Abstract
Background:
Hepatectomy presents unique challenges potentially heightening acute kidney injury risk, but the full spectrum of risk factors have not been identified.
Methods:
Data for hepatectomy patients in the 2016 American College of Surgeons National Surgical Quality Improvement Program (N=3814) was randomly split into derivation (70%) and validation (30%) cohorts. AKI was defined as an increase in serum creatinine ≥0.3 mg/dl or ≥1.5-fold above the preoperative value within 30 days of surgery. Multivariable logistic regression assessed preoperative and intraoperative risk factors for AKI.
Results:
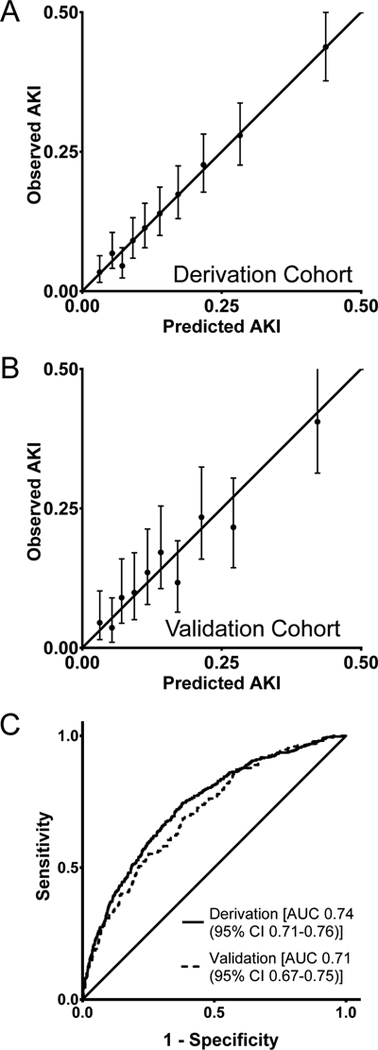
Of 2,692 patients (derivation cohort), 432 (16%) developed AKI. Risk factors were: age (years; adjusted odds ratio [aOR] 1.016 [95% confidence interval 1.006–1.026], female sex (aOR 0.65 [0.51–0.82]), body mass index (kg/m2; aOR 1.043 [1.024–1.062]), diabetes (aOR 1.71 [1.31–2.24]), hypertension (aOR 1.66 [1.30–2.13]), hematocrit (%; aOR 0.944 [0.924–0.966]), operative time (min; aOR 1.004 [1.003–1.004]), planned open procedure (aOR 2.00 [1.47–2.73]), and Pringle maneuver (aOR 1.36 [1.07–1.72]). The areas under the curve of the receiver operator characteristic curves were 0.74 (95% CI 0.71–0.76) and 0.71 (95% CI 0.67–0.75) in the derivation and validation cohorts, respectively.
Conclusions:
Postoperative AKI affects one in six hepatectomy patients; preoperative and intraoperative factors can predict the risk of postoperative AKI.
Keywords: Acute Kidney Injury, Hepatectomy, Risk Assessment, Surgical Procedures, Operative
Introduction
Postoperative acute kidney injury (AKI) is well-recognized as a significant contributor to morbidity and mortality in patients undergoing intraabdominal general surgery and other major non-cardiac surgery procedures.1,2 While prediction models derived from broad surgical populations are useful in defining general risk factors, specific surgical procedures may have unique aspects affecting AKI risk that are not applicable to other procedures but should nonetheless be accounted for in the development of these models. Hepatectomy is a major intraabdominal general surgery procedure with a high risk of postoperative AKI that has unique features due to the underlying diseases of the patients as well as surgical and anesthetic management considerations.3 To date, studies evaluating AKI in hepatectomy patients have been smaller, single-institution studies4–6 but a large, multicenter analysis of patients developing AKI after hepatectomy would allow for a more generalized application of these findings.
Using data from the 2016 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), risk factors for postoperative AKI in patients undergoing hepatectomy were identified, including preoperative variables as well as variables related to the surgery. In addition, a risk score for AKI was derived based on the model, allowing for a simple and easily interpretable application of AKI risk-prediction. This tool should help clinicians identify patients at high-risk for AKI after hepatectomy and provide a basis for future studies evaluating interventions that might reduce this risk.
