Abstract
As the interest in DNA nanotechnology increases, so does the need for larger and more complex DNA structures. In this work we describe two methods of using large, double-stranded (ds) DNA to self-assemble sequence-specific, non-repetitive microscale structures. A model system restructures T7 DNA (40 kb) through sequence-specific biotinylation followed by intramolecular binding to a 40 nm diameter Neutravidin bead to create T7 “rosettes.” This model system informed the creation of “nodal DNA” where “nodes” with single-stranded DNA flaps are attached to a large dsDNA insert so that a complementary oligonucleotide “strap” bridges the two nodes for restructuring to form a DNA “bolo.” In order to do this in high yield, several methodologies were developed, including a protection / deprotection scheme using RNA / RNase H and dialysis chambers that remove excess straps while retaining large DNA molecules. To assess these restructuring processes, the DNA was adsorbed onto supported lipid bilayers, allowing for a visual assay of their structure using single-molecule fluorescence microscopy. Good agreement between the expected and observed fluorescence intensity measurements of the individual features of restructured DNA for both the DNA rosettes and bolos gives us a high degree of confidence that both processes give sequence-specific restructuring of large, dsDNA molecules to create microscale objects.
Keywords: DNA nanotechnology, self-assembly, microscale, double-stranded DNA, supported lipid bilayer
Graphical Abstract

INTRODUCTION
The patterning of materials with increasing spatial resolution, precision and functionalities is driving modern society and will play an increasingly central role in virtually all future systems and technologies. From microelectronics1–3 to microfluidics4–6 to micro arrays7,8 and beyond, the ability to directly pattern material at both the micro- and nanoscale enables technological advances touching many scientific fields. Traditionally, chemical patterning has been done with photolithography and more recently with improved resolution through processes such as block copolymer lithography,9–12 soft lithography stamps,13–16 dip-pen lithography,17–19 among others. In recent years, DNA has become a material of great interest as a modality for enabling new nanopatterned technologies in fields such as biomedicine,20–22 nanoelectronics,23–25 optical materials26–28 and DNA assembly lines.29–32 As a self-assembling, double-stranded, sequence-defined polymer, DNA supports base-by-base template-directed patterning during replication and transcription, and its hybridization is thermodynamically governed by complementary strands. Such attributes make DNA a facile “addressable” material for the fabrication of complex objects bearing sub-nanometer features. In the 1980’s, these advantages were first recognized by Seeman and colleagues through their use of non-natural DNA junctions for construction of DNA polyhedra with edge lengths of ~7 nm with applications aimed at patterning hard to crystallize proteins for X-ray crystallography.33,34 DNA materials gained in popularity with the invention of DNA origami by Rothemund in 200635 and DNA tiles36 and bricks37, 38 in 2012 by Yin. Objects made with DNA take advantage of the addressability of DNA to template a wide variety of particles, from enzymes,29 to gold nanoparticles,26 to carbon nanotubes24 and even create DNA machines that sort molecular cargo.39
Although, double-stranded (ds) DNA molecules may present contour lengths spanning multiple centimeters, objects made with DNA origami and DNA tiles/bricks are typically restricted in size to hundreds of nm across, as limited by the use of ssDNA. Accordingly, methods have been developed to increase the size of DNA origami objects, from denaturing long dsDNA molecules to use as a scaffold,40 to increasing the length of the single-stranded scaffold,41, 42 to making multi-origami constructs—via hierarchical assembly schemes to make 2-dimensional43 and 3-dimensional structures,44 to adding proteins to the origami objects.45 DNA has also been used to make large, repetitive structures such as macroscopic 3-dimensional crystals46 and 2-dimensional lattices.47, 48 However, no method of DNA assembly has made non-repetitive structures larger than a micron.
Given these considerations and the need for new ways to construct even larger structures from DNA, we reasoned that large, dsDNA molecules would offer new routes and advantages to self-assembly unfettered by constraints imposed by single-stranded scaffolds. Single-stranded DNA is inherently less stable than dsDNA because each nick quantitively cleaves such molecules into several fragments, whereas dsDNA is structurally robust, requiring a high density of nicks on complementary strands before fragmentation is apparent. To make such structures, we have invented a new method of DNA “soft-assembly” where dsDNA molecules self-assemble into very large, but deformable constructs, in ways inspired by the “soft sculptures” made by artist Claes Oldenburg.49 Our DNA “soft constructs” are made by leveraging the inherent mechanical advantages of dsDNA to make truly micro-scale objects. dsDNA is an incredibly stiff polymer,50 boasting a huge persistence length, Lp, of ~50 nm, and thus a Kuhn segment length (2Lp) spanning ~100 nm, dimensions that are comparable in size to many origami objects. However, these same properties that make dsDNA an attractive building material also render it difficult to make objects with. Issues include: molecular addressability, since single-stranded regions are not present on native dsDNA; and shear mediated breakage,51 an inherent vulnerability due to expansive random chain sizes presenting molecules that are microns in diameter. Such stiff, molecular expanses also diminish intramolecular hybridization rates, especially when joining distant loci.
As our group has studied and manipulated large dsDNA molecules for decades,5, 52–54 we focused our efforts to understand and overcome these issues. Our utilization of nicking restriction enzymes55 for genome analysis via the Nanocoding system,4, 56, 57 in particular, gave us insight into how to modify dsDNA at specific loci to create addressable nodes for soft-assembly. To consider hybridization issues involving long-range intramolecular diffusivity, we created a model system, where specifically incorporated patches of biotinylated nucleotides on T7 DNA (40 kb) bind to a Neutravidin coated bead (40 nm) in dilute solution. Resulting intramolecular binding events create “rosette”-like structures. To allow for more exact control over which loci are bridged, we enable directed intramolecular hybridization of modified lambda DNA (48.5 kb) by ligation of three-arm junctions to the 12 base single-stranded 5’ overhangs naturally present on λ DNA (cos sites). This junction construct, termed a “node”, also bears a hybridizable strand that when bridged by a complementary oligonucleotide, termed a “strap”, enables circularization, and a homopolymer strand [poly(dA)] that supports attachment of large (~20 kb) tails. We achieve our final construct, resembling the “bolo tie” of traditional western United States fashion, through a multi-step fabrication workflow that we present here. Soft constructs made with this workflow make truly microscale objects that combine fixed linkages, accomplished at the base pair level, with very large, dynamic regions: attributes that create both challenges and opportunities for their use. Because these structures have the molecular addressability of DNA spanning multiple microns while retaining specific molecular linkages, they have the potential to act as an interface between nanoscale objects (e.g. DNA origami, enzymes, gold nanoparticles, etc.) and microscale objects such as cells, viruses or microelectronic circuits. The adaptability inherent in the dynamic, non-rigid regions in particular provides opportunities for these soft-constructs to act as an interface, especially to the adaptable surfaces of biological materials such as cells and viruses. We envision such adaptability harnessed for molecular machines and switches, where a restructured molecule in response to an external stimulus could partially deconstruct itself or further restructure itself to do different jobs under different environmental conditions.
