Abstract
The Revised International Staging System (R-ISS) combines ISS with genetic markers and lactate dehydrogenase, and can prognosticate newly diagnosed multiple myeloma (MM). Early relapse (<24 months) after upfront autologous hematopoietic cell transplantation (AHCT) strongly predicts inferior overall survival (OS). We examined the ability of R-ISS in predicting early relapse and its independent prognostic effect on post-relapse survival after an early relapse. Using the Center for International Blood and Marrow Transplant Research database, we identified MM patients receiving first AHCT within 18 months after diagnosis with available R-ISS stage at diagnosis (n= 628). Relative risks of relapse/progression, progression-free survival (PFS) and OS were calculated with R-ISS group as a predictor in multivariate analysis. Among early relapsers, post-relapse survival was tested to identify factors affecting post-relapse OS. The cumulative incidence of early relapse was 23%, 39% and 50% for R-ISS I, R-ISS II and R-ISS III, respectively (p <0.001). Shorter PFS and OS were seen with higher stage R-ISS. R-ISS was independently predictive for inferior post-relapse OS among early relapsers, as was the presence of ≥3 comorbidities and the use of ≥2 induction chemotherapy lines. R-ISS stage at diagnosis predicts early post-AHCT relapse and independently affects post-relapse survival among early relapsers.
Keywords: myeloma stage, post-relapse survival, transplant
Introduction
Novel anti-myeloma agents, including proteasome inhibitors and immunomodulatory drugs, incorporated into induction therapy before high-dose therapy and autologous hematopoietic cell transplant (AHCT) have significantly improved survival in multiple myeloma (MM) patients over the past two decades, as shown by randomized trials and retrospective series. (1,2) Nonetheless, responses to novel agents vary among various biological subgroups. (3) A clearer understanding of the important prognostic factors for survival can improve risk stratification and therapeutic decision-making.
Available evidence shows a strong association between pre-transplant depth of response and post-transplant progression-free survival (PFS) and overall survival (OS). (4,5) Despite achieving deep pre-AHCT responses, some patients relapse early and have very poor OS. (6,7) Similarly, data from the Arkansas group suggest that the loss of an established complete response in the first 3 years confers an inferior prognosis when compared with a lower level of response that is sustained over time. (8) Thus early relapse represents a dynamic high-risk marker that is unknown at diagnosis and available only during the natural course of disease evolution.
The revised International Staging System (R-ISS), proposed in 2015 as a more accurate prognostic model for newly diagnosed MM, incorporates ISS stage, serum lactate dehydrogenase (LDH) and high-risk cytogenetics assessed by interphase fluorescent in-situ hybridization (FISH). High-risk cytogenetic abnormalities defined as the presence of del(17p) and/or t(4;14) and/or t(14;16), or an elevated LDH above the upper limit of normal are risk factors that upstage patients in the R-ISS system. At a median follow-up of 46 months, the 5-year OS rate was 82% in the R-ISS I, 62% in the R-ISS II, and 40% in the R-ISS III groups; the 5-year PFS rates were 55%, 36%, and 24%, respectively. (9)
We analyzed the impact of R-ISS stage at diagnosis to predict early post-AHCT relapse (defined as relapse/progression within 24 months after AHCT) and the influence of the R-ISS stage on post-relapse survival using the Center for International Blood and Marrow Transplant Research (CIBMTR®) database.
Patients and methods
Data Sources
The CIBMTR® (Center for International Blood and Marrow Transplant Research®) is research collaboration between the National Marrow Donor Program®/Be The Match® and the Medical College of Wisconsin. It comprises a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on allogeneic and autologous hematopoietic cell transplantation. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits and patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on site audits of participating centers ensure data quality. Studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
The CIBMTR collects data at two levels: Transplant Essential Data (TED) level and Comprehensive Report Form (CRF) level. The TED-level data is an internationally accepted standard data set that contains a limited number of key variables for all consecutive transplant recipients. TED-level data, with some additional details of donor and graft characteristics, comprise the obligatory data submitted to the SCTOD (Stem Cell Therapeutic Outcomes Database). When a transplant is registered with the CIBMTR, a subset of patients are selected for the CRF level of data collection through a weighted randomization scheme. The CRF-level captures additional patient, disease and treatment-related data. TED and CRF level data are collected pre-transplant, 100 days and six months post-transplant, annually until year 6 post-transplant and biannually thereafter until death.
