Abstract
Adoptive cell therapy of tumor infiltrating lymphocytes has shown promise for treatment of refractory melanoma and other solid malignancies; however, challenges to manufacturing have limited its widespread use. Traditional manufacturing efforts were lengthy, cumbersome, and employed open culture systems. We describe changes in testing and manufacturing that decreased the process cycle time, enhanced the robustness of critical quality attribute testing, and facilitated a functionally closed system. These changes have enabled export of the manufacturing process to support multi-center clinical trials.
Keywords: Adoptive immunotherapy, Cell Therapy, Melanoma, Tumor infiltrating lymphocytes
Introduction
In 2013, Science listed ‘Cancer Immunotherapy’ as the breakthrough of the year [1]; however, immunotherapy, specifically adoptive cell therapy (ACT) with tumor infiltrating lymphocytes (TIL) have been used as an experimental treatment for cancer patients for the past three decades. Pioneered by Dr. Steven Rosenberg at the National Cancer Institute, infusion of TIL as a treatment for melanoma has shown promise as a salvage therapy for refractory disease [2]. The first preliminary report, published in the New England Journal of Medicine in 1988, summarized the results of 20 patients treated with TIL and showed responses in nine of 15 patients not previously treated with interleukin 2 (IL-2) and two of 5 patients who failed previous IL-2 therapy [3]. Consistent results in melanoma patients have been maintained over the years with objective response rates occurring in 40 – 50% of patients treated, and complete response rates occurring in 10 – 20% of patients [4–9]. ACT with TIL has curative potential and those patients who do mount a complete response have durable responses out three to five years post treatment.
The principle of ACT with TIL is to utilize ex vivo expanded T lymphocytes isolated directly from the tumor sample and infuse the cells back to the patient to elicit an immune response against the tumor. T lymphocytes isolated directly from the tumor sample are thought to be specific to tumor associated antigens but have been silenced by inhibitory signaling pathways via malignant and/or other cell types present in the tumor microenvironment. TIL used to date for clinical trials have not been genetically modified and represent a polyclonal population of cells capable of recognizing and responding to a variety of tumor associated antigens. Prior identification of the tumor associated antigens is not required and concerns over off-target tissue damage are negated as target antigen recognition has occurred via natural mechanisms of T cell development in response to the malignant cells. In these regards, TIL are uniquely distinct from chimeric antigen receptor T cells which have been genetically modified to express one or a limited number of target antigens, which may also occur on normal, healthy tissue leading to untoward off-tumor effects.
At the Moffitt Cancer Center (MCC), the initial TIL trial opened in 2010 with 19 metastatic melanoma patients combining non-myeloablative chemotherapy, ACT with TIL and high-dose IL-2 [5]. Thirteen of 19 patients enrolled successfully completed treatment with a demonstrated response rate of 38%. However, progression of disease occurred in six patients (21%) before receiving TIL. Trial dropout due to progression occurred during the time required for TIL production in the laboratory (generally 6 weeks). To address the drop-out rate of nearly one-third of patients, a second trial was initiated at MCC [10]. It was hypothesized that systemic use of antibodies that block immune checkpoints, in combination with ACT with TIL, would decrease drop-out rates since patients would be receiving an active in vivo therapy during TIL manufacturing in vitro. Ipilimumab, an antibody that inhibits cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) was administered 2 weeks prior to and 1 week following tumor harvest. TIL were successfully expanded from all 13 patients enrolled and twelve patients were treated, significantly reducing the drop-out rate of patients due to disease progression. An overall objective response rate of 38% by intention to treat analysis was observed at 12 weeks. Together these trials at MCC demonstrated that adequate numbers of TIL could be produced from patient tumors in sufficient time to avoid disease progression, leading to predictable clinical outcomes.
Despite impressive clinical results, only a few centers outside of the National Cancer Institute and MCC have conducted ACT TIL clinical trials and TIL therapy has not been licensed by the FDA. This is due in large part to the complexity and cost of the manufacturing procedure. This review is focused on streamlining and shortening the TIL manufacturing process to facilitate export to other manufacturing sites and commercialization.