RESULTS AND DISCUSSION
Biotin-Bead Model System.
We explored how multiple, biotin-labeled sites on large molecules such as T7 DNA (~13 μm contour length) would intramolecularly interact when binding to a large common target—a 40 nm (diameter), fluorescent, Neutravidin-conjugated latex bead. The idea behind this model is to first establish a system where sequence-dependent hybridization effects are obviated in order to focus on issues concerning intramolecular diffusivity and image-based assessment of restructured products. Consider that the 40 nm bead presents a ~5,000 nm2 area available for biotinylated patches to bind to Neutravidin, a factor that increases the likelihood of attachment. Figure 1 shows biotinylation of specific sites on T7 DNA placed and visualized using adaptations of protocols previously developed in our group for Nanocoding.4, 56, 57 Briefly, nick-translation with E. coli polymerase I incorporates biotin-dUTP in a template directed fashion starting at nick sites created by Nt.BspQI (GCTCTTCN▼) while excising the nucleotides from the impeding strand. Labeling yields within stretched molecules6 were quantitated by FRET (Förster Resonance Energy Transfer) imaging, enabled by the additional binding of Alexa Fluor 647-streptavidin (acceptor) to the biotinylated patches and staining entire molecules with the intercalating fluorochrome, YOYO-1 (donor). This yielded punctates in analyzed molecules at ~83% of expected sites with ~40% having 4 punctates (SI Figure S1), similar to a Nanocoded control using Alexa Fluor 647-dUTP modified nucleotides (obviating the need for the Alexa Fluor 647-streptavidin to visualize punctates), which yielded punctates in analyzed molecules at ~92% of expected sites with ~43% having 4 punctates (SI Figure S2). This suggests that the lower yield of punctates in the biotinylated sample could be due to the less efficient transfer of energy from the YOYO-1 to the Alexa Fluor 647 through FRET since Alexa Fluor 647 attached to streptavidin attached to biotin attached to DNA will be farther away from the YOYO-1 donor than Alexa Fluor 647 directly attached to the DNA. This means that the biotinylation yield of the T7 DNA is probably significantly higher than the ~83% measured. Because of this, the fact that this was a model system and the expectation that significantly longer incubation times would increase the number of non-specific nicks, no further optimization was deemed necessary.
Figure 1.
Scheme for sequence-specific biotinylation of T7 DNA and subsequent FRET assay. Step 1 removes random nicks and gaps naturally present in the DNA, step 2 introduces sequence-specific nicks on the DNA, step 3 adds biotin-modified nucleotides to these sequence-specific loci through nick translation, and step 4 binds the fluorescent dye Alexa Fluor 647 to the biotin through its interaction with streptavidin to localize the Alexa Fluor 647 fluorochrome in close proximity to the double-stranded DNA backbone, allowing us to use the intercalating dye YOYO-1 to do FRET with YOYO-1 as the donor and Alexa Fluor 647 as the acceptor. The fluorescence micrograph shows an overlay of the YOYO-1 (green) and Alexa Fluor 647 (red) channels of a typical, fully-biotinylated T7 DNA molecule presenting the expected pattern of Alexa Fluor 647 labeled punctates. See SI Figure S3 for additional molecules.
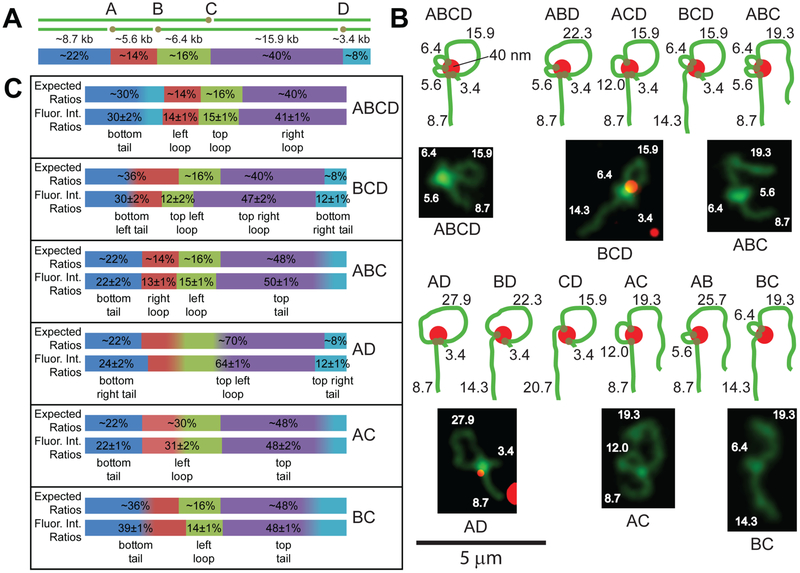
Given validated labeling yields, a highly dilute sample (~10−5 pmol/μl) of biotinylated DNA was mixed with a ~25-fold excess of red fluorescent Neutravidin-labeled 40 nm diameter polystyrene beads so that the biotin patches would intramolecularly bind to the bead and restructure the T7 DNA (Figure 2) to form “rosette”-like structures. T7 DNA was chosen specifically because it only has 4 Nt.BspQI nick sites, all of which are >5 kb away from each other, making for relatively few possible conformations after restructuring where all features are larger than the resolution of epifluorescence microscopy. Because restructured products are conformationally dynamic, complex and much larger than the depth of focus of high numerical aperture microscope objectives (~300 nm), we used a supported lipid bilayer for their presentation and imaging. Positively charged supported lipid bilayers adsorb DNA molecules through electrostatic interactions, thus immobilizing the DNA in the Z-dimension while still allowing for planar diffusion,58 thereby increasing the average polymer end-to-end distance to roughly scale as ~M0.8,59, 60 where M is DNA size (bp), compared to ~M0.5 for DNA in free-solution. This dynamic immobilization stretches DNA, attenuates chain overlaps and allows for a visual assay of fluorochrome stained DNA to determine its structure. Because the labeling is not 100% efficient (~83% of nick sites labeled), multiple, differently restructured conformations are possible using biotinylated T7 DNA. However, given the low rate of spurious labeling (SI Figure S1), labeling errors mostly stem from a combination of missing Nt.BspQI nick sites and those lacking biotin. Accordingly, our image datasets comprise T7 soft-rosette features representing combinations of available patches and their binding probabilities. This means that the percent of DNA of each feature in each conformation is predictable, as enumerated in Figure 2B, and comparable to the integrated fluorescence intensity ratios of the measured object features.53 By measuring the integrated fluorescence intensities of each feature across multiple frames of a movie collected as each object diffused on the supported lipid bilayer, we find very good agreement between the expected and measured fluorescence intensity ratios (Figure 2).