Patient selection
Patients with MM receiving an upfront AHCT defined as AHCT within 18 months after diagnosis, with melphalan conditioning following a novel agent-based induction, and transplanted between 2008 and 2014 were included in this analysis. Based on the availability of all components of the R-ISS schema, 628 patients were included. The same data set was used for a recently published CIBMTR analysis. (10)
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the study population using the median value and range for continuous variables and the frequency and percentage for categorical variables. Group comparisons were done by Kruskall-Wallis test, chi-square test, and Fisher exact test. The endpoints of interest included disease response, progression-free survival (PFS), and OS after transplant. Transplant-related mortality (TRM) was defined as mortality after transplant in the absence of disease relapse or progression. Disease response and progression were assessed using the international Myeloma Working Group (IMWG) consensus criteria. (11) PFS was defined as the interval without progressive disease with patients alive and without progression/relapse censored at last follow-up. OS was defined as time interval from diagnosis or time of relapse (in the case of post relapse OS) till death from any cause with survivors censored at last follow-up. Survival probabilities were calculated by using the Kaplan-Meier estimator with the variance estimated by Greenwood’s formula. We also examined post-relapse survival from the date of relapse/progression in patients with documented relapse or myeloma progression occurring within 24 months of AHCT. Age, gender, Karnofsky score, R-ISS, HCT comorbidity index, clinical trial enrollment, novel versus non-novel induction treatment, lines of chemotherapy, disease status at transplant, time from diagnosis to transplant, year of transplant and melphalan conditioning dose were tested in multivariate analysis.
Results
Patient characteristics and treatment details are shown in table 1. More patients in R-ISS III cohort required more than 1 line of chemotherapy prior to AHCT (29% compared to 20% for R-ISS II and 15% for R-ISS I). Eighty seven percent of patients proceeded to AHCT within 12 months of diagnosis. Pre transplant disease status of ≥VGPR was similar across the R-ISS stages I,II and III at 49%,53% and 52%, respectively. Post-transplant maintenance was administered to 74, 68 and 70%, respectively, within each R-ISS stage cohort.
Table 1.
Characteristics of US adult patients who underwent melphalan base first auto PB MM transplant from 2008–2014 and reported with CIBMTR
| R-ISS I | R-ISS II | R-ISS III | |
|---|---|---|---|
| Number of patients | 199 | 360 | 69 |
| Number of centers | 55 | 64 | 33 |
| Median age at HCT (range) | 59 (41–76) | 60 (40–78) | 60 (43–75) |
| Male Gender | 115 (58) | 213 (59) | 41 (59) |
| Karnofsky score | |||
| 90–100 | 120 (60) | 198 (55) | 35 (51) |
| <90 | 73 (37) | 156 (43) | 29 (42) |
| Missing | 6 (3) | 6 (2) | 5 (7) |
| HCT-CI | |||
| 0 | 85 (43) | 108 (30) | 15 (22) |
| 1 | 28 (14) | 61 (17) | 9 (13) |
| 2 | 26 (13) | 66 (18) | 15 (22) |
| ≥3 | 60 (30) | 122 (33) | 30 (43) |
| Missing | 0 | 3 (<1) | 0 |
| Clinical Trial Enrollment | 76 (38) | 118 (33) | 18 (26) |
| LDH at diagnosis ≥ upper limit | 0 | 71 (20) | 58 (84) |
| ISS stage at diagnosis | |||
| Stage I | 199 | 45 (13) | 0 |
| Stage II | 0 | 214 (59) | 0 |
| Stage III | 0 | 101 (28) | 69 |
| Cytogenetic abnormality (conventional or FISH) | |||