Traditional TIL Manufacturing Process
The classical schema for procuring, expanding and testing naturally occurring autologous TIL consists of a lengthy multistep open system process, termed pre-Rapid Expansion Protocol (pre-REP) followed by a 14-day Rapid Expansion Protocol (REP). Figure 1 demonstrates that the resected tumor is carefully dissected into multiple tumor fragments of 1–3 mm in size. Individual fragments are placed into single wells of a 24-well culture plate in the presence of IL-2 enriched (3000 – 6000 IU/mL) culture media. The total number of fragments plated depends on the tumor size and is dictated by the clinician performing the tumor resection. However, the targeted fragment number is typically 48 resulting in two primary 24-well plates for pre-REP culture. The goal is to prepare fragments for plating from all areas of the tumor excluding necrotic and fibrous tissue areas. Any remaining tumor is enzymatically digested and cryopreserved for future use in ex vivo co-culture with TIL to detect tumor specificity. Each well requires microscopic examination and feeding or splitting every three to 4 days. Over time, any residual tumor cells die out since the culture conditions are supportive of lymphocytes only. Wells with greater than 80% confluency of TIL are split into secondary plates prior to feeding. Each secondary plate is limited to only one primary fragment for further splitting during the pre-REP phase. Feeding consists of a 50% media change while splitting is at a 1:1 ratio. In a successful expansion the tumor cells are out grown by TIL within three to 5 weeks. By the end of the pre-REP phase, the culture plate number has increased from two primary plates to between 60 to one hundred 24-well plates. The large number of plates present in the later pre-REP phase requires technologists to spend approximately 6 hours examining, splitting and feeding the wells for one patient every three to 4 days. Ideally, expansion of at least 6 fragments into more than 6 wells of a secondary plate should occur prior to testing for tumor specificity. One or 2 wells of each expanded fragment are harvested and co-cultured with autologous tumor digest, HLA matched (positive control) and human leukocyte antigen (HLA) mismatched (negative control) tumor cell lines for 20–24 hours. Interferon-γ (IFN-γ) production is measured in each co-culture supernatant by enzyme-linked immunosorbent assay (ELISA). TIL that are reactive to autologous or HLA-matched tumor cells are considered specific to tumor associated antigens. TIL that react to HLA-mismatched tumor cells are excluded due to lack of specificity. The goal is to have 60 × 106 viable TIL for the subsequent REP stage. Reactive TIL are pooled and then expanded in the presence of IL-2 (3,000 IU/ml), agonistic anti-CD3 (30ng/ml) and irradiated allogeneic peripheral blood mononuclear cell (PBMC) feeder cells at a ratio of 1 TIL: 200 PBMC for 14 days in REP phase. Traditionally, REP processing begins in multiple T175 flasks with subsequent feeding at day three to 4 and splitting of flasks at day 7. TIL are split by transferring from the T175 into as many as thirty gas permeable 3 liter cell culture bags. At day 14, cells are harvested, volume reduced, washed and prepared for fresh administration. The cumulative time from tumor resection to final TIL production is typically five to 7 weeks.
Figure 1.
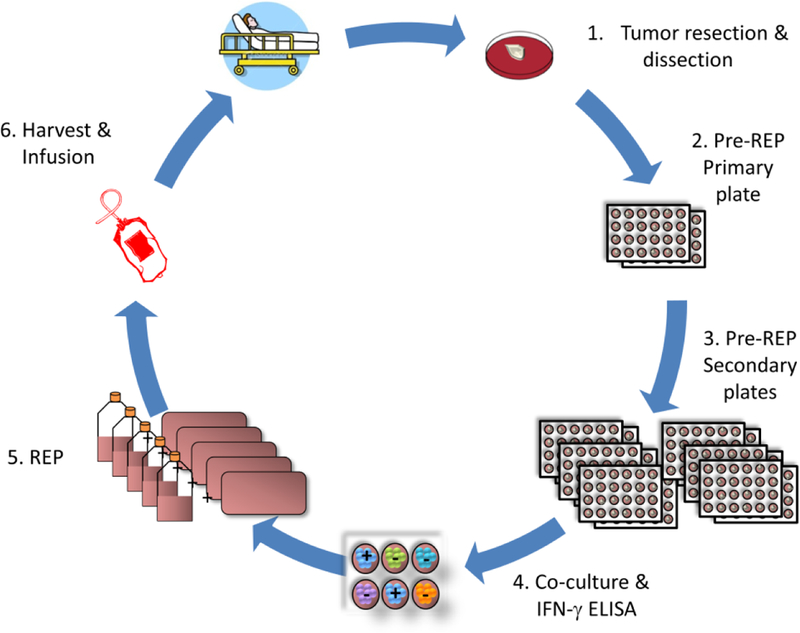
Traditional TIL Manufacturing Process
Challenges to TIL Manufacturing
Obstacles encountered in the traditional TIL manufacturing process have limited the widespread adoption of TIL ACT. In addition to the technical difficulty of the processing procedure and the manufacturing time and cost, a significant limitation impeding commercialization of the TIL manufacturing process is the lack of availability of qualified reagents. Human AB serum is required to supplement the culture media throughout TIL manufacturing and we have determined that the TIL response to commercially available human AB serum varies significantly based on source and lot. To decrease the impact of this variation MCC completes an extensive validation study to determine the optimal serum for the TIL process. Multiple different manufacturer lots are acquired and tested, coincidentally with the current lot in use, against a minimum of two tumor samples. The number of fragments that expand, total cell number and viability, IFN-γ production in response to tumor challenge and the phenotype of the final TIL