Methods
Overview
This study was designated as Not Human Subjects Research Under 45 CFR 46 by the Columbia University Medical Center Institutional Review Board (IRB-AAAI9650) as it did not require access to protected health information. The ACS-NSQIPa is a validated, prospectively collected national dataset designed to improve surgical quality and outcomes. This is a retrospective, observational cohort study of patients undergoing hepatectomy in the 2016 Procedure Targeted Participant Use File (PTPUF) for hepatectomy.b In addition to the standard ACS-NSQIP variables, the PTPUF provides additional data on variables specific to hepatectomy patients. Patients are followed for 30 days after surgery, including post-discharge, by the surgical clinical reviewer at each participating site to monitor postoperative outcomes.
Patient selection
The 2016 hepatectomy PTPUF contained 4,325 cases from 116 sites (Supplemental Figure 1). The primary CPT codes for these patients were: 47120 (Hepatectomy, resection of liver; partial lobectomy), 47122 (Hepatectomy, resection of liver; trisegmentectomy), 47125 (Hepatectomy, resection of liver; total left lobectomy), or 47130 (Hepatectomy, resection of liver; total right lobectomy). After removing patients with preoperative dialysis (N=9) or missing a pre- or postoperative creatinine (mg/dL) measurement, the final hepatectomy cohort contained 3,814 observations. After a random 70/30 split, the derivation and validation cohorts contained 2,692 and 1,122 observations, respectively.
Pre- and intraoperative risk factor variables
Pre- and intraoperative risk factor variables were collected from both the main ACS-NSQIP participant use file as well as the hepatectomy PTPUF. Body mass index (BMI; kg/m2) was calculated from height and weight data; the estimated glomerular filtration rate (ml/min/1.73m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula incorporating creatinine, sex, age, and race7 and categorized into groups corresponding to the stages of chronic kidney disease:8 <30, ≥30 and <60, ≥60 and <90, or ≥90. We visualized the relationships between continuous variables and the primary outcome by plotting the deciles of each variable by the log(odds ratio) of the primary outcome. Based on these analyses, age (years), BMI, hematocrit (%), albumin (g/dL), alkaline phosphatase (U/L), international normalized ratio (INR), operative time (min), platelet count (103/μL), sodium (mEq/L), and white blood cell count (103/μL) were modeled as continuous variables. Aspartate transaminase (AST; U/L) was categorized as <29 or ≥29 and total bilirubin (mg/dL) was categorized as ≤0.8 or >0.8.
Definition of primary outcome: acute kidney injury
The dataset provides the most recent creatinine measurement prior to surgery as well as the peak postoperative creatinine measurement. Therefore, our primary outcome was the difference between the peak postoperative creatinine and the preoperative creatinine. AKI was defined as a creatinine change ≥0.3 mg/dL or ≥1.5-fold, according to the KDIGO guidelines.9 It was not possible to determine from the dataset the postoperative day on which the peak creatinine measurement occurred.
Statistical analysis
Differences in the proportions or means of preoperative characteristics, comorbidities, and intraoperative variables between patients with and without AKI were compared with the χ2-test or the t-test as appropriate. Logistic regression was used to model the risk of postoperative AKI. Each pre- and intraoperative variable was separately analyzed in a univariable logistic regression model. All preoperative variables with P<0.1 in univariable analyses were entered into a multivariable logistic regression model, and variables with P<0.1 in this multivariable model were retained. Then, all intraoperative variables with P<0.1 in univariable analyses were added to the retained preoperative variables, and subsequently, intraoperative variables with P<0.1 in this combined multivariable model were retained. The final model consisted of variables with P<0.05 in the multivariable model with the retained preoperative and intraoperative variables.
AKI Risk Score
An AKI risk score model was developed from the final multivariable logistic regression model based on previously published methodology.10 In brief, point values for each risk factor were proportional to the β coefficients in the multivariable logistic regression model. Continuous variables were grouped into quartiles, except for age, which was grouped by decade.
Model performance and validation
Model calibration was assessed using calibration plots by decile of predicted AKI risk. Model discrimination was assessed by the area under the curve (AUC) of the receiver operator characteristic (ROC) curve. Internal validation was assessed by comparing the performance of the model on the validation dataset.
Statistical analyses were performed using SAS Software version 9.4 (SAS Institute, Cary, NC) and GraphPad Prism 7.04 (GraphPad Software, Inc, La Jolla, CA).
Results
Characteristics of patients undergoing hepatectomy, with and without AKI
Of the 2,692 patients in the derivation cohort, 432 (16%) developed AKI after hepatectomy (Table 1). Compared to those without AKI, patients who developed AKI were more likely to be older, male, and have comorbidities such as diabetes, hypertension, and reduced renal function. In addition, those with AKI had longer procedures and were more likely to have had intra/postoperative transfusions, a planned open procedure, Pringle maneuver, biliary stenting, biliary reconstruction, and abnormal liver texture.