| t(4;14)only | 0 | 16 (4) | 8 (12) |
| t(14; 16) only | 0 | 4 (1) | 3 (4) |
| Del17p only | 0 | 14 (4) | 4 (6) |
| 1q abnormality | 13 (7) | 25 (7) | 5 (7) |
| ≥ 2 High risk | 0 | 9 (3) | 5 (7) |
| No high risk Abnormality | 186 (93) | 292 (81) | 44 (64) |
| Lines of chemotherapy | |||
| 1 | 170 (85) | 289 (80) | 49 (71) |
| ≥2 | 29 (15) | 71 (20) | 20 (29) |
| Pre-transplant induction chemotherapy* | |||
| VTD | 15 (8) | 18 (5) | 5(7) |
| VRD | 89 (45) | 162 (45) | 31 (45) |
| VCD | 29 (15) | 59 (16) | 15 (22) |
| VD | 17 (9) | 37 (10) | 9 (13) |
| RD | 35 (18) | 68 (19) | 5 (7) |
| TD | 14 (7) | 16 (4) | 4 (6) |
| Disease status prior to HCT** | |||
| sCR/CR | 41 (21) | 70 (19) | 12 (17) |
| VGPR | 55 (28) | 122 (34) | 24 (35) |
| PR/SD/PD | 103 (52) | 168 (47) | 11 (48) |
| Melphalan dose (mg/m2) | |||
| 140 | 46 (23) | 89 (25) | 19 (28) |
| 200 | 153 (77) | 271 (75) | 50 (72) |
| Time from diagnosis to transplant | |||
| ≤ 6 months | 69 (35) | 139 (39) | 31 (45) |
| 6–12 months | 110 (55) | 172 (48) | 29 (42) |
| 12–18 months | 20 (10) | 49 (14) | 9 (13) |
| Year of transplant | |||
| 2008 | 44 (22) | 94 (26) | 11 (16) |
| 2009 | 17 (9) | 32 (9) | 6 (9) |
| 2010 | 8 (4) | 23 (6) | 10 (14) |
| 2011 | 30 (15) | 46 (13) | 6 (9) |
| 2012 | 39 (20) | 46 (13) | 8 (12) |
| 2013 | 30 (15) | 70 (19) | 9 (13) |
| 2014 | 31 (16) | 49 (14) | 19 (28) |
| Planned post-transplant treatment | 147 (74) | 245 (68) | 48 (70) |
| Median follow-up of survivors (range), months | 47 (6–97) | 48 (3–99) | 40 (12–97) |
Abbreviation: Bortezomib (V), Thalidomide (T), Dexamethasone (D), Lenalidomide (R), Cyclophosphamide (C)
Abbreviation: Stringent complete response (sCR), Complete response (CR), Very good partial response (VGPR), Partial response (PR), Stable disease (SD), Progressive disease (PD)
Univariate analysis for Relapse/Progression, PFS and OS (Table 2):
Table 2.
Univariate analysis (R-ISS stage)
| Stage I | Stage II | Stage III | |||||
|---|---|---|---|---|---|---|---|
| (N = 199) | (N = 360) | (N = 69) | |||||
| N | Prob | N | Prob | N | Prob | ||
| Outcomes | Eval | (95% CI) | Eval | (95% CI) | Eval | (95% CI) | p-value |
| Relapse/progression | 198 | 358 | 69 | <0.001 | |||
| 1-year | 10 (6–14)% | 21 (17–26)% | 38 (27–49)% | <0.001 | |||
| 2-year | 23 (17–29)% | 39 (33–44)% | 50 (38–62)% | <0.001 | |||
| 3-year | 35 (28–42)% | 50 (44–55)% | 65 (51–78)% | <0.001 | |||
| PFS | 198 | 358 | 69 | <0.001 | |||
| 1-year | 90 (85–94)% | 77 (72–81)% | 61 (49–72)% | <0.001 | |||
| 2-year | 77 (70–82)% | 59 (54–64)% | 47 (35–59)% | <0.001 | |||
| 3-year | 64 (57–71)% | 47 (41–53)% | 32 (20–45)% | <0.001 | |||
| Overall survival | 199 | 360 | 69 | <0.001 | |||
| 1-year | 97 (95–99)% | 93 (90–95)% | 88 (80–95)% | 0.005 | |||
| 2-year | 96 (92–98)% | 85 (81–88)% | 71 (59–82)% | <0.001 | |||
| 3-year | 88 (83–93)% | 75 (70–80)% | 56 (43–69)% | <0.001 | |||
Abbreviation: N, number; Prob, probability; Eval, evaluable; CI, confidence interval; PFS, progression-free survival
The incidence of relapse/progression was higher, and PFS/OS were inferior with higher R-ISS stage (table 2). The cumulative incidence of early relapse within 24 months of AHCT was 23% for R-ISS I, 39% for R-ISS II and 50% for R-ISS III groups (p <0.001). Table 2 summarizes the survival data. 3-year PFS for R-ISS stages I, II and III were 64 (57–71), 47 (41–53) % and 32 (20–45) % (p <0.001) respectively. The 3-year OS for R-ISS stages I, II and III were 88 (95% CI:83–93) %; 75 (95% CI:70–80)%; 56 (95% CI:43–69)% (p<0.001), respectively.