product are compared across the different serums evaluated. Once the best performing serum is identified, the entire lot is purchased from the vendor. This requires both a considerable financial output as well as a large amount of controlled and monitored storage space. Identification of a serum substitute or, at a minimum, of a component with consistent lot-to-lot performance, would obviate the need for extensive testing and storage of large quantities of reagents. We are currently evaluating commercially available human platelet lysate as a serum substitute and although we have identified sources that exhibit comparable or superior TIL growth characteristics, to date we continue to see unacceptable lot-to-lot variation. As a consequence we have opted to continue use with human AB serum.
Expansion of TIL in the REP phase requires the addition of mononuclear cells (MNC) isolated from normal donor apheresis products. The donors must meet eligibility requirements, as determined by the FDA, and undergo a four blood volume apheresis procedure. The apheresis product is subjected to ficoll gradient separation via an automated process to prepare enriched MNCs which are then cryopreserved. A minimum of three individual donor’s cells are thawed, pooled and irradiated to be used for a single TIL expansion at a ratio of 1 TIL to 200 normal donor MNC. In order to sustain a bank of normal donor MNC at our institution, we are required to maintain an IRB-approved protocol, retain a pool of qualified donors, and incur the costs of screening, testing, processing and storing the reagent. Access to a better defined source of lymphocyte stimulation, one that consistently meets minimum quality control standards, would eliminate the need to maintain normal donor MNC and likely exclude a significant variable in the expansion protocol.
In our experience, tumor quality is the single most important quality attribute for successful TIL growth. Due to the small fragment size placed in the initial pre-REP cultures, the tumor must be carefully resected by an experienced surgeon with care to exclude non-tumor tissue. Fatty or necrotic tissue remaining in the specimen provided to the processing facility negatively impacts TIL growth in the pre-REP culture. Importantly, the sterility of the specimen must be carefully maintained throughout surgical resection to limit the risk of microbial contamination. Antibiotics may be used in the transport medium as a precautionary measure.
In-process and Release Testing of TIL
Additional critical quality attributes determined during the TIL manufacturing process, or for lot release testing, include sterility, endotoxin, mycoplasma, purity and viability as defined by the FDA. Specific tests and acceptable results at the end of Pre-REP and at release are summarized in Table 1. In-process testing is completed at the end of Pre-REP to ensure sterility, determine total cell number and viability, and to determine fragments showing tumor specificity. Final sterility results are not available prior to release of the product; therefore, a Gram stain test is completed to detect gross contamination of the product. In the event that a sterility culture is positive after product infusion, an action plan is in place to notify the Principal Investigator and Medical Director to ensure appropriate patient care. Purity of the final product is determined by endotoxin testing using the Endosafe® test system. Development of a potency assay representative of the function of the final TIL product has been a primary objective of our development efforts. Ultimately, we seek to expand a population of TIL with specificity to the tumor antigens of the patient and which have cytotoxic function in vivo. Although the identity of the final TIL product is tested by flow cytometry for lymphocyte-specific phenotypic markers, including CD45, CD3, CD4 and CD8, this testing does not predict tumor specificity. Incorporation of IFN-γ secretion to autologous tumor challenge by ELISA currently represents the best in vitro assay for prediction of tumor specificity. However, the assay is limited in application as it does not always correlate with clinical outcome measures. None the less, we have painstakingly validated the IFN-γ assay to determine accuracy, precision, sensitivity and specificity, as defined by the FDA and United States Pharmacopeia (USP). Development of a potency assay predictive of individual patient tumor specificity represents an on-going effort by our development team.