Table 1.
Characteristics of hepatectomy patients, American College of Surgeons National Surgical Quality Improvement Program, 2016.
| No AKI | AKI | P-value | |||
|---|---|---|---|---|---|
| 2,260 | (84) | 432 | (16) | ||
| Preoperative Variables | |||||
| Age (years) | 58 | (14) | 62 | (13) | * |
| Female | 1,166 | (52) | 168 | (39) | * |
| White | 1,409 | (62) | 257 | (59) | 0.3 |
| Body Mass Index (kg/m2) | 28.1 | (6.5) | 30.2 | (7.1) | * |
| Emergency | 13 | (0.6) | 2 | (0.5) | 1.0 |
| Diabetes | 318 | (14) | 126 | (29) | * |
| Mechanical Ventilation | 1 | (0.0) | 2 | (0.5) | 0.07 |
| Dyspnea | 103 | (4.6) | 26 | (6.0) | 0.2 |
| Chronic Obstructive Pulmonary Disease | 79 | (3.5) | 22 | (5.1) | 0.11 |
| Current Smoker | 389 | (17) | 60 | (14) | 0.09 |
| Congestive Heart Failure | 6 | (0.3) | 1 | (0.2) | 0.9 |
| Hypertension | 929 | (41) | 264 | (61) | * |
| Sepsis/Septic Shock | 30 | (1.3) | 3 | (0.7) | 0.3 |
| Wound Infection | 12 | (0.5) | 7 | (1.6) | 0.02 |
| Functionally Dependent | 20 | (0.9) | 1 | (0.2) | 0.2 |
| Ascites | 13 | (0.6) | 4 | (0.9) | 0.4 |
| Steroid Use | 59 | (2.6) | 18 | (4.2) | 0.08 |
| Disseminated Cancer | 942 | (42) | 179 | (41) | 0.9 |
| Preoperative Weight Loss | 87 | (3.9) | 29 | (6.7) | 0.01 |
| Bleeding Disorder | 74 | (3.3) | 18 | (4.2) | 0.3 |
| Preoperative Transfusion | 11 | (0.5) | 6 | (1.4) | 0.04 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | * | ||||
| eGFR <30 | 12 | (0.5) | 5 | (1.2) | |
| eGFR ≥30 and <60 | 213 | (9.4) | 82 | (19) | |
| eGFR ≥60 and <90 | 929 | (41) | 181 | (42) | |
| eGFR ≥90 | 1,103 | (49) | 162 | (38) | |
| Sodium (mEq/L) | 139 | (3) | 139 | (3) | 0.2 |
| Albumin (g/dL) | 4.0 | (0.5) | 3.8 | (0.5) | * |
| Bilirubin (mg/dL) | 0.6 | (0.7) | 0.7 | (0.9) | 0.03 |
| Total Bilirubin (mg/dL) >0.8 | 362 | (17) | 97 | (24) | 0.001 |
| AST (U/L) ≥29 | 835 | (40) | 188 | (47) | 0.01 |
| Alkaline Phosphatase (U/L) | 115 | (90) | 128 | (104) | 0.02 |
| Hematocrit (%) | 40 | (4.9) | 38 | (5.0) | * |
| White Blood Cells (103/μL) | 6.9 | (2.7) | 6.9 | (3.0) | 0.9 |
| Platelets (103/μL) | 234 | (91) | 225 | (84) | 0.05 |
| International Normalized Ratio | 1.04 | (0.16) | 1.07 | (0.19) | 0.01 |
| Intraoperative Variables | |||||
| Operative Time (minutes) | 235 | (113) | 299 | (141) | * |
| Intra/Postoperative Transfusion | 357 | (16) | 129 | (30) | * |
| Planned Open Procedure | 1,677 | (74) | 372 | (87) | * |
| Pringle Maneuver | 544 | (24) | 142 | (33) | 0.0001 |
| Biliary Stent | 110 | (4.9) | 47 | (11) | * |
| Biliary Reconstruction | 137 | (6.1) | 51 | (12) | * |
| Liver Texture | 0.002 | ||||
| Cirrhotic | 200 | (8.9) | 57 | (13) | |
| Congested | 55 | (2.4) | 16 | (3.7) | |
| Fatty | 260 | (12) | 65 | (15) | |
| Normal | 664 | (29) | 110 | (25) | |
| Not Documented | 1,081 | (48) | 184 | (43) | |
| Procedure (CPT Code) | 0.005 | ||||
| Hepatectomy, resection of liver; partial lobectomy (47120) | 1,517 | (67) | 257 | (59) | |
| Hepatectomy, resection of liver; total left lobectomy (47125) | 219 | (9.7) | 40 | (9.3) | |
| Hepatectomy, resection of liver; total right lobectomy (47130) | 340 | (15) | 89 | (21) | |
| Hepatectomy, resection of liver; trisegmentectomy (47122) | 184 | (8.1) | 46 | (11) | |
AKI, acute kidney injury.