Figure 2.
T7 DNA “soft”-rosettes form after binding to a 40 nm Neutravidin-conjugated red fluorescent bead. A): Diagram shows a dsT7 DNA molecule (green lines) with 4 biotin patches (brown dots) incorporated into 4 Nt.BspQI nick sites (A-D; labeled in kb and % of total mass). B): These biotin patches bind to a Neutravidin-labeled microsphere (red dot) and restructure into 11 possible conformations. Depending on exactly which patches bind to the bead, different soft-rosettes are formed where each feature on each rosette comprises a known amount of DNA. A gallery of fluorescence micrographs shows T7 DNA molecules on supported lipid bilayers restructured into a variety of different rosettes; feature masses are noted in kb. Bright spots in the center of each object are believed to be ~0.5 kb of each biotin patch, spatially coalesced by the 40 nm bead. Images of structures BCD and AD are colorized, overlapped green and red channels showing the colocalization of the red bead within the site of restructuring (see SI Figure S4 for two-color images of the other constructs). See the supplementary videos to view the conformations over time of these restructured molecules (scale bar = 5 μm; the fluorescent microspheres are shown at the beginning and end). C): The expected and measured fluorescence intensity ratios (± 1 S.D.) of each feature of the 6 objects imaged in (B) averaged over 16 frames. Colors correspond to the DNA segments in (A) estimated to contribute to the fluorescence of each feature (classified as a “tail” or “loop”). See SI Figure S5 for example segmentations.
A control where dTTP was added during nick translation (step 3 in Figure 1) instead of the biotinylated dUTP was found not to bind to streptavidin or Neutravidin. Thus, no punctates were seen after addition of the Alexa Fluor 647-streptavidin and no restructured molecules were seen after adding the Neutravidin labeled microspheres (SI Figures S6, and S7).
Nodes.
Building on the new knowledge gained from our bead/biotin-patch model system, we designed three-arm junctions,33, 34 or “nodes” bearing single-stranded features to establish a more addressable system through hybridization (Figure 3) in place of non-selective binding via Neutravidin-biotin. Node components and their functionalities include: (i) a linker with a 5’ overhang for enabling ligation of a large dsDNA insert, (ii) a single-stranded arm supporting circularization of the insert via hybridization of a bridging “strap,” and (iii) a 3’ poly(dA) homopolymer strand for additional hybridization of “tails,” or large dsDNA molecules (Figure 4).
Figure 3.
Schema for node constructs. Nodes functionalize a large DNA insert for subsequent restructuring steps. Flanking 12 base overhang linkers enable insert ligation; other node strands support bridging and circularization (flap strands) via an added “strap”, leaving the available poly(dA) strands (template strands) to then hybridize to added, large, poly(dT) tailed dsDNA (Figure 4).
Figure 4.
Schema for restructuring large, dsDNA molecules using nodes and straps. Workflow steps: (1) Addition of excess strap, blocked with an RNA oligonucleotide with same sequence as Node 1 (flap strand). (2) Excess strap dialyzed away leaving straps hybridized to the flap strand on Node 2. (3) Added RNase H degrades RNA blocker, leaving a single-stranded region complementary to Node 1. (4–6) Added spermidine may stabilize the hybridization of the strap to Node 1 as well as the poly(dA)-poly(dT) hybrids between the nodal λ DNA and the T7-BanII tails before ligase joins them all together to form a microscale object; inset shows a fluorescence micrograph of a fully-formed, restructured nodal λ DNA “bolo” electrostatically adsorbed onto a positively charged glass surface.
To create the nodes, three oligos for each node (the template, flap oligo and completing oligo; Figure 3) are annealed together by heating a ~1:1.1:1.1 mol:mol:mol mixture at 95° C and slowly cooling to 16° C over 2.66 hours to form two nodes in quantitative yield (assessed with a native polyacrylamide gel, SI Figure S8). Nodes were hybridized and ligated to a large DNA insert (λ DNA) by adding an excess of Node 1 (~60:1; mol:mol) in high salt buffer (100 mM NaCl, 10 mM MgCl2) in the presence of T4 DNA ligase followed by an even higher excess of Node 2 (~100:1; mol:mol) to give quantitative yield of “nodal λ.” This yield was assessed through a gel shift assay (SI Figure S9) by methylating nodal λ with M.SssI to block CpG containing restriction sites61 so that only cut sites near the ends of λ DNA were available, thus simplifying electrophoretic fingerprinting after digesting with BanII and PshAI (cognate sequence: GACNNNNGTC).
Enablement of Nodes.
We designed a molecular strap that hybridizes to and thereby joins a pair of distant nodes flanking a large DNA insert (Figure 4). To ensure a high yield of hybridization while preventing addition of multiple straps to a single molecule construct, now harboring a large insert, we developed a protection / deprotection scheme using an RNA oligonucleotide to reversibly protect one side of our strap. Leveraging RNA-DNA heteroduplex stability, we hybridize a complementary RNA blocker to the portion of our strap complementary to Node 1, which selectively blocks strap hybridization to Node 1, but does not block strap hybridization to Node 2 (Figure 4, 1st step; see SI Figure S10 for gel shift assay). Excess strap is then dialyzed away using a dialysis chamber fashioned from PDMS and polycarbonate track-etched membranes with pores 400 nm in diameter (Figure 4, 2nd step). Our sample with excess strap was added to this chamber and dialyzed in TE overnight. As the strap is much smaller than the pores (<25 nm), while the hydrodynamic diameter of λ DNA (~700 nm) is larger than the pores, the excess strap passes through the membrane, enriching the nodal λ DNA content and any strap already hybridized to Node 2. The RNA blocker is then removed by RNase H digestion (Figure 4, 3rd step), which selectively degrades the hybridized RNA strand, thus allowing the strap to hybridize to Node 1 and restructure the molecule. Large DNA tails (T7 BanII fragments: 19.6 kb and 20.4 kb) are hybridized to the 20 dA 3’ overhangs of our nodal λ DNA via short poly-dT stretches, placed by controlled nucleotidyl terminal transferase (TdT) addition. We found that adding dTTP to our T7 BanII fragments in a ~300:1 mol:mol ratio in the presence of TdT and letting the reaction sit at room temperature overnight gave the best hybridization efficiency to our nodal λ DNA when in the presence of T4 DNA ligase.
