagaki N, Hinohara K, Takahashi M, Manatsu S-I, Sasaoka T, Izumi T, Bonne G, Schwartz K, Kimura A. Structural analysis of four and half LIM protein-2 in dilated cardiomyopathy. Biochem Biophys Res Commun. 2007;357:162–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.