Continuous variables expressed as mean (SD). Categorical variables expressed as counts (%).
P<0.0001
Logistic regression model for the development of AKI following hepatectomy
The logistic regression model for AKI was developed in stages using the derivation cohort. Of the initial preoperative variables (Table 1) that were significant at a P<0.1 level in univariable logistic regression analyses, the following remained significant at a P<0.1 level in multivariable analysis: sex, BMI, diabetes, hypertension, preoperative weight loss, hematocrit, and alkaline phosphatase. In addition, age was retained even though it did not meet this criteria (P=0.2). The adjusted ORs (aORs) and 95% CIs for the multivariable logistic regression model with preoperative variables are shown in Table 2 (first column) and the AUC of the ROC curve was 0.68 [0.66–0.71].
Table 2.
Logistic regression models for acute kidney injury after hepatectomy, derivation cohort, American College of Surgeons National Surgical Quality Improvement Program, 2016.
| Preoperative Variables | All Variables | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Age (years) | 1.014 | [1.005, 1.024] | 1.016** | [1.006, 1.026] |
| Female | 0.59*** | [0.47, 0.74] | 0.65** | [0.51, 0.82] |
| Body Mass Index (kg/m2) | 1.050*** | [1.032, 1.069] | 1.043*** | [1.024, 1.062] |
| Diabetes | 1.50** | [1.15, 1.97] | 1.71*** | [1.31, 2.24] |
| Hypertension | 1.56** | [1.21, 2.00] | 1.66*** | [1.30, 2.13] |
| Preoperative Weight Loss | 1.606* | [1.002, 2.575] | ||
| Hematocrit (%) | 0.945*** | [0.924, 0.967] | 0.944*** | [0.924, 0.966] |
| Alkaline Phosphatase (U/L) | 1.002** | [1.000, 1.003] | ||
| Operative Time (min) | 1.004*** | [1.003, 1.004] | ||
| Planned Open Procedure | 2.00*** | [1.47, 2.73] | ||
| Pringle Maneuver | 1.36* | [1.07, 1.72] | ||
| AUC | 95% CI | AUC | 95% CI | |
| 0.68 | [0.66, 0.71] | 0.74 | [0.71, 0.76] | |
OR, odds ratio; CI, confidence interval; AUC, area under the curve of the receiver operator characteristic curve.
P<0.0001
P<0.01
P<0.05
Next, the following intraoperative variables were significant at a P<0.1 level in multivariable analysis when combined with the previously retained preoperative variables: operative time, planned open procedure, and Pringle maneuver. Of note, intra/postoperative transfusion was not a candidate variable in this model. After the addition of these intraoperative variables to the retained preoperative variables, alkaline phosphatase (P=0.5) and preoperative weight loss (P=0.09) were removed as they were no longer statistically significant. The final logistic regression model included the following preoperative and intraoperative variables: age, sex, BMI, diabetes, hypertension, hematocrit, operative time, planned open procedure, and Pringle maneuver. The aORs and 95% CIs are shown in Table 2 (second column) and the AUC of the ROC curve was 0.74 [0.71–0.76].
Logistic regression model calibration and validation
The Hosmer-Lemeshow test had a P-value of 0.8, indicating good model fit, and the calibration plot also indicated good model fit (Figure 1A). To validate the model, we applied parameters from the multivariable logistic regression on the validation dataset (N=1,122) to estimate the predicted probability of AKI. Calibration in the validation dataset was reasonable (Figure 1B) and the AUC of the ROC curve was 0.71 [0.67–0.75] and not significantly different from the AUC of the ROC curve from the derivation cohort (P=0.25) (Figure 1C).
Figure 1.
Calibration plots (by decile of predicted mortality risk) (A-B) and receiver operator characteristic curves (C) of the logistic regression model for acute kidney injury (AKI) in patients undergoing hepatectomy in the derivation cohort (A, C) and the validation cohort (B, C), American College of Surgeons National Surgical Quality Improvement Program, 2016. In the calibration plots, the solid line represents perfect calibration the error bars represent the 95% confidence interval for predicted AKI risk. AUC, area under the curve.