Multivariate analysis for OS and post-relapse survival for early relapses:
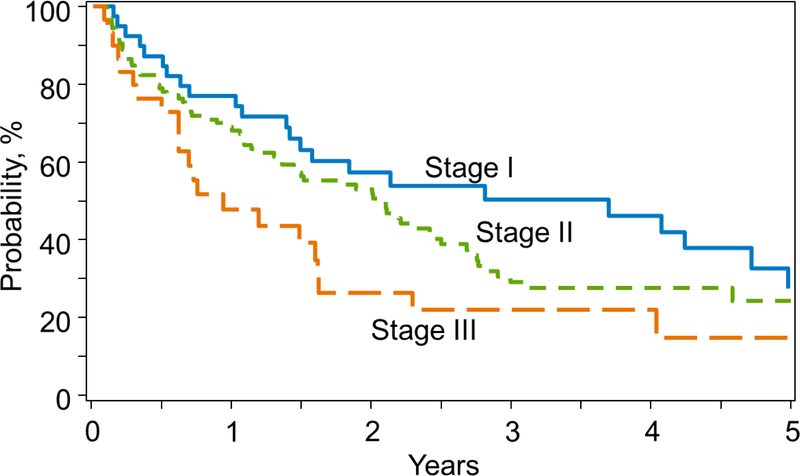
Multivariate analysis showed that R-ISS III at diagnosis was independently prognostic for both OS from transplant (Table 3) and post-relapse survival in early relapses (table 4). Higher R-ISS stage; HCT-comorbidity index of ≥3 and ≥2 lines of pre-ASCT chemotherapy and relapse after full dose melphalan conditioning with 200 mg/m2 dose were significant factors associated with shorter OS overall. While treatment era (2008–2011 vs 2012–2014) was a significant prognostic factor for OS in the whole cohort, it was not significant for survival in the early relapse cohort. Figure 1 shows post-relapse survival by R-ISS groups. Median post relapse survival after an early relapse was 4.1 years, 2.5 years and 1.5 years for R-ISS I,II and III at diagnosis respectively.
Table 3.
Multivariate analysis for OS for the whole cohort (R-ISS)
| 606 | |||||||
| R-ISS | Overall | 0.0004 | |||||
| I | 193 | 1.00 | |||||
| II | 349 | 1.83 | 1.225 | 2.726 | 0.003 | ||
| III | 64 | 2.82 | 1.657 | 4.813 | 0.0001 | ||
| HCT Comorbidity Index | Overall | 0.013 | |||||
| 0 | 204 | 1.00 | |||||
| 1–2 | 199 | 1.02 | 0.687 | 1.526 | 0.907 | ||
| 3+ | 203 | 1.66 | 1.129 | 2.444 | 0.010 | ||
| Lines of Chemotherapy | Overall | 0.007 | |||||
| 1 | 491 | 1.00 | |||||
| 2+ | 115 | 1.65 | 1.14 | 2.30 | 0.007 | ||
| Year of Transplant | Overall | 0.006 | |||||
| 2012–2014 | 296 | 1.00 | |||||
| 2008–2011 | 310 | 1.77 | 1.179 | 2.652 | 0.006 |
Abbreviation: OS, overall survival; R-ISS, Revised-International Staging System; N, number; HCT, hematopoietic cell transplantation
Table 4.