Analysis of Nodal λ Soft-Construct.
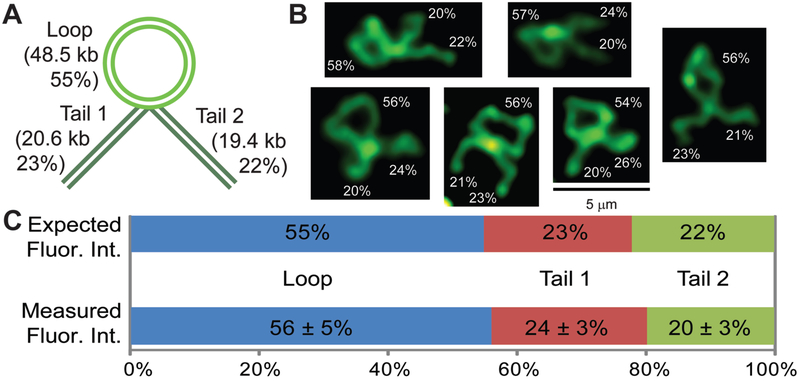
After putting our nodal λ DNA through the workflow summarized in Figure 4, the samples were stained with YOYO-1 and added to a supported lipid bilayer for structural visualization using epifluorescence microscopy. By leveraging DNA mobility on a supported lipid bilayer, we were able to spread and image these structures over time and confirm the structural persistence of each molecule. Through a visual assay of the bilayer, we estimated the yield of the restructuring process as well as the addition of the T7-BanII tails (Table 1). To ensure that the molecules were in fact restructured as expected, a sample of 29 molecules that appeared to be restructured with two full-length tails from the “with strap” sample were analyzed for the integrated fluorescence intensity ratios of each feature (loop, tail 1 and tail 2) in the same manner as described above for the analysis of molecules restructured with biotin and Neutravidin-labeled beads, except that five movie frames were analyzed. Doing so, we found one molecule (3.5%) where the fluorescence intensity ratios correspond to that expected of a restructured λ dimer where the nodes were 97 kb apart rather than 48.5 kb apart. For the other 28 molecules, the average fluorescence intensity of the loop was found to be 55 ± 5%, the average fluorescence intensity of the brighter tail was found to be 24 ± 3% and the average fluorescence intensity of the dimmer tail was found to be 20 ± 3% of the total fluorescence (Figure 6, SI Table S3), which is in good agreement with the expected fluorescence intensities (55% for the loop and 23% or 22% for the tails). The structure where the loop was comprised of 97 kb could either be formed by our oligo nodes hybridizing to a λ dimer during the initial formation of our nodal λ molecule, or from an intermolecular strapping event between two nodal λ molecules followed by an intramolecular strapping event, which we see strong evidence for in some of the molecules classified as “other” in Table 1 (see SI Figure S12 for examples). This suggests that our method of restructuring can also be used with nodes that are significantly farther apart than the 48.5 kb used here.
Table 1.
Restructuring Yields.a
| Restructured | Linearf | Otherg | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 0 Tailsb | 1 Tailc | 2 Tailsd | Combinede | |||||
| With Strap | Number of Molecules |
50 | 112 | 78 | 240 | 212 | 86 | 538 |
| Percentage of Molecules |
9.3% | 21.1% | 14.7% | 45.1% | 38.9% | 16.0% | ||
| No Strap | Number of Molecules |
2 | 4 | 2 | 8 | 66 | 14 | 88 |
| Percentage of Molecules |
2.3% | 4.5% | 2.3% | 9.1% | 75.0% | 15.9% | ||
Number of molecules and corresponding percentages determined to be restructured by an image-based assay of the experimental condition (with strap) and control (no strap; see SI Figure S11 for scheme).
0-tailed structures appear as a λ circle.
1-tailed structures look like a lollipop.
2-tailed structures are bolos as described in the text.
”Combined” column is a sum of all of the restructured molecules with 0, 1 or 2 tails.
Linear molecules are nodal λ DNA molecules that were not restructured, either with or without tails.
“Other” molecules were molecules that could not be classified in any of the other groups, including intermolecular strapping events (SI Figure S12) and nodal λ DNA molecules that had more than one T7-BanII tail attached to a single node.
CONCLUSION
Using the approaches described here, we show that we can construct non-repetitive microscale objects out of dsDNA through sequence-specific self-assembly. As such, new methodologies were developed or adapted to handle and analyze these structures. Chief among these were the use of a reversible RNA blocker and a PDMS dialysis chamber, which allowed the use of excess strap to drive the hybridization to the single-stranded flaps, and a supported lipid bilayer that allowed the DNA to diffuse in two-dimensions only, allowing us to use single-molecule fluorescence microscopy to assess the conformations of the restructured molecules. Two different methods were found to be able to restructure large, dsDNA molecules in a sequence-specific manner. First, DNA can be restructured through sequence-specific biotinylation followed by restructuring around a Neutravidin-labeled bead, which gives a variety of different structures depending on which biotin patches bound to the bead. Second, dsDNA can be restructured in a much more programmable fashion by the addition of nodes to the DNA, which puts flaps of unique sequences on the dsDNA, thus allowing for the use of oligonucleotide straps to self-assemble the DNA into a pre-determined shape. Fluorescence intensity measurements of all the restructured objects confirm that the molecules were indeed in the expected conformations, thus verifying that both restructuring processes are in fact sequence-specific in their self-assembly.
Future efforts are centering on designing dsDNA molecules featuring engineered sequences for enabling creation of multiple internal flaps or single stranded regions, which would support hybridization of nodes. Such engineered functionalities could be enabled by sequence-specific nicks placed by nicking restriction enzymes, followed by strand displacement synthesis using DNA polymerases, such as Klenow exo- to form flaps, or by exonuclease action (e.g., T7 exonuclease62) that would create directed, single stranded patches along large dsDNA molecules. However, as the number of single-stranded features increases, the yield of each strapping event also becomes more important. While our overall strapping yield of ~45% is respectable, when multiple strapping events need to happen simultaneously, a much higher yield of each individual strapping event will be needed. Fortunately, the more strapping events that happen, the smaller the object becomes and the more likely it is for another strapping event to happen, thus increasing the number of straps added should actually increase the yield of each strapping event.