Table 1.
In-Process and Lot Release Testing for TIL
| Test | Method | Acceptable Results | |
|---|---|---|---|
| In-Process Testing (Pre-REP) | Sterility | Aerobic, Anaerobic culture (BACT/ALERT®) Fungal culture | No growth |
| Tumor-specific T cell activation | IFN-γ ELISA | >200 pg/ml | |
| Viability | Cellometer AO/PI or Manual Trypan Blue | >70% viable cells | |
| Cell Count | Cellometer AO/PI or Manual Trypan Blue | >60 × 106 cells | |
| Lot Release Testing (Post-REP) | Mycoplasma contamination | PCR (VenorGeM) or qPCR (MycoTOOL) | Negative |
| Sterility* | Aerobic, Anaerobic culture (BacT/ALERT®) Fungal culture | No growth | |
| Gross Contamination | Gram stain | No organisms seen | |
| Purity | Endosafe® | <5 EU/kg | |
| Identity | Flow cytometry | >90% CD45+ CD3+ cells | |
| Viability | Cellometer AO/PI or Manual Trypan Blue | >70% viable cells | |
| Cell Count | Cellometer AO/PI or Manual Trypan Blue | 0.1 – 2 × 1011 cells | |
| Tumor-specific T cell activation | IFN-γ ELISA | >200 pg/ml |
Sterility test results are not available prior to release of the final product.
Improvements to TIL Manufacturing
Our attempts to advance the TIL manufacturing process have been squarely focused on decreasing the production time by enhancing TIL expansion capacity in vitro. The pre-REP phase represents the longest part of the TIL production process, requiring three to 5 weeks of culture to obtain the desired number of cells (60 × 106) needed to go into the REP phase. In an attempt to shorten pre-REP culture time and increase cell number, manipulation of co-stimulatory signaling pathways was examined. Preclinical laboratory studies demonstrated that TIL isolated from solid tumors express the activation/co-stimulatory molecule 4–1BB/CD137, a sign of recent antigenic stimulation in the tumor microenvironment, which can be exploited ex vivo to enhance TIL expansion through direct addition of anti-4-1BB antibody to tumor fragments in culture [11]. Addition of agonistic anti-4-1BB antibody during pre-REP resulted in increased survival and expansion of T effector functions of TIL cultured ex vivo [12]. In a large-scale validation study performed in our laboratory, adding agonistic anti-4-1BB antibody to pre-REP TIL cultures significantly increased the percentage of tumor fragments exhibiting TIL outgrowth (Figure 2A) as well as the total number of viable TIL harvested (Figure 2B). Importantly, tumor reactivity, as determined by IFN-γ secretion from TIL challenged with autologous tumor, was markedly elevated in the presence of anti-4-1BB antibody as compared to IL-2 alone (Figure 2C). Finally, the addition of anti-4-1BB antibody commonly led to an increased number of CD8 positive cells in the pre-REP cultures (Figure 2D). These results indicated that addition of activating agents to the pre-REP TIL culture significantly enhanced proliferation resulting in production of more TIL in a reduced time frame. Phenotypic and functional studies are ongoing to determine the consequence of enhanced in vitro TIL expansion as it applies to differentiation of the cells.
Figure 2.
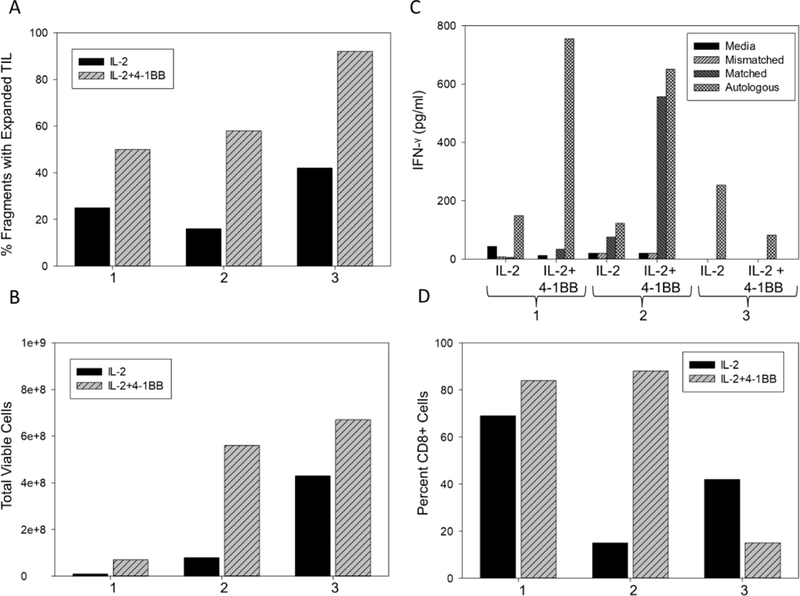
Pre-REP TIL expansion in tissue culture plates with and without 4-1BB. Equal numbers of 48 melanoma tumor fragments from three separate patients were plated in tissue culture wells in IL-2 with or without 4-1BB. The addition of 4-1BB consistently increased the number of tumor fragments with TIL outgrowth (A) and the total number of viable TIL (B). (C) TIL harvested at day 14 were cultured in complete media alone, with melanoma cell lines mismatched or matched for MHC class I antigens or with autologous tumor digests. Culture supernatants were tested for IFN-γ levels by ELISA. The presence of 4-1BB augmented IFN-γ production in 2 of three samples tested. (D) The percent CD8+ cells was increased with 4-1BB in 2 or three samples tested.