Multivariate Analysis of OS in patients who relapse early (Analysis limited patients relapsing <24 mo post transplant)
| 197 | |||||||
| R-ISS | Overall | 0.036 | |||||
| I | 42 | 1.00 | |||||
| II | 126 | 1.31 | 0.824 | 2.089 | 0.252 | ||
| III | 29 | 2.15 | 1.190 | 3.879 | 0.011 | ||
| HCT-CI | Overall | 0.017 | |||||
| 0 | 65 | 1.000 | |||||
| 1–2 | 61 | 0.95 | 0.593 | 1.520 | 0.827 | ||
| 3+ | 71 | 1.69 | 1.091 | 2.624 | 0.019 | ||
| Lines of Chemotherapy | Overall | 0.037 | |||||
| 1 | 146 | 1.00 | |||||
| 2+ | 51 | 1.52 | 1.026 | 2.252 | 0.037 | ||
| Melphalan Dose | Overall | 0.023 | |||||
| 140 | 54 | 1.00 | |||||
| 200 | 143 | 1.65 | 1.072 | 2.537 | 0.023 |
Abbreviation: N, number; R-ISS, Revised-International Staging System; HCT-CI, hematopoietic cell transplantation-comorbidity index
Figure 1.
Post-relapse survival of early relapsers by R-ISS
Impact of Maintenance therapy:
Intent to post-transplant maintenance therapy was reported in 74, 68 and 70% of R-ISS I, II and III patients, respectively (Table 1). Comparison of survival after early relapse between those who received maintenance and no maintenance (table 5) indicated that there was no difference in survival after early relapse regardless of utilization of maintenance post AHCT(p=0.86). The median time to relapse post transplant was similar in the maintenance and no maintenance arms. As our analysis was specifically looking at impact of maintenance in post relapse OS, this manuscript does not report on response or rate of relapse in the maintenance vs no maintenance cohorts. Likewise, as all maintenance strategies were taken together, impact of proteasome inhibitors vs immunomodulatory agents in maintenance is not included in this analysis.
Table 5.
Univariate analysis of impact of maintenance in the early relapse group (Post relapse overall survival in maintenance vs no maintenance)
| No planned post-maintenance | Planned post-maintenance | ||||
|---|---|---|---|---|---|
| Number of patients | 127 | 70 | |||
| Best response post-transplant | |||||
| ≥VGPR | 59 (46) | 39 (56) | |||
| <VGPR | 68 (54) | 31 (44) | |||
| Median time to relapse after | 10 (<l-24) | 12 (1–24) | |||
| transplant | |||||
| Overall survival post relapse | |||||
| N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | P-value | |
| 127 | 70 | 0.86 | |||
| 1-year | 67 (59–76)% | 66 (54–77)% | 0.82 | ||
| 2-year | 48 (39–57)% | 51 (39–64)% | 0.67 | ||
| 3-year | 30 (21–39)% | 38 (24–53)% | 0.33 | ||
Abbreviation: N, number; Eval, evaluable; Prob, probability; CI, confidence interval
Discussion
In this contemporaneous CIBMTR study, we make the following clinically important observations: 1) R-ISS stage group predicts for early relapse after an upfront AHCT; 2) R-ISS independently predicts for post-relapse survival among those relapsing early; 3) in addition to R-ISS, higher HCT-CI, number of lines of induction chemotherapy pre-AHCT and relapse after standard dose melphalan when compared to lower dose of melphalan, were associated with inferior post-relapse survival among early relapse patients and 4) maintenance treatment did not impact post-relapse survival among early relapsers.