Because dsDNA may exist as millimeter length molecules, once the limitations discussed above have been worked out, this work can be adapted to construct complex structures reminiscent of the nanoscale DNA objects made with DNA origami, but orders of magnitude larger in scale. In ways analogous to DNA origami fabrication, we expect that different functionalities could also be added to these microscale objects through hybridization to flaps not used in the restructuring process to create ordered 3-dimensional superstructures of enzymes/gold nanoparticles/DNA origami/etc. This will create objects featuring well-defined linkages via nodes and straps connecting long dynamic parts. This defines the overall topography of the object in ways akin to a “net-like” structure. This is analogous to the structure of intrinsically disordered proteins where portions of the protein are rigid and well-defined, but many parts are dynamic, allowing the protein to interact with multiple substrates.63, 64 Intrinsically disordered proteins have important roles such as hubs in protein-interaction networks, the assembly of macromolecular machines, and the regulation of gene-expression.65, 66 We believe that our soft-constructs will be similarly valuable for creating adaptive structures useful for broad range applications once they become widely available. However, if a more rigid structure is necessary, dsDNA can also be modified to make it stiffer by coating it in substances such as the protein RecA,45 metals,67–69 or synthetic polymers.70
METHODS
All chemicals, buffers, oligos, equipment and step-by-step instructions are listed in the supplementary data.
Biotin-Bead Restructuring.
T7 DNA was biotinylated through a modification of a published protocol4, 56, 57 where engodenous nicks and gaps are removed through the addition of ten units of E. coli ligase (New England Biolabs, M0205L) and five units of E. coli polymerase I (Roche Applied Science, 10642720001) in the presence of a healing buffer (see SI) at 16° C for a total of 6 hours (2 hours with just the ligase, 4 hours with both the ligase and the polymerase). Sequence-specific nicks are then created through the addition of five units of the nicking enzyme Nt.BspQI (New England Biolabs, R0644S) in the presence of 1X NEBuffer 3.1 buffer and incubating at 16 °C for 4 hours. To add the biotin to these nicks, we added five units of E. coli polymerase I in the presence of a biotinylation buffer (see SI) and incubated at 16 °C for 2 hrs. The Alexa Fluor 647-streptavidin (Invitrogen, S21374) was then bound to the biotinylated DNA by adding an excess of Alexa Fluor 647-streptavidin and incubating on ice for at least 15 minutes. The DNA was then mounted on an optical mapping surface with a PDMS microchannel device and imaged using previously described methods.5
To restructure, ~1.5 ng of biotinylated DNA in TE was combined with 1 μl 100X diluted red fluorescent (580 nm / 605 nm) neutravidin-labeled “FluoSpheres” (Life Technologies, F8770) and incubated at room temperature ~30 minutes before adding 0.5 μl 10 μM YOYO-1 in sterile ddiH2O, incubating at room temperature in the dark for ~20 minutes and then adding to a supported lipid bilayer rinsed with 25 ml of ~40 mM NaCl, 10 mM Na2HPO4, 5 mM Ascorbic acid, pH 7.5.
Assembling Nodal λ.
To assemble the oligo nodes, we combined the Template, Impeding and Flap oligos (IDT; see SI Table S1 for sequences) of each construct in ~1:1.1:1.1 mol:mol:mol ratio, heated at 95 °C and slowly cool to 16 °C over 2.66 hours on a thermocycler. Nodal λ DNA was assembled by combining ~500 ng (~0.016 pmol) λ DNA (New England Biolabs, N3011S) in its monomeric form with ~1 pmol of Node 1 in 1 mM ATP, 1X NEBuffer 3.1 in the presence of 400 units of T4 DNA ligase (New England Biolabs, M0202L) and incubating at room temperature for ~8 hours before adding ~1.5 pmol Node 2 and incubating at room temperature ~16 hours. To remove the excess nodes, the DNA was dialyzed in a 25 μl PDMS dialysis chamber with 0.4 μm pore track-etched polycarbonate membranes (Fisher Scientific, HTTP01300) in 55 ml TE at 4 °C ~24 hours (unpublished experiments).
T7-BanII Tail Creation.
~30 base homopolymer dT tails were added to the 3’ overhangs of the T7-BanII fragments by incubating T7-BanII digests in the presence of a ~300 molar excess of dTTP, 0.25 mM CoCl2 and 4 units of terminal transfersae (New England Biolabs, M0315L) and incubating at room temperature overnight. Excess reagents were then removed by dialyzing the sample against 55 ml TE in a 35 μl PDMS dialysis chamber with 0.1 μm pores (Fisher Scientific, VCTP01300) at 4 °C for ~6 hours.
Blocked Strap Assembly.
To form the non-blocked strap, we combined both strap oligos (IDT; see SI Table S2 for sequences) in a ~1:1 mol:mol ratio in TE at a ~10 μM concentration of each oligo and heated to 95 °C before slowly cooling to 16 °C over 2.66 hours in a thermocycler. To block the strap, we combined the strap (~1 μM) with the RNA blocker oligo (IDT) in IDT nuclease free duplex buffer in a 1:1.1 mol:mol ratio in the presence of 1 mM DTT (BioRad, 1610611) and 30 units of RNase inhibitor, Murine (New England Biolabs, M0314S), then heated at 37 °C for 30 minutes and slowly cooled to room temperature over 3.5 hours in a thermocycler.
Node-Strap Restructuring Protocol.
To restructure the nodal λ DNA, an excess of the blocked strap was hybridized to the nodal λ DNA at room temperature overnight in the presence of 1X NEBuffer 3.1 (100 mM NaCl, 10 mM MgCl2). Excess strap was then removed by dialyzing against 55 ml TE at 4 °C overnight in a 25 μl PDMS dialysis chamber with 0.4 μm pore track-etched polycarbonate membranes. The RNA blocker was then removed with RNase H (New England Biolabs, M0297S). A buffer exchange was then performed by dialyzing the sample in 55 ml 0.1X TE at 4 °C on a rocker for ~6 hours. The sample was then incubated at room temperature for ~18.5 hours in the presence of 0.1 mM spermidine (Fisher Scientific, AC21510–0050) before adding a ~2-fold molar excess of T7-BanII tails and incubating at room temperature for ~24 hours. The sample was then incubated in 1X NEBuffer 3.1 supplemented with 1 mM ATP and 400 units of T4 DNA ligase at room temperature for ~2 days before adding to an SLB for analysis.
Supported Lipid Bilayer Presentation.
Supported lipid bilayers were created using a modification of a published solvent-exchange protocol.67 Briefly, this involves mixing together 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, Avanti Polar Lipids, 850375C), a net neutral lipid, and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP, Avanti Polar Lipids, 890890C), a net positively charged lipid, in the desired proportion (usually 2–4% DOTAP by weight). This mixture of lipids is dissolved in 50% isopropanol to a final concentration of ~1 mg/ml and 500 μl is added to a ~2 ml Teflon/Plexiglass chamber with a Hellmanex (Fisher Scientific, 14-385-944) cleaned glass coverslip bottom. Water is slowly added to the lipid mixture in seven 750 μl aliquots until the solution is >90% water. The chamber is then washed with 25 ml of sterile water to remove any residual isopropanol and non-precipitated lipid, then washed with the desired mobility buffer (10 mM Na2HPO4, pH 7.5, 5 mM Ascorbic acid and ~40–50 mM NaCl) before adding the DNA sample pre-stained with YOYO-1 (~0.5 ng of DNA stained with ~7 pmol YOYO-1 at room temperature for at least 15 minutes) and letting the DNA adsorb overnight.