Missing data
Generally, the rate of missing data was low except for certain preoperative laboratory values (INR 11%, albumin 10%, AST 7.3%, alkaline phosphatase 5.6%, total bilirubin 5.5%). Missing data only affected 41 observations (1.5%) in the final logistic regression model and due to the low rate of missing data, imputation analyses to account for the missing data were not performed.
Development of AKI Risk Score from logistic regression model
We developed an AKI risk score to estimate the risk of AKI based on the multivariable logistic regression model (Table 3). The continuous variable age was categorized as <40, 40–49, 50–59, 60–69, and ≥70 years, and the remaining continuous variables (BMI, hematocrit, and operative time) were categorized by quartiles. Possible values of the AKI risk score ranged from −8 to 30, and the corresponding predicted AKI risk ranged from 0.9% to 78.7% (Supplemental Table 1). In the derivation cohort, observed AKI risk scores ranged from −7 to 28 and the median [interquartile range (IQR)] was 9 [IQR 5–13]. In the validation cohort, the AKI risk scores ranged from −6 to 27 and the median was 9 [IQR 5–13].
Table 3.
Acute kidney injury risk score in patients undergoing hepatectomy, American College of Surgeons National Surgical Quality Improvement Program, 2016.
| Risk Factor | Category | Points |
|---|---|---|
| Age (years) | <40 | 0 |
| 40–49 | 1 | |
| 50–59 | 2 | |
| 60–69 | 3 | |
| ≥70 | 5 | |
| Sex | Female | −3 |
| Male | 0 | |
| Body Mass Index (m/kg2) | <24 | 0 |
| ≥24 to <28 | 1 | |
| ≥28 to <32 | 2 | |
| ≥32 | 5 | |
| Diabetic | Yes | 3 |
| No | 0 | |
| Hypertension | Yes | 3 |
| No | 0 | |
| Hematocrit (%) | <37 | 0 |
| ≥37 to <40 | −3 | |
| ≥40 to <43 | −4 | |
| ≥43 | −5 | |
| Operative Time (min) | <154 | 0 |
| 155–218 | 2 | |
| 219–298 | 4 | |
| ≥299 | 8 | |
| Planned Open Procedure | Yes | 4 |
| No | 0 | |
| Pringle Maneuver | Yes | 2 |
| No | 0 |
Risk score ranges from −8 to 30.
The calibration plot for the AKI risk score model displays values for each level of the score; however, scores < 0 were combined into one group and scores > 19 were combined into one group due to small sample sizes within each individual level. Calibration plots demonstrate good calibration for the derivation cohort (Supplemental Figure 2A) and validation cohort (Supplemental Figure 2B). The AUC of the ROC curve in the derivation and validation cohorts were 0.72 [95% CI 0.70–0.75] and 0.70 [95% CI 0.66–0.75], respectively, and not significantly different (P=0.4) (Supplemental Figure 2C).
Acute kidney injury and other postoperative complications
Compared to patients without AKI, patients with postoperative AKI had higher rates of other complications following hepatectomy, including 30-day mortality (6.3% vs 0.3%, P<0.0001), postoperative liver failure (17% vs 3.5%, P<0.0001), bile leak (15% vs 7.5%, P<0.0001), and additional invasive interventions (21% vs 8.0%, P<0.0001) (Table 4).
Table 4.
Postoperative complications in hepatectomy patients with and without acute kidney injury, American College of Surgeons National Surgical Quality Improvement Program, 2016.
| No AKI | AKI | P-value | |||
|---|---|---|---|---|---|
| 2,260 | (84) | 432 | (16) | ||
| 30-Day Mortality | 7 | (0.3) | 27 | (6.3) | * |
| Postoperative Liver Failure | 78 | (3.5) | 75 | (17) | * |
| Bile Leak | 169 | (7.5) | 64 | (15) | * |
| Additional Invasive Intervention | 180 | (8.0) | 89 | (21) | * |
AKI, acute kidney injury.
P<0.0001
Discussion
In a large, multi-center dataset with 116 participating institutions, we identified risk factors—including both preoperative and intraoperative variables—that were significantly associated with an increased risk of postoperative AKI in patients undergoing hepatectomy. The risk factors were: age, sex, BMI, diabetes, hypertension, hematocrit, operative time, planned open procedure, and Pringle maneuver. In addition, we developed a risk score model that assigned point values for each risk factor, allowing for an easy comparison of the relative contributions of each factor to the increase in AKI risk, and the risk score can be calculated immediately at the conclusion of the procedure. The range of predicted risk for AKI was between 0.9% and 79%, and the model was well-calibrated.