Early relapse defined as relapse within 24 months after AHCT for newly diagnosed MM remains an area of therapeutic challenge even in the modern era of myeloma. While high risk cytogenetics and depth of response post AHCT have been identified as high risk for early relapse, (12) these alone have not been able to characterize the group of patients who have high-risk MM. We, and others, have shown that early relapse post-AHCT is an important prognostic factor determining survival. (6, 12) In this current analysis, we found that despite achievement of deep responses pre-AHCT (approximately 50% of patients achieve ≥VGPR status pre-AHCT), a significant proportion of patients in all R-ISS stages (23% stage I; 39% stage II; 50% stage III) relapse in under 24 months. A recent CIBMTR analysis studying early relapse during three different time periods found that the incidence of early relapse has remained unchanged at ~ 35% between 2001–2004,2005–2008 and 2009–2013 (13) in spite of more patients receiving planned maintenance (72% compared to 6%) between the 2009–2013 and 2001–2004 period. The apparent constancy in the incidence of early relapse despite widespread adoption of novel agents and early AHCT is consistent with the notion that the majority of the benefit from novel agents for MM has accrued to biologically standard risk patients. (14–16) This also means that our current armamentarium for frontline management of myeloma does not address the innate biological features that contribute toward early relapse. Prospective trials of monoclonal antibodies such as daratumumab or elotuzumab added to triplet regimens are ongoing and it remains to be seen whether they can reduce the incidence of early relapse. (17–20)
In addition to R-ISS, HCT-CI >2, receipt of >1 line of pre-AHCT chemotherapy and year of AHCT 2008–2011 (compared to 2012–2014) were associated with worse OS in this cohort. HCT-CI and lines of treatment have been shown to be associated with OS in MM in multiple studies. (21–23) Similarly, receipt of more than 1 line of therapy prior to AHCT has not shown to be of benefit even among patients who achieve suboptimal response to first line of treatment. (24) Year of transplant is a close surrogate for use of maintenance therapy during this period. (24)(25) Indeed, in our study, 29% of patients were reported to receive maintenance in 2008–2011 compared to 51% in 2012–2014.
Survival following early relapse is poor despite availability of novel agents. The median overall survival of R-ISS I, II and III are 4.1, 2.5 and 1.5 years, respectively, in our analysis. R-ISS III at diagnosis was an independent prognostic factor even at the time of early relapse. Kumar et al. reported that in myeloma patients treated between 1994 and 2006, relapse within a year after AHCT confers poor prognosis, with median OS of 10.8 months from the time of relapse. (6) In our recent CIBMTR analysis, the median post relapse survival following early relapse was 24 months for those transplanted after 2005 compared with 16 months prior to 2005. (13) While there was improvement of post-relapse survival after 2005, the improvement has been minimal in the recent years and is similar to what we have observed in the current analysis. The marginal improvement in survival in this setting is likely due to access to new drugs such as pomalidomide, carfilzomib, daratumumab, elotuzumab and other clinical trials. We are able to show the robustness of R-ISS in predicting post-relapse survival with R-ISS III patients showing a far inferior OS of 1.5 years compared to R-ISS I patients showing 4.5 years post-relapse survival.
In our cohort of patients with an early post-AHCT relapse, two groups were identified, those who had received maintenance post-AHCT (N=127) or not (N=70). It is important to note that this analysis did not compare the rate of early relapse in maintenance versus non-maintenance groups. However, the receipt of maintenance did not affect survival in the early relapse group or the median duration to relapse after AHCT. This speaks to the fact that disease biology driving early relapse is probably the most important prognostic factor and newer strategies need to be devised for patients at risk of experiencing early relapse.
The year of transplant was not of prognostic significance in the early relapse group although it was significant for OS in the entire cohort. A significant majority of patients in the era spanning 2012–2014 received maintenance and may reflect the improved survival from maintenance strategies. However, this improvement in survival did not accrue to the early relapse group and again reflects the aggressive biology of disease that led to early relapse. HCT-CI and lines of pre-AHCT treatment remained significant predictors of post-relapse survival even among early relapses. Lastly, we observed that early relapse patients who had received full dose melphalan conditioning (200 mg/m2) had inferior post-relapse survival compared with early relapse following lower dose melphalan (140 mg/m2). Notably melphalan dose was not correlated with OS in the entire cohort. This intriguing finding suggests that early relapse of MM despite full conditioning intensity may behave more aggressively. This analysis is unable to determine the mechanism of this phenomenon: i.e. if early relapse after full melphalan dosage indicates relative refractoriness to subsequent therapies or clonal evolution with the addition of high risk markers induced by high dose melphalan in the setting of genomic instability or if there are other mechanisms mediating this observation.
A recent study by Kastritis et.al reported prognosis of unselected patients who were treated with novel agents using R-ISS staging. The conclusion was verified that R-ISS is a robust tool for risk stratification of newly diagnosed patients with symptomatic myeloma. (26)
Our analysis is limited by the relatively small sample size for the early relapse cohort. This may be reflective of the years of transplant that we studied when the R-ISS was not in full clinical use. The R-ISS was developed in 2015, while our dataset extending between 2008–2014 captures the real-world practice of MM patients receiving AHCT, and we remain optimistic that LDH and FISH studies will be more universally adopted.