Supplementary Material
Figure 5.
Nodal λ DNA restructured into a bolo object. A): Cartoon showing a fully restructured molecule comprising three features: a loop made of λ DNA (48.5 kb, ~55% of the total amount of DNA in the structure) and two tails made of two T7-BanII fragments (20.6 kb/19.4 kb each, ~22–23% of the total amount of DNA in the structure). B): Fluorescence micrographs of some restructured molecules with the average integrated fluorescence intensity ratios of each feature. See the supplementary videos to view the conformations over time of these restructured molecules (scale bar = 5 μm). C): Bar plot showing the expected and measured integrated fluorescence intensity ratios. Measured fluorescence intensity ratios are averaged across 28 molecules (±1 S.D.). See SI Figure S13 for example segmentations.
ACKNOWLEDGMENT
We thank G. Potamousis and M. Ray for their lab instruction and insightful discussions on experimental design and data analysis, P. Ravindran for his help with the analysis of the biotinylated DNA, E. Winden for his help developing the ImageJ Macro used to analyze the restructured molecules as well as his insightful discussions on experimental design and data analysis and our undergraduate assistants S. Abraham, K. Nakamura and M. Gotteiner for their assistance in the lab with supported lipid bilayers.
Funding Sources
Work was supported by grants from National Human Genome Research Institute: NIH R01-HG-000225 (DCS and SJWK) and T32 HG002760 (SJWK).
ABBREVIATIONS
- dsDNA
double-stranded DNA
- ssDNA
single-stranded DNA
- SLB
supported lipid bilayer
Footnotes
Supporting Information. Detailed protocols, oligo sequences, fluorescence intensity data, the ImageJ macro used to analyze restructured molecules and 13 supplementary figures of the biotinylation yield of T7 DNA, the schema for the controls, the gels mentioned in the text, extra fluorescence micrographs of various DNA molecules, and example segmentations of the features of restructured molecule fluorescent micrographs can be found in the supporting information (PDF). Video micrographs of example restructured molecules (scale bar = 5 μm) have also been made available. This material is available free of charge on the ACS Publications website at DOI:.
REFERENCES
- 1.Davis RF, Kelner G, Shur M, Palmour JW and Edmond JA, Thin Film Deposition and Microelectronic and Optoelectronic Device Fabrication and Characterization in Monocrystalline Alpha and Beta Silicon Carbide. Proceedings of the IEEE 1991, 79, 677–701. [Google Scholar]
- 2.Liang J, Chen Y, Xu Y, Liu Z, Zhang L, Zhao X, Zhang X, Tian J, Huang Y and Ma Y, Toward All-Carbon Electronics: Fabrication of Graphene-Based Flexible Electronic Circuits and Memory Cards Using Maskless Laser Direct Writing. ACS applied materials & interfaces 2010, 2, 3310–3317. [DOI] [PubMed] [Google Scholar]
- 3.Penzo E, Palma M, Wang R, Cai H, Zheng M and Wind SJ, Directed Assembly of End-Functionalized Single Wall Carbon Nanotube Segments. Nano letters 2015, 15, 6547–6552. [DOI] [PubMed] [Google Scholar]
- 4.Jo K, Dhingra DM, Odijk T, de Pablo JJ, Graham MD, Runnheim R, Forrest D and Schwartz DC, A Single-Molecule Barcoding System Using Nanoslits for DNA Analysis. Proceedings of the National Academy of Sciences 2007, 104, 2673–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimalanta ET, Lim A, Runnheim R, Lamers C, Churas C, Forrest DK, de Pablo JJ, Graham MD, Coppersmith SN, Goldstein S and Schwartz DC, A Microfluidic System for Large DNA Molecule Arrays. Analytical chemistry 2004, 76, 5293–5301. [DOI] [PubMed] [Google Scholar]
- 6.Duffy DC, McDonald JC, Schueller OJA and Whitesides GM, Rapid Prototyping of Microfluidic Systems in Poly (Dimethylsiloxane). Analytical chemistry 1998, 70, 4974–4984. [DOI] [PubMed] [Google Scholar]
- 7.Heller MJ, DNA Microarray Technology: Devices, Systems, and Applications. Annual review of biomedical engineering 2002, 4, 129–153. [DOI] [PubMed] [Google Scholar]
- 8.Kierzek R, Turner DH and Kierzek E, Microarrays for Identifying Binding Sites and Probing Structure of RNAs. Nucleic acids research 2014, 43, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz R, Kang H, Detcheverry F. o. A., Dobisz E, Kercher DS, Albrecht TR, de Pablo JJ and Nealey PF, Density Multiplication and Improved Lithography by Directed Block Copolymer Assembly. Science 2008, 321, 936–939. [DOI] [PubMed] [Google Scholar]
- 10.Chang T-H, Xiong S, Jacobberger RM, Mikael S, Suh HS, Liu C-C, Geng D, Wang X, Arnold MS and Ma Z, Directed Self-Assembly of Block Copolymer Films on Atomically-Thin Graphene Chemical Patterns. Scientific reports 2016, 6, 31407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji S, Liu C-C, Liu G and Nealey PF, Molecular Transfer Printing Using Block Copolymers. ACS nano 2009, 4, 599–609. [DOI] [PubMed] [Google Scholar]
- 12.Park M, Harrison C, Chaikin PM, Register RA and Adamson DH, Block Copolymer Lithography: Periodic Arrays of ~1011 Holes in 1 Square Centimeter. Science 1997, 276, 1401–1404. [Google Scholar]
- 13.Loo Y-L, Willett RL, Baldwin KW and Rogers JA, Interfacial Chemistries for Nanoscale Transfer Printing. Journal of the American Chemical Society 2002, 124, 7654–7655. [DOI] [PubMed] [Google Scholar]
- 14.Yoon J, Lee S-M, Kang D, Meitl MA, Bower CA and Rogers J, Heterogeneously Integrated Optoelectronic Devices Enabled by Micro-Transfer Printing. Advanced Optical Materials 2015, 3, 1313–1335. [Google Scholar]
- 15.Liao W-S, Cheunkar S, Cao HH, Bednar HR, Weiss PS and Andrews AM, Subtractive Patterning Via Chemical Lift-Off Lithography. Science 2012, 337, 1517–1521. [DOI] [PubMed] [Google Scholar]
- 16.Qin D, Xia Y and Whitesides GM, Soft Lithography for Micro-and Nanoscale Patterning. Nature protocols 2010, 5, 491. [DOI] [PubMed] [Google Scholar]
- 17.Piner RD, Zhu J, Xu F, Hong S and Mirkin CA, “Dip-Pen” Nanolithography. Science 1999, 283, 661–663. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Keiper T, Wang X, Yang G, Hallinan D, Zhao J and Xiong P, Molecular Patterning and Directed Self-Assembly of Gold Nanoparticles on Gaas. ACS applied materials & interfaces 2017, 9, 43363–43369. [DOI] [PubMed] [Google Scholar]