A second priority has been to minimize the number of TIL manufacturing steps performed in an open system. Traditionally, every step of the TIL manufacturing process including tumor dissection, pre-REP and REP was performed in an open system, albeit in a biological safety cabinet located in a monitored Class 10,000 clean room. Currently, the REP phase is the step most amenable to closure by replacing the tissue culture flasks and bags with G-Rex®100MCS culture flasks and GatheRex Cell Harvest Pump. The design of the G-Rex culture flasks with a gas-permeable membrane at the base supports large media volumes without compromising gas exchange. The GatheRex pump functions to replace media when necessary and to harvest cells in a functionally closed system. When used together, the G-Rex®100MCS flasks and GatheRex pump represent a culture system more amenable to good manufacturing practices. A multicenter study confirmed that this fully optimized cell culture system can reliably produce a 100-fold cell expansion in only 10 days using 1liter of medium without additional medium exchange [13]. When applied to TIL expansion culture utilizing a minimal median exchange approach, Figure 3A demonstrates that by day 14 of culture TIL expanded 2310-fold in the REP phase in G-Rex®100MCS versus 729-fold in the flask-to-bag method while maintaining excellent viability (Figure 3B). To assess tumor specificity of the expanded TIL, an IFN-γ ELISA assay was performed on TIL harvested at day 14. TIL were cultured in conditioned media alone or with melanoma cell lines that were either mismatched or matched at the MHC Class I locus and compared to pre-REP cells prior to the expansion phase. Figure 3C demonstrates that TIL expanded in either flask-to-bag or G-Rex®100MCS culture vessels had comparable IFN-γ production in response to matched tumor cell lines (autologous tumor unavailable), albeit higher background was observed in media only or mismatched tumor cell line cultures with TIL grown in flasks. These data suggest that IFN-γ production in response to tumor cell lines represent a surrogate assay for tumor specificity and advocate for development of a more specific potency assay when autologous tumor is unavailable. In summary, in addition to production of greater numbers of viable TIL, transition to G-Rex®100MCS flasks has resulted in fewer total vessels, less media, less incubator space and less labor than traditional culture methods using flasks and bags.
Figure 3.
