Abstract
Background:
Infections and graft-versus-host disease have historically resulted in high mortality among children undergoing umbilical cord blood transplantation (UCBT). However, recent advances in clinical practice have likely improved outcomes of these patients.
Methods:
We conducted a retrospective cohort study of children (<18 years of age) undergoing UCBT at Duke University between January 1, 1995 and December 31, 2014. We compared two-year all-cause and cause-specific mortality during three time periods based on year of transplantation (1995–2001, 2002–2007, 2008–2014). We used multivariable Cox regression to identify demographic and UCBT characteristics that were associated with all-cause mortality, transplantation-related mortality, and death from invasive aspergillosis after adjustment for time period.
Results:
During the 20-year study period, 824 children underwent UCBT. Two-year all-cause mortality declined from 48% in 1995–2001 to 30% in 2008–2014 (P=0.0002). White patient race and non-malignant UCBT indications were associated with lower mortality. Black children tended to have a higher risk of death for which graft-versus-host disease (18% vs 11%; P=0.06) or graft failure (9% vs 3%; P=0.01) were contributory than white children. Comparing 2008–2014 to 1995–2001, more than half (59%) of the reduced mortality was attributable to a reduction in infectious mortality, with 45% specifically related to reduced mortality from invasive aspergillosis. Antifungal prophylaxis with voriconazole was associated with lower mortality from invasive aspergillosis than low-dose amphotericin B lipid complex [hazard ratio (HR): 0.09; 95% confidence interval (CI): 0.01–0.76]. With the decline in mortality from invasive aspergillosis, adenovirus and cytomegalovirus have become the most frequent infectious causes of death in children after UCBT.
Conclusions:
Advances in clinical practice over the past 20 years improved survival of children after UCBT. Reduced mortality from infections, particularly invasive aspergillosis, accounted for the largest improvement in survival and was associated with use of voriconazole for antifungal prophylaxis.
Keywords: umbilical cord blood transplantation, children, survival, race, aspergillosis
Introduction
Allogeneic hematopoietic stem cell transplantation is used to treat a growing number of malignant and non-malignant conditions in children. When available, bone marrow from a human leukocyte antigen (HLA)-matched sibling is the preferred donor source for allogeneic stem cell transplantation. However, less than 40% of children requiring a stem cell transplant have a matched sibling donor.1 Banked unrelated donor umbilical cord blood has been utilized for the past 25 years as an alternative donor for patients lacking a matched sibling or matched unrelated adult donor.2 Historically, infections, graft-versus-host disease (GVHD), graft failure or delayed engraftment, and regimen-related toxicity resulted in high mortality following unrelated donor umbilical cord blood transplantation (UCBT).3–5 However, advances in donor selection and clinical care over the past several decades have likely improved outcomes of UCBT.6,7 Other advances included the introduction of several new antifungal medications, use of steroid-sparing GVHD prophylaxis, and access to defibrotide for the treatment of severe veno-occlusive disease (VOD).8
In this study, we examined mortality over time in the largest single-center cohort of pediatric UCBT recipients reported to date. As secondary objectives, we evaluated for temporal changes in cause-specific mortality and sought to identify changes in clinical practice that influenced survival of children after UCBT.
Methods
Study Population
We performed a retrospective cohort study of children less than 18 years of age undergoing their first UCBT through the Duke University Pediatric Blood and Marrow Transplant (PBMT) program between January 1, 1995 and December 31, 2014. The study protocol was approved by the Duke University Institutional Review Board.