- 19.Wu C-C, Reinhoudt DN, Otto C, Subramaniam V and Velders AH, Strategies for Patterning Biomolecules with Dip-Pen Nanolithography. Small 2011, 7, 989–1002. [DOI] [PubMed] [Google Scholar]
- 20.Peng Q, Shao X-R, Xie J, Shi S-R, Wei X-Q, Zhang T, Cai X. x. and Lin Y-F, Understanding the Biomedical Effects of the Self-Assembled Tetrahedral DNA Nanostructure on Living Cells. ACS applied materials & interfaces 2016, 8, 12733–12739. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Tu J, Wang D, Zhu H, Maity SK, Qu X, Bogaert B, Pei H and Zhang H, Programmable and Multifunctional DNA-Based Materials for Biomedical Applications. Advanced Materials 2018, 30, 1703658. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Q, Song C, Nangreave J, Liu X, Lin L, Qiu D, Wang Z-G, Zou G, Liang X and Yan H, DNA Origami as a Carrier for Circumvention of Drug Resistance. Journal of the American Chemical Society 2012, 134, 13396–13403. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z-G, Liu Q and Ding B, Shape-Controlled Nanofabrication of Conducting Polymer on Planar DNA Templates. Chemistry of Materials 2014, 26, 3364–3367. [Google Scholar]
- 24.Maune HT, Han S. p., Barish RD, Bockrath M, Goddard Iii WA, Rothemund PWK and Winfree E, Self-Assembly of Carbon Nanotubes into Two-Dimensional Geometries Using DNA Origami Templates. Nature nanotechnology 2010, 5, 61. [DOI] [PubMed] [Google Scholar]
- 25.Teschome B, Facsko S, Schonherr T, Kerbusch J, Keller A and Erbe A, Temperature-Dependent Charge Transport through Individually Contacted DNA Origami-Based Au Nanowires. Langmuir 2016, 32, 10159–10165. [DOI] [PubMed] [Google Scholar]
- 26.Shen X, Song C, Wang J, Shi D, Wang Z, Liu N and Ding B, Rolling up Gold Nanoparticle-Dressed DNA Origami into Three-Dimensional Plasmonic Chiral Nanostructures. Journal of the American Chemical Society 2011, 134, 146–149. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Huh J-H, Kim K and Lee S, DNA Origami-Guided Assembly of the Roundest 60–100 Nm Gold Nanospheres into Plasmonic Metamolecules. Advanced Functional Materials 2018, 28, 1707309. [Google Scholar]
- 28.Kuzyk A, Jungmann R, Acuna GP and Liu N, DNA Origami Route for Nanophotonics. ACS Photonics 2018, 5, 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J, Liu M, Liu Y, Woodbury NW and Yan H, Interenzyme Substrate Diffusion for an Enzyme Cascade Organized on Spatially Addressable DNA Nanostructures. Journal of the American Chemical Society 2012, 134, 5516–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Wang F, Lu C-H, Girsh J, Golub E and Willner I, Switchable Enzyme/Dnazyme Cascades by the Reconfiguration of DNA Nanostructures. Chemistry-A European Journal 2014, 20, 16203–16209. [DOI] [PubMed] [Google Scholar]
- 31.Linko V, Eerikainen M and Kostiainen MA, A Modular DNA Origami-Based Enzyme Cascade Nanoreactor. Chemical Communications 2015, 51, 5351–5354. [DOI] [PubMed] [Google Scholar]
- 32.Timm C and Niemeyer CM, Assembly and Purification of Enzyme-Functionalized DNA Origami Structures. Angewandte Chemie International Edition 2015, 54, 6745–6750. [DOI] [PubMed] [Google Scholar]
- 33.Seeman NC, Nucleic Acid Junctions and Lattices. Journal of theoretical biology 1982, 99, 237–247. [DOI] [PubMed] [Google Scholar]
- 34.Seeman NC, DNA Engineering and Its Application to Nanotechnology. Trends in biotechnology 1999, 17, 437–443. [DOI] [PubMed] [Google Scholar]
- 35.Rothemund PWK, Folding DNA to Create Nanoscale Shapes and Patterns. Nature 2006, 440, 297–302. [DOI] [PubMed] [Google Scholar]
- 36.Wei B, Dai M and Yin P, Complex Shapes Self-Assembled from Single-Stranded DNA Tiles. Nature 2012, 485, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ke Y, Ong LL, Shih WM and Yin P, Three-Dimensional Structures Self-Assembled from DNA Bricks. Science 2012, 338, 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong LL, Hanikel N, Yaghi OK, Grun C, Strauss MT, Bron P, Lai-Kee-Him J, Schueder F, Wang B, Wang P, Jocelyn KY, Myrhvold C, Zhu A, Jungmann R, Bellot G, Ke Y and Yin P, Programmable Self-Assembly of Three-Dimensional Nanostructures from 10,000 Unique Components. Nature 2017, 552, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thubagere AJ, Li W, Johnson RF, Chen Z, Doroudi S, Lee YL, Izatt G, Wittman S, Srinivas N and Woods D, A Cargo-Sorting DNA Robot. Science 2017, 357, eaan6558. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Han D, Nangreave J, Liu Y and Yan H, DNA Origami with Double-Stranded DNA as a Unified Scaffold. ACS nano 2012, 6, 8209–8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchi AN, Saaem I, Vogen BN, Brown S and LaBean TH, Toward Larger DNA Origami. Nano letters 2014, 14, 5740–5747. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Wang Q, Peng J, Long Q, Yu H and Li Z, Self-Assembly of Large DNA Origami with Custom-Designed Scaffolds. ACS applied materials & interfaces 2018, 10, 24344–24348. [DOI] [PubMed] [Google Scholar]
- 43.Rajendran A, Endo M, Katsuda Y, Hidaka K and Sugiyama H, Programmed Two-Dimensional Self-Assembly of Multiple DNA Origami Jigsaw Pieces. ACS nano 2011, 5, 665–671. [DOI] [PubMed] [Google Scholar]
- 44.Wagenbauer KF, Sigl C and Dietz H, Gigadalton-Scale Shape-Programmable DNA Assemblies. Nature 2017, 552, 78. [DOI] [PubMed] [Google Scholar]
- 45.Schiffels D, Szalai VA and Liddle JA, Molecular Precision at Micrometer Length Scales: Hierarchical Assembly of DNA-Protein Nanostructures. ACS nano 2017, 11, 6623–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paukstelis PJ, Nowakowski J, Birktoft JJ and Seeman NC, Crystal Structure of a Continuous Three-Dimensional DNA Lattice. Chemistry & biology 2004, 11, 1119–1126. [DOI] [PubMed] [Google Scholar]
- 47.Yan H, Park SH, Finkelstein G, Reif JH and LaBean TH, DNA-Templated Self-Assembly of Protein Arrays and Highly Conductive Nanowires. Science 2003, 301, 1882–1884. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki Y, Endo M and Sugiyama H, Lipid-Bilayer-Assisted Two-Dimensional Self-Assembly of DNA Origami Nanostructures. Nature communications 2015, 6, 8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Https://En.Wikipedia.Org/Wiki/Claes_Oldenburg.