AKI is a serious postoperative complication that is associated with both short-term and long-term sequelae. Early AKI research focused on patients undergoing cardiac and major vascular surgeries, but AKI is also a major consideration in non-cardiac surgery.1 Postoperative AKI leads to greater mortality and other undesired effects such as longer hospital stays and higher costs11 as well as an increased risk of developing chronic kidney disease.12 Even with a full recovery of renal function at discharge, patients with AKI face an increased risk of long-term mortality.13 Unfortunately, there are no perioperative therapeutic interventions that have clearly been shown to reduce the risk of postoperative AKI,14 although hemodynamic optimization appears to decrease the risk of AKI15 and there are continued efforts to identify the most optimal perioperative management strategies.16 Due to this lack of effective therapies, it is imperative that clinicians identify patients with the highest risk of postoperative AKI.
Hepatectomy procedures are complex abdominal surgeries in patients with significant comorbidities and risk factors predisposing them to the development of AKI. The reported incidence of AKI after hepatectomy ranges from 0.9% to 15.1%,3 and the analyses vary with respect to the factors that are identified as contributing to AKI risk. Our AKI rate was 16%, though this may be higher compared to other studies as the period of time when AKI could be diagnosed in our study was up to 30 days after surgery.
A patient’s risk of postoperative AKI is affected by their baseline comorbidities, and patient factors found in our study (age, sex, BMI, diabetes, hypertension, and hematocrit) are largely consistent with studies in general surgery patients2 and hepatectomy patients.17 Interestingly, no preoperative comorbidities or laboratory values typically associated with liver disease was retained in the final AKI risk score. In particular, the Model for End-Stage Liver Disease (MELD) score4,6,18 was identified as risk factors for AKI in prior studies, but in our analysis, the individual components of MELD—INR, bilirubin, and creatinine (via eGFR)—while significantly associated with AKI in univariable analyses, did not meet criteria for inclusion in the risk score. In a sensitivity analysis, the MELD score was also not a significant predictor of AKI. This lack of association between MELD variables and AKI may reflect differences in underlying disease and comorbidity severity in our sample compared to prior studies, and these differences will need further investigation.
Many AKI risk models incorporate only variables known preoperatively,17 but intraoperative factors also contribute to the risk of AKI and other major complications.3 Anesthetic management for hepatectomy may include the use of hypovolemia and vasodilation to maintain a low central venous pressure (CVP) to reduce operative blood loss, but this may come at the expense of kidney hypoperfusion and resultant AKI.19 While an early study reported an AKI rate below 1% with low CVP management,20 in a more recent study, AKI occurred in 16% of patients, though the decrease in eGFR was reported to be transient and clinically insignificant in most instances.21 Detailed anesthetic management data is not available in our dataset so we cannot determine if any patients were managed in this fashion.
Prior studies identified major hepatic resection as a risk factor for postoperative AKI18 and other morbidities22 but the type of surgery was not a significant predictor in the multivariable model. Our procedure categories are based on CPT codes, while major hepatectomy is defined by the number of liver segments resected,22 and the CPT codes may not fully capture the magnitude of liver mass that is resected. Liver texture at resection (e.g., cirrhotic, congested, fatty) also was not a significant predictor in the multivariable model. Compared to a study with ~80% of patients with cirrhosis,23 the rate of abnormal liver texture was low in our sample (~24%) and it is possible that this accounts for the lack of association between liver texture and AKI. It should be noted that liver texture was not documented in almost half of patients (~47%), but the rate of AKI was similar to that in normal patients, indicating that they likely had normal texture.
Blood loss and transfusions are associated with morbidity in hepatectomy patients,24,25 but we were limited by the fact that the dataset only indicates if blood was administered within the first 72 hours of surgery. Our goal was to create a risk score that could be applied immediately after surgery so this variable was not included in the multivariable analysis, but a sensitivity analysis demonstrated that intra/postoperative transfusions would have been an additional significant risk factor for postoperative AKI in our model (data not shown).
There is limited data in the literature regarding the relationship between vascular clamping, a maneuver undertaken to reduce intraoperative blood loss, and AKI risk in hepatectomy patients. Xu et al.26 did not find increased risks of postoperative complications, including renal failure, with the Pringle maneuver. However, in our study, the Pringle maneuver was associated with a moderately increased risk of AKI and remained a significant predictor in the risk score. We are unable to determine the mechanisms underlying this finding, but vascular clamping can be accompanied by hypotension and ischemia-reperfusion injury27 which may potentially contribute to increased AKI risk. It may also reflect confounding with variables not measured in our study, such as blood loss and transfusions, the extent of liver resection (i.e., major vs. minor), and severity of cirrhosis. If there are significant risks associated with the Pringle maneuver, they will have to be weighed against the benefits of using the technique, but further research is necessary to clarify these effects.