Transplantation Practices
Throughout the study period, patients were cared for on a dedicated unit containing positive-pressure, high efficiency particulate air (HEPA)-filtered rooms. Cyclosporine in combination with corticosteroids was the most frequent GVHD prophylaxis regimen before 2006 while, during more recent years, the majority of children received cyclosporine plus mycophenolate mofetil. Low-dose heparin (100 units/kg/day administered as a continuous intravenous infusion) was used for VOD prophylaxis until 2013, after which prophylaxis with ursodiol became standard practice. Defibrotide was available for the treatment of severe VOD either through a clinical trial or a compassionate use program beginning in 1998. Throughout the study period, low-dose acyclovir (250 mg/m2/dose given intravenously every 12 hours) was administered to UCBT recipients with serological evidence of prior infection with herpes simplex viruses. For Pneumocystis jirovecii prophylaxis, children received trimethoprim-sulfamethoxazole starting at the time of hospital admission and continuing until two days before UCBT, followed by inhaled or intravenous pentamidine starting 30 days after transplantation. Prior to September 2003, antifungal prophylaxis was most frequently provided with low-dose amphotericin B lipid complex (0.2 mg/kg IV once daily). In September 2003, prophylaxis with voriconazole (4 mg/kg IV or PO twice daily) was implemented for most UBCT recipients; no loading dose was provided and plasma trough voriconazole levels were not routinely monitored. Antifungal prophylaxis was started on the day after the transplantation date and continued for 100 days after UCBT or while the patient remained on immunosuppressive prophylaxis or therapy for GVHD. Throughout the study period, routine antibacterial prophylaxis was not used.
Data Sources and Measures
Patient demographic data and transplant characteristics were obtained from the Duke Enterprise Data Unified Content Explorer (DEDUCE) research portal and a secure database maintained by the Duke University PBMT program.9 Causes of death were identified through review of the PBMT database and the electronic medical record, including autopsy reports (when available), provider notes, and the results of microbiological testing. We considered transplantation-related mortality to be death from any cause other than progressive or recurrent malignancy (primary disease). In order to accurately determine the contribution of infections to the mortality of children after UCBT, we chose to classify fatal infections and GVHD as the cause of death when patients were receiving corticosteroids or other treatment for GVHD in the one month preceding death from an infection. Invasive fungal diseases were classified as possible, probable, or proven according to definitions developed by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group.10 Only probable and proven invasive fungal diseases were included in these analyses. For consistency during the 20-year study period, allele-level typing for HLA-A, -B, and -DRB1 was performed to determine the degree of matching regardless of UCBT year.
Statistical Analysis
We categorized patients into three time periods based on year of UCBT (1995–2001, 2002–2007, 2008–2014). The probability of overall survival was estimated within these groups using the Kaplan-Meier method. We used χ2 or Fisher’s exact tests to evaluate for differences in patient and transplant characteristics across these time periods. We used a log-rank test to evaluate for a difference in overall survival by time period and a Gray’s test to assess for a change in transplantation-related mortality. Time at risk for analyses of overall survival was from the date of UCBT until death from any cause or censoring (e.g. survival two years after UCBT). In analyses for which transplantation-related mortality was the outcome of interest, patients with a malignant UCBT indication who died of primary disease were additionally censored. We examined temporal trends in cause-specific mortality using Cochran-Armitage tests. We used multivariable Cox regression to identify demographic and UCBT clinical practices that were associated with all-cause mortality, transplantation-related mortality, and death from invasive aspergillosis. In constructing these models, we first evaluated for associations between the outcome and the following variables: age, sex, race, UCBT graft type (single vs. double), UCBT indication (malignancy vs. non-malignancy), conditioning intensity (myeloablative vs. reduced-intensity or non-myeloablative), GVHD prophylaxis (regimen with corticosteroids vs. regimen without corticosteroids), and antifungal prophylaxis regimen (amphotericin B lipid complex vs. voriconazole). We used multivariable Cox regression to evaluate demographic and donor selection factors that were associated with all-cause mortality. This analysis excluded transplants that included multiple umbilical cord blood units or for which sex, race, or HLA data were missing from either the recipient or the donor. Final multivariable models included variables that were associated with the outcome in univariable analyses (P<0.20). Using the time-dependent covariate approach, we determined that the proportional odds assumption was violated for the models of all-cause and transplantation-related mortality. To account for this, we included time period and an interaction term between time period and days after UCBT in these models.11 Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC) and R version 3.3.1.