- 50.Manning GS, The Persistence Length of DNA Is Reached from the Persistence Length of Its Null Isomer through an Internal Electrostatic Stretching Force. Biophys J 2006, 91, 3607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reese HR and Zimm BH, Fracture of Polymer Chains in Extensional Flow: Experiments with DNA, and a Molecular-Dynamics Simulation. The Journal of Chemical Physics 1990, 92, 2650–2662. [Google Scholar]
- 52.Schwartz DC and Cantor CR, Separation of Yeast Chromosome-Sized Dnas by Pulsed Field Gradient Gel Electrophoresis. Cell 1984, 37, 67–75. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz DC, Li X, Hernandez LI, Ramnarain SP, Huff EJ and Wang Y-K, Ordered Restriction Maps of Saccharomyces Cerevisiae Chromosomes Constructed by Optical Mapping. Science 1993, 262, 110–114. [DOI] [PubMed] [Google Scholar]
- 54.Gupta A, Place M, Goldstein S, Sarkar D, Zhou S, Potamousis K, Kim J, Flanagan C, Li Y, Newton MA, Callander NS, Hematti P, Bresnick EH, Ma J, Asimakopoulos F and Schwartz DC, Single-Molecule Analysis Reveals Widespread Structural Variation in Multiple Myeloma. Proceedings of the National Academy of Sciences 2015, 112, 7689–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang P, Too PH-M, Samuelson JC, Chan S-H, Vincze T, Doucette S, Bäckström S, Potamousis KD, Schramm TM, Forrest D, Schwartz DC and Xu S-Y, Engineering Bspqi Nicking Enzymes and Application of N. Bspqi in DNA Labeling and Production of Single-Strand DNA. Protein expression and purification 2010, 69, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kounovsky-Shafer KL, Hernandez-Ortiz JP, Potamousis K, Tsvid G, Place M, Ravindran P, Jo K, Zhou S, Odijk T, de Pablo JJ and Schwartz DC, Electrostatic Confinement and Manipulation of DNA Molecules for Genome Analysis. Proceedings of the National Academy of Sciences 2017, 114, 13400–13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kounovsky-Shafer KL, Hernandez-Ortiz JP, Jo K, Odijk T, de Pablo JJ and Schwartz DC, Presentation of Large DNA Molecules for Analysis as Nanoconfined Dumbbells. Macromolecules 2013, 46, 8356–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hohner AO, David MPC and Rädler JO, Controlled Solvent-Exchange Deposition of Phospholipid Membranes onto Solid Surfaces. Biointerphases 2010, 5, 1–8. [DOI] [PubMed] [Google Scholar]
- 59.Maier B and Rädler JO, Conformation and Self-Diffusion of Single DNA Molecules Confined to Two Dimensions. Physical review letters 1999, 82, 1911. [Google Scholar]
- 60.Maier B and Rädler JO, DNA on Fluid Membranes: A Model Polymer in Two Dimensions. Macromolecules 2000, 33, 7185–7194. [Google Scholar]
- 61.Ananiev GE, Goldstein S, Runnheim R, Forrest DK, Zhou S, Potamousis K, Churas CP, Bergendahl V, Thomson JA and Schwartz DC, Optical Mapping Discerns Genome Wide DNA Methylation Profiles. BMC molecular biology 2008, 9, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramanathan A, Huff EJ, Lamers CC, Potamousis KD, Forrest DK and Schwartz DC, An Integrative Approach for the Optical Sequencing of Single DNA Molecules. Anal Biochem 2004, 330, 227–41. [DOI] [PubMed] [Google Scholar]
- 63.Berlow RB, Dyson HJ and Wright PE, Functional Advantages of Dynamic Protein Disorder. FEBS letters 2015, 589, 2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berlow RB, Dyson HJ and Wright PE, Hypersensitive Termination of the Hypoxic Response by a Disordered Protein Switch. Nature 2017, 543, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright PE and Dyson HJ, Intrinsically Disordered Proteins in Cellular Signalling and Regulation. Nature reviews Molecular cell biology 2015, 16, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma R, Raduly Z, Miskei M and Fuxreiter M, Fuzzy Complexes: Specific Binding without Complete Folding. FEBS letters 2015, 589, 2533–2542. [DOI] [PubMed] [Google Scholar]
- 67.Ongaro A, Griffin F, Beecher P, Nagle L, Iacopino D, Quinn A, Redmond G and Fitzmaurice D, DNA-Templated Assembly of Conducting Gold Nanowires between Gold Electrodes on a Silicon Oxide Substrate. Chemistry of Materials 2005, 17, 1959–1964. [Google Scholar]
- 68.Berti L, Alessandrini A and Facci P, DNA-Templated Photoinduced Silver Deposition. Journal of the American Chemical Society 2005, 127, 11216–11217. [DOI] [PubMed] [Google Scholar]
- 69.Monson CF and Woolley AT, DNA-Templated Construction of Copper Nanowires. Nano letters 2003, 3, 359–363. [Google Scholar]
- 70.Watson S, Hedley JH, Galindo MA, Al-Said SAF, Wright NG, Connolly BA, Horrocks BR and Houlton A, Synthesis, Characterisation and Electrical Properties of Supramolecular DNA-Templated Polymer Nanowires of 2, 5-(Bis-2-Thienyl)-Pyrrole. Chemistry-A European Journal 2012, 18, 12008–12019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.