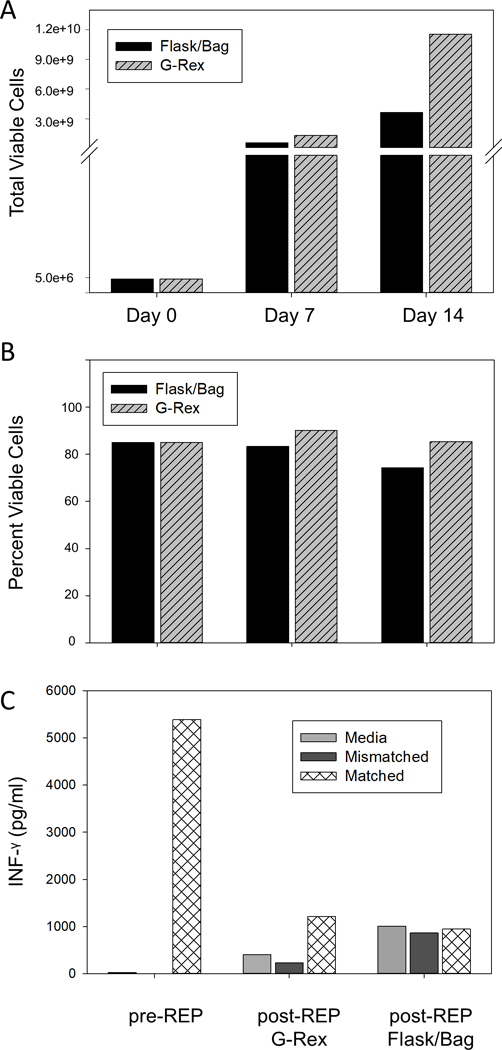
REP TIL expansion in tissue culture flasks and bags compared to G-Rex®100MCS. 5 × 106 pre-REP TIL isolated from melanoma tumors were cultured in T175 flasks or G-Rex®100MCS in the presence of IL-2 (3,000 IU/ml), normal donor mononuclear cells and agonistic anti-CD3 (30 ng/ml). On day 7 TIL cultured in T175 flasks were transferred to tissue culture bags. On day 14 TIL were harvested from culture vessels and cell counts and viability were determined. (A) 5 × 106 TIL were set up in each culture system at day 0. Cell counts at day 7 and 14 were 5.7 × 108 and 3.7 × 109, respectively for T175 flask to bag cultures and 1.32 × 109 and 1.16 × 1010, respectively for G-Rex®100MCS cultures. (B) Viability of pre-REP TIL were 85% at day 0, 83% and 74% at day 7 and 14, respectively, for T175 flask to bag cultures and 90% and 85%, respectively, for G-Rex®100MCS cultures. Data represent the mean of two independent experiments using expanded TIL from different patients. (C) TIL harvested at day 14 were cultured for 24 hours in complete media alone, or with melanoma cell lines mismatched or matched for MHC class I antigens. Culture supernatants were tested for IFN-γ levels by ELISA and compared to IFN-γ levels from the same pre-REP cells prior to REP expansion. Data denote results from a single representative experiment.
Given the benefits shown by addition of anti-4-1BB antibody during pre-REP and culturing TIL in G-Rex®100MCS during REP, we designed a Phase 1 clinical trial to incorporate both process changes. When taken together, the optimization decreased the culture period from 51 to 37 days and limited the technical intervention to 14 days. Most importantly, optimizing the TIL culture expansion allowed for consistent achievement of the optimal therapeutic dose of 60 × 109 cells, for all patients compared to achievement of optimal dose in only 40% of patients using traditional culture methods. This clinical trial has recently completed accrual and is in the follow-up stage. Our team has subsequently moved forward to replace 24-well polystyrene plates used in the culture of tumor fragments in the pre-REP phase with 24-well gas permeable GRex plates [14]. In these experiments comparable cell doses were obtained a full 7 days earlier in GRex versus polystyrene plates and demonstrated superior viability (91±3% versus 79±5%, respectively). In addition, TIL grown in GRex plates showed a higher percentage of CD8+ cells (94±8% versus 76±14%, p=<0.001) and lower percentage of NK cells (2.4±3% versus 12±9%, p=<0.001) than those grown in polystyrene. Tumor‐ specific activity was comparable, as measured by IFN‐ γ secretion between the two culture conditions.
Ongoing efforts at MCC to improve the TIL manufacturing process include exploring techniques to identify tumor specific TIL in the original tumor sample in order to maximize expansion of this precise subset of lymphocytes. To accomplish this goal we prepared an enzymatically digested sample from the original tumor biopsy and subjected the cells to high throughput rapid screening flow cytometric analysis of extra and intracellular markers representing various activation and functional stages. The purpose of the screening analysis is to identify cells that phenotypically suggest recent exposure to tumor specific antigens and to preferentially isolate and expand that subpopulation. A second major goal is to replace the normal donor feeder cells utilized during the REP phase of TIL expansion with an artificial antigen presenting cell (aAPC) that has been genetically engineered to express T cell stimulating molecules. Our laboratory is currently in the process of preparing GMP-grade Master and Working Cell Banks of the aAPC for use in production of TIL for clinical trials. Further manufacturing/processing revisions will be evaluated as the interest in the use of TIL for ACT continues to grow and new indications for treatment of solid tumors beyond melanoma are investigated. In 2017, our industry partner Iovance Biotherapeutics announced that the U.S. FDA granted Fast Track designation for LN-144, the company’s ACT using novel TIL technology, for the treatment of advanced melanoma. Significant improvements made to the manufacturing process included a total manufacturing time of 22 days and incorporation of cryopreservation of the final cell product. Preliminary results suggest products from the improved manufacturing protocol have comparable quality attributes [15] and safety profiles when administered to patients [16]. Incorporation of the freezing step offers flexibility in the timing of dosing allowing the availability of the product for infusion to the patient at the optimal time.