Results
Patient Characteristics
During the 20-year study period, 824 children (<18 years) underwent their first UCBT. The most frequent malignant indications for UCBT were acute lymphoblastic leukemia (n=182, 22%), acute myelogenous leukemia (n=140, 17%), and myelodysplastic syndrome (n=39, 5%). The most common non-malignant indications were genetic or metabolic disorders (n=245, 30%), non-malignant hematological disorders (n=64, 8%), and immunodeficiencies (n=93, 11%). Table 1 presents characteristics of the study population. We observed no significant differences in age, sex, or race by time period. In contrast, UCBT graft type (P<0.0001), UCBT indication (P<0.0001), conditioning intensity (P<0.0001), GVHD prophylaxis regimen (P<0.0001), and antifungal prophylaxis regimen (P<0.0001) differed by time period. The proportion of children undergoing UCBT for malignancies declined during the study period. In the most recent time period (2008–2014), the number of patients receiving reduced-intensity conditioning regimens rose substantially.
Table 1.
Characteristics of the study population
| Time Period | P | ||||||
|---|---|---|---|---|---|---|---|
| 1995–2001 (n=298) | 2002–2007 (n=323) | 2008–2014 (n=203) | |||||
| n | % | n | % | n | % | ||
| Age | 0.29 | ||||||
| <1 year | 47 | 16 | 43 | 13 | 29 | 14 | |
| 1 to 4 years | 100 | 34 | 138 | 43 | 72 | 35 | |
| 5 to 11 years | 97 | 33 | 98 | 30 | 71 | 35 | |
| ≥12 years | 54 | 18 | 44 | 14 | 31 | 15 | |
| Sex | 0.48 | ||||||
| Female | 121 | 41 | 116 | 36 | 79 | 39 | |
| Male | 177 | 59 | 207 | 64 | 124 | 61 | |
| Race | 0.13 | ||||||
| White | 219 | 73 | 230 | 71 | 134 | 66 | |
| Black | 44 | 15 | 51 | 16 | 29 | 14 | |
| Other1 | 35 | 12 | 42 | 13 | 40 | 20 | |
| UCBT graft type | <0.0001 | ||||||
| Single | 289 | 100 | 324 | 97 | 156 | 77 | |
| Double | 0 | 0 | 9 | 3 | 47 | 23 | |
| UCBT indication2 | <0.0001 | ||||||
| Genetic or inherited metabolic disorders | 50 | 17 | 117 | 36 | 78 | 39 | |
| Immunodeficiency | 35 | 12 | 28 | 9 | 30 | 15 | |
| Malignancy | 187 | 63 | 158 | 49 | 76 | 38 | |
| Non-malignant hematological disorder | 26 | 9 | 20 | 6 | 18 | 9 | |
| Conditioning intensity3 | <0.0001 | ||||||
| Myeloablative | 293 | 99 | 311 | 96 | 173 | 85 | |
| Reduced-intensity | 0 | 0 | 5 | 2 | 30 | 15 | |
| Non-myeloablative | 3 | 1 | 7 | 2 | 0 | 0 | |
| GVHD prophylaxis regimen4 | <0.0001 | ||||||
| Cyclosporine and mycophenolate | 0 | 0 | 113 | 35 | 137 | 67 | |
| Cyclosporine and corticosteroids | 283 | 96 | 198 | 61 | 36 | 18 | |
| Other | 12 | 4 | 12 | 4 | 30 | 15 | |
| Antifungal prophylaxis regimen5 | <0.0001 | ||||||
| Amphotericin B lipid complex | 275 | 98 | 117 | 37 | 0 | 0 | |
| Voriconazole | 4 | 1 | 190 | 60 | 196 | 97 | |
| Other | 3 | 1 | 11 | 3 | 6 | 3 | |
UCBT, umbilical cord blood transplantation; GVHD, graft-versus-host disease
Subjects of other racial minorities were Hispanic (n=54), Asian (n=37), Middle Eastern (n=25), and Native American (n=1)
One subject underwent UCBT for an autoimmune disorder and was excluded from these analyses
Conditioning intensity was missing for two subjects
Graft-versus-host disease prophylaxis regimen was missing for three subjects
Nineteen subjects had a preceding fungal infection and were excluded; three subjects were missing data
Patient Outcomes