In summary, we report that R-ISS at diagnosis predicts the risk of early relapse post-AHCT. Further, the outcomes of patients with R-ISS III disease continue to be poor even in the era of novel drugs in the setting of early relapse and maintenance. Lastly, early relapse a dynamic marker for high risk disease, remains a therapeutic challenge and future studies should also address the prevention of early relapse. Myeloma therapy continues to advance with newer modes of targeted therapies such as monoclonal antibodies, bispecific antibodies, chimeric antigen receptor T-cells, and dendritic cell-based cancer vaccines. (27) Clinical trials targeting patients at risk for early relapse using such newer agents and novel combinations are necessary in order to effect a meaningful improvement in high risk disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sonneveld P, Goldschmidt H, Rosiñol L, Bladé J, Lahuerta JJ, Cavo M, et al. Bortezomib-Based Versus Nonbortezomib-Based Induction Treatment Before Autologous Stem-Cell Transplantation in Patients With Previously Untreated Multiple Myeloma: A Meta-Analysis of Phase III Randomized, Controlled Trials. JCO. American Society of Clinical Oncology; 2013. September 10;31(26):3279–87. [DOI] [PubMed] [Google Scholar]
- 2.Harousseau J-L, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib Plus Dexamethasone Is Superior to Vincristine Plus Doxorubicin Plus Dexamethasone As Induction Treatment Prior to Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: Results of the IFM 2005–01 Phase III Trial. JCO. 2010. October 20;28(30):4621–9. [DOI] [PubMed] [Google Scholar]
- 3.Chng WJ, Dispenzieri A, Chim C-S, Fonseca R, Goldschmidt H, Lentzsch S, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. Nature Publishing Group; 2014. February 1;28(2):269–77. [DOI] [PubMed] [Google Scholar]
- 4.Lahuerta JJ, Paiva B, Vidriales M-B, Cordón L, Cedena M-T, Puig N, et al. Depth of Response in Multiple Myeloma: A Pooled Analysis of Three PETHEMA/GEM Clinical Trials. JCO. American Society of Clinical Oncology; 2017. September;35(25):2900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. Nature Publishing Group; 2013. August 9;28(2):258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. Nature Publishing Group; 2008. June 30;42(6):413–20. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Zepeda VH, Reece DE, Trudel S, Chen C, Tiedemann R, Kukreti V. Early relapse after single auto-SCT for multiple myeloma is a major predictor of survival in the era of novel agents. Bone Marrow Transplant. 2014. October 27;50(2):204–8. [DOI] [PubMed] [Google Scholar]
- 8.Hoering A, Crowley J, Shaughnessy JD, Hollmig K, Alsayed Y, Szymonifka J, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. American Society of Hematology; 2009. August 13;114(7):1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosiñol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. JCO. American Society of Clinical Oncology; 2016. September 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott EC, Hari P, Kumar S, Fraser R, Davila O, Shah N, et al. Staging Systems for Newly Diagnosed Myeloma Patients Undergoing Autologous Hematopoietic Cell Transplantation: The Revised International Staging System Shows the Most Differentiation between Groups. Biology of Blood and Marrow Transplantation. 2018. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International uniform response criteria for multiple myeloma. Leukemia. Nature Publishing Group; 2006. July 20;20(9):1467–73. [DOI] [PubMed] [Google Scholar]