Conclusions
We approached a complex manufacturing protocol for tumor infiltrating lymphocyte production with the goal of reducing cycle time by shortening the culture period without adversely affecting product quality or function. Simultaneously, we modified the process by incorporating a more closed system to minimize contamination and align with good manufacturing practices. These process improvements were instrumental in facilitating export of the technology to other manufacturing sites to support multicenter clinical trials. A focus on critical quality attributes in early stage manufacturing at the academic cell processing laboratory provides seamless technology transfer to partners looking to commercialize the cell therapy product.
Acknowledgments
The authors thank Dr. Nermin Gerges for her work in optimization of the TIL manufacturing process and Dr. Amod Sarnaik for his continued collaboration. This work was supported in part by NCI 3P30CA076292-18S5.
Abbreviations
- ACT
adoptive cell therapy
- TIL
tumor infiltrating lymphocytes
- IL-2
interleukin 2
- MCC
Moffitt Cancer Center
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- FDA
U.S. Food and Drug Administration
- REP
rapid expansion protocol
- HLA
human leukocyte antigen
- IFN-γ
interferon-γ
- PBMC
peripheral blood mononuclear cells
- MNC
mononuclear cells
- USP
U.S. Pharmacopeia
- NK cells
natural killer cells
- aAPC
artificial antigen presenting cell
- GMP
good manufacturing practices
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Moffitt Cancer Center has licensed Intellectual Property related to the proliferation and expansion of tumor infiltrating lymphocytes (TILs) to Iovance Biotherapeutics. SPT is an inventor on such Intellectual Property.
References
- 1.Couzin-Frankel J, Breakthrough of the year 2013. Cancer immunotherapy. Science, 2013. 342(6165): p. 1432–3. [DOI] [PubMed] [Google Scholar]
- 2.Phan GQ and Rosenberg SA, Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy. Cancer Control, 2013. 20(4): p. 289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, et al. , Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med, 1988. 319(25): p. 1676–80. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, et al. , Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res, 2011. 17(13): p. 4550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilon-Thomas S, et al. , Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother, 2012. 35(8): p. 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besser MJ, et al. , Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res, 2013. 19(17): p. 4792–800. [DOI] [PubMed] [Google Scholar]
- 7.Andersen R, et al. , Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin Cancer Res, 2016. 22(15): p. 3734–45. [DOI] [PubMed] [Google Scholar]
- 8.Wu R, et al. , Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J, 2012. 18(2): p. 160–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goff SL, et al. , Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol, 2016. 34(20): p. 2389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullinax JE, et al. , Combination of Ipilimumab and Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes for Patients with Metastatic Melanoma. Front Oncol, 2018. 8: p. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chacon JA, et al. , Triggering co-stimulation directly in melanoma tumor fragments drives CD8(+) tumor-infiltrating lymphocyte expansion with improved effector-memory properties. Oncoimmunology, 2015. 4(12): p. e1040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chacon JA, et al. , Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res, 2015. 21(3): p. 611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajgain P, et al. , Optimizing the production of suspension cells using the G-Rex “M” series. Mol Ther Methods Clin Dev, 2014. 1: p. 14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerges N, et al. A rapid and streamlined method for the culture of Tumor Infiltrating Lymphocytes (TIL) from melanoma tumor fragments. in Society for Immunotherapy of Cancer 2017. National Harbor, Maryland. [Google Scholar]
- 15.Wardell S, et al. A cryopreserved TIL product, LN-144, generated with an abbreviated method suitable for high throughput commercial manufacturing exhibits favorable quality attributes for adoptive cell transfer. in Society for Immunotherapy of Cancer 2017. National Harbor, Maryland. [Google Scholar]
- 16.Sarnaik A, et al. Novel cryopreserved tumor infiltrating lymphocytes (LN-144) administered to patients with metastatic melanoma demonstrates efficacy and tolerability in a multicenter Phase 2 clinical trial. . in Society for Immunotherapy of Cancer 2017. National Harbor, Maryland. [Google Scholar]