Laparosopic hepatectomy is associated with improved outcomes vs. open hepatectomy, possibly via reduced inflammation28 and reduced blood loss and transfusion requirement.29 Our results confirm that a planned open procedure doubled the adjusted odds of postoperative AKI. The length of surgery has clearly been demonstrated to be associated with surgical complications,30 and operative time is a major component of our risk score.
AKI was associated with higher mortality, postoperative liver failure, bile leak, and the need for additional invasive interventions. Hepatorenal syndrome is a known complication of liver failure31 but AKI may also develop independently of liver failure. Due to the retrospective nature of our analysis, we cannot determine the causal pathways for these associations, and the mechanisms leading to these associations will need to be clarified. Additionally, as a retrospective analysis, we cannot determine if the components of the risk score directly increase the AKI risk, but at the very least, they are significant markers for patients with a higher risk.
Prior studies evaluating the risk of AKI after hepatectomy procedures are mainly studies conducted at a single institution.4,17,18,28,32 A major strength of our study is the use of a well-established, national surgical dataset with data from over 100 institutions, allowing for greater generalizability, though the ACS-NSQIP tends to reflect mainly larger, academic medical centers. Although the main ACS-NSQIP data file underestimates the incidence of postoperative AKI,33 the hepatectomy PTPUF allows for the direct calculation of the maximal change between pre- and postoperative creatinine. However, we do not know when in the 30-day postoperative period that this maximal change occurred, so our definition of AKI does not completely align with the standard KDIGO guidelines9 and this remains the major limitation of the study. Another limitation of the dataset is missing data. Approximately 12% of the PTPUF sample had either a missing preoperative or postoperative creatinine measurement and had to be excluded from the analysis. As these variables are involved in determining the primary outcome, it would not be prudent to impute their values.
With a large, multicenter dataset, it is difficult to capture many important clinical variables that may be relevant to the development of postoperative AKI, including hemodynamics,4 fluid management, and the use of low CVP management.19 In addition, other important perioperative factors for increased AKI risk include impaired glycemic control34 and poor nutritional status.35 Our dataset does not provide data on these variables, but their effects on postoperative AKI should be evaluated in future studies. A recent study demonstrated that the use of additional data beyond that contained in the ACS-NSQIP dataset improved the prediction of postoperative complications after hepatectomy,36 so additional data may improve our model. We cannot assess differences among hospitals, though this may significantly contribute to postoperative complications.37
In conclusion, we have identified significant preoperative and intraoperative predictors of AKI following hepatectomy using a large, multi-institution cohort of patients. A risk score that can be calculated at the conclusion of the procedure was developed to stratify the risk of postoperative AKI. This score may assist the operative team on the optimal postoperative destination (i.e., intensive care unit vs. post-anesthesia care unit) and identify patients who may require early consultation from a nephrologist. The Pringle maneuver was a significant risk score in our model, and it will need to be determined if there is a direct causal effect with AKI or if the Pringle maneuver is simply a marker for a high-risk patient. In addition, there appear to be associations between postoperative AKI and liver failure and these relationships will need to be explored further. While there are no known therapeutic measures to prevent AKI, perioperative management must be optimized in the highest risk patients and this tool can facilitate the early identification of these patients.
Supplementary Material
Footnotes
Reprints will not be available from the authors.
This work was presented at the American Society of Anesthesiologists’ Annual Meeting, October 2018, San Francisco, CA.
Conflict of Interest
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through Grant Number KL2TR001874 (MK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors otherwise declare no conflicts of interest.
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
https://www.facs.org/~/media/files/quality%20programs/nsqip/pt_nsqip_puf_userguide_2016.ashx; Accessed January 31, 2018.