- 12.Scott EC, Hari P, Sharma M, Le-Rademacher J, Huang J, Vogl D, et al. Post-Transplant Outcomes in High-Risk Compared with Non–High-Risk Multiple Myeloma: A CIBMTR Analysis. Biology of Blood and Marrow Transplantation. Elsevier; 2016. October 1;22(10):1893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar SK, Dispenzieri A, Fraser R, Mingwei F, Akpek G, Cornell R, et al. Early relapse after autologous hematopoietic cell transplantation remains a poor prognostic factor in multiple myeloma but outcomes have improved over time. Leukemia. Nature Publishing Group; 2018. January 5;:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood. American Society of Hematology; 2013. February 7;121(6):884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haessler J, Shaughnessy JD, Zhan F, Crowley J, Epstein J, van Rhee F, et al. Benefit of Complete Response in Multiple Myeloma Limited to High-Risk Subgroup Identified by Gene Expression Profiling. Clinical Cancer Research. 2007. December 1;13(23):7073–9. [DOI] [PubMed] [Google Scholar]
- 16.Kazmi SM, Nusrat M, Gunaydin H, Cornelison AM, Shah N, Kebriaei P, et al. Outcomes Among High-Risk and Standard-Risk Multiple Myeloma Patients Treated With High-Dose Chemotherapy and Autologous Hematopoietic Stem-Cell Transplantation. Clinical Lymphoma Myeloma and Leukemia. 2015. November;15(11):687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubowiak AJ, Chari A, Lonial S, Weiss BM, Comenzo RL, Wu K, et al. Daratumumab (DARA) in combination with carfilzomib, lenalidomide, and dexamethasone (KRd) in patients (pts) with newly diagnosed multiple myeloma (MMY1001): An open-label, phase 1b study. JCO. American Society of Clinical Oncology; 2017. May 30. [Google Scholar]
- 18.A Study to Evaluate Daratumumab in Transplant Eligible Participants With Previously Untreated Multiple Myeloma - Full Text View - ClinicalTrials.gov.
- 19.A Phase III Trial on the Effect of Elotuzumab in VRD Induction /Consolidation and Lenalidomide Maintenance in Patients With Newly Diagnosed Myeloma - Full Text View - ClinicalTrials.gov.
- 20.Study Comparing Daratumumab, Lenalidomide, Bortezomib, and Dexamethasone (D-RVd) Versus Lenalidomide, Bortezomib, and Dexamethasone (RVd) in Subjects With Newly Diagnosed Multiple Myeloma - Full Text View - ClinicalTrials.gov.
- 21.Berro M, Arbelbide JA, Rivas MM, Basquiera AL, Ferini G, Vitriu A, et al. Hematopoietic Cell Transplantation–Specific Comorbidity Index Predicts Morbidity and Mortality in Autologous Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2017. October;23(10):1646–50. [DOI] [PubMed] [Google Scholar]
- 22.Nadiminti K, Farooq U, Dozeman L, Mott S, Tricot GJ, Jethava Y, et al. Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI) Score Predicts Non-Relapse Mortality (NRM) and Overall Survival (OS) in Multiple Myeloma Patients Undergoing Intensive Preparative Regimen with Autologous Stem Cell Transplantation (ASCT) Followed By Maintenance Therapy Using Novel Agents. Blood. 2017. December 7;130(Suppl 1):3281. [Google Scholar]
- 23.Saad A, Mahindra A, Zhang M-J, Zhong X, Costa LJ, Dispenzieri A, et al. Hematopoietic Cell Transplant Comorbidity Index Is Predictive of Survival after Autologous Hematopoietic Cell Transplantation in Multiple Myeloma. Biology of Blood and Marrow Transplantation. 2014. March;20(3):402–408.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vij R, Kumar S, Zhang M-J, Zhong X, Huang J, Dispenzieri A, et al. Impact of Pretransplant Therapy and Depth of Disease Response before Autologous Transplantation for Multiple Myeloma. Biology of Blood and Marrow Transplantation. Elsevier Inc; 2015. February 1;21(2):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Souza A, Zhang M-J, Huang J, Fei M, Pasquini M, Hamadani M, et al. Trends in pre- and post-transplant therapies with first autologous hematopoietic cell transplantation among patients with multiple myeloma in the United States, 2004–2014. Leukemia. 2017. June 30;31(9):1998–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastritis E, Terpos E, Roussou M, Gavriatopoulou M, Migkou M, Eleutherakis-Papaiakovou E, et al. Evaluation of the Revised International Staging System in an independent cohort of unselected patients with multiple myeloma. Haematologica. Haematologica; 2017. February 28;102(3):593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podar K, Jäger D. Targeting the immune niche within the bone marrow microenvironment: The rise of immunotherapy in Multiple Myeloma. Current Cancer Drug Targets. 2017. February 14;17(999):1–1. [DOI] [PubMed] [Google Scholar]