References
- 1.Kim M, Brady JE, Li G. Variations in the risk of acute kidney injury across intraabdominal surgery procedures. Anesth Analg. 2014;119:1121–32. [DOI] [PubMed] [Google Scholar]
- 2.Gameiro J, Fonseca JA, Neves M, Jorge S, Lopes JA. Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care. 2018;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peres LA, Bredt LC, Cipriani RF. Acute renal injury after partial hepatectomy. World J Hepatol. 2016;8:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredt LC, Peres LAB. Risk factors for acute kidney injury after partial hepatectomy. World J Hepatol. 2017;9:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kambakamba P, Slankamenac K, Tschuor C, Kron P, Wirsching A, Maurer K et al. Epidural analgesia and perioperative kidney function after major liver resection. Br J Surg. 2015;102:805–12. [DOI] [PubMed] [Google Scholar]
- 6.Cho E, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: a prospective observational study. BMC Nephrol. 2014;15:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 9.National Kidney Foundation: Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:19–36. [Google Scholar]
- 10.Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–60. [DOI] [PubMed] [Google Scholar]
- 11.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–8. [DOI] [PubMed] [Google Scholar]
- 14.Zacharias M, Mugawar M, Herbison GP, Walker RJ, Hovhannisyan K, Sivalingam P et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. 2013;9:CD003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–90. [DOI] [PubMed] [Google Scholar]
- 16.Gocze I, Jauch D, Gotz M, Kennedy P, Jung B, Zeman F et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann Surg. 2018;267:1013–20. [DOI] [PubMed] [Google Scholar]
- 17.Slankamenac K, Breitenstein S, Held U, Beck-Schimmer B, Puhan MA, Clavien PA. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg. 2009;250:720–8. [DOI] [PubMed] [Google Scholar]
- 18.Lim C, Audureau E, Salloum C, Levesque E, Lahat E, Merle JC et al. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford). 2016;18:540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–5. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong T, Welsh FK, Wells J, Chandrakumaran K, John TG, Rees M. The impact of pre-operative serum creatinine on short-term outcomes after liver resection. HPB (Oxford). 2009;11:622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa-Gallego C, Berman A, Denis SC, Langdon-Embry L, O’Connor D, Arslan-Carlon V et al. Renal function after low central venous pressure-assisted liver resection: assessment of 2116 cases. HPB (Oxford). 2015;17:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford). 2011;13:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bressan AK, James MT, Dixon E, Bathe OF, Sutherland FR, Ball CG. Acute kidney injury following resection of hepatocellular carcinoma: prognostic value of the acute kidney injury network criteria. Can J Surg. 2018;61:E11–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259–64. [DOI] [PubMed] [Google Scholar]
- 25.Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth. 2015;62:753–61. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Xu H, Yang H, Liao W, Ge P, Ren J et al. Continuous Pringle maneuver does not affect outcomes of patients with hepatocellular carcinoma after curative resection. Asia Pac J Clin Oncol. 2017;13:e321–e30. [DOI] [PubMed] [Google Scholar]
- 27.Chouker A, Schachtner T, Schauer R, Dugas M, Lohe F, Martignoni A et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204–11. [DOI] [PubMed] [Google Scholar]
- 28.Moon YJ, Jun IG, Kim KH, Kim SO, Song JG, Hwang GS. Comparison of acute kidney injury between open and laparoscopic liver resection: Propensity score analysis. PLoS One. 2017;12:e0186336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siniscalchi A, Ercolani G, Tarozzi G, Gamberini L, Cipolat L, Pinna AD et al. Laparoscopic versus Open Liver Resection: Differences in Intraoperative and Early Postoperative Outcome among Cirrhotic Patients with Hepatocellular Carcinoma-A Retrospective Observational Study. HPB Surg. 2014;2014:871251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Procter LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. 2010;210:60–5e1–2. [DOI] [PubMed] [Google Scholar]
- 31.Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066–79. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa S, Tanaka M, Maruyama F, Fukagawa A, Shiota N, Matsumura S et al. Effects of acute kidney injury after liver resection on long-term outcomes. Korean J Anesthesiol. 2017;70:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bihorac A, Brennan M, Ozrazgat-Baslanti T, Bozorgmehri S, Efron PA, Moore FA et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013;41:2570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez CE, Der Mesropian PJ, Mathew RO, Slawski B. Hyperglycemia and Acute Kidney Injury During the Perioperative Period. Curr Diab Rep. 2016;16:10. [DOI] [PubMed] [Google Scholar]
- 35.Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017 : Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017;43:730–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fruscione M, Kirks R, Cochran A, Murphy K, Baker EH, Martinie JB et al. Developing and validating a center-specific preoperative prediction calculator for risk of outcomes following major hepatectomy procedures. HPB (Oxford). 2018;20:721–8. [DOI] [PubMed] [Google Scholar]
- 37.Eppsteiner RW, Csikesz NG, Simons JP, Tseng JF, Shah SA. High volume and outcome after liver resection: surgeon or center? J Gastrointest Surg. 2008;12:1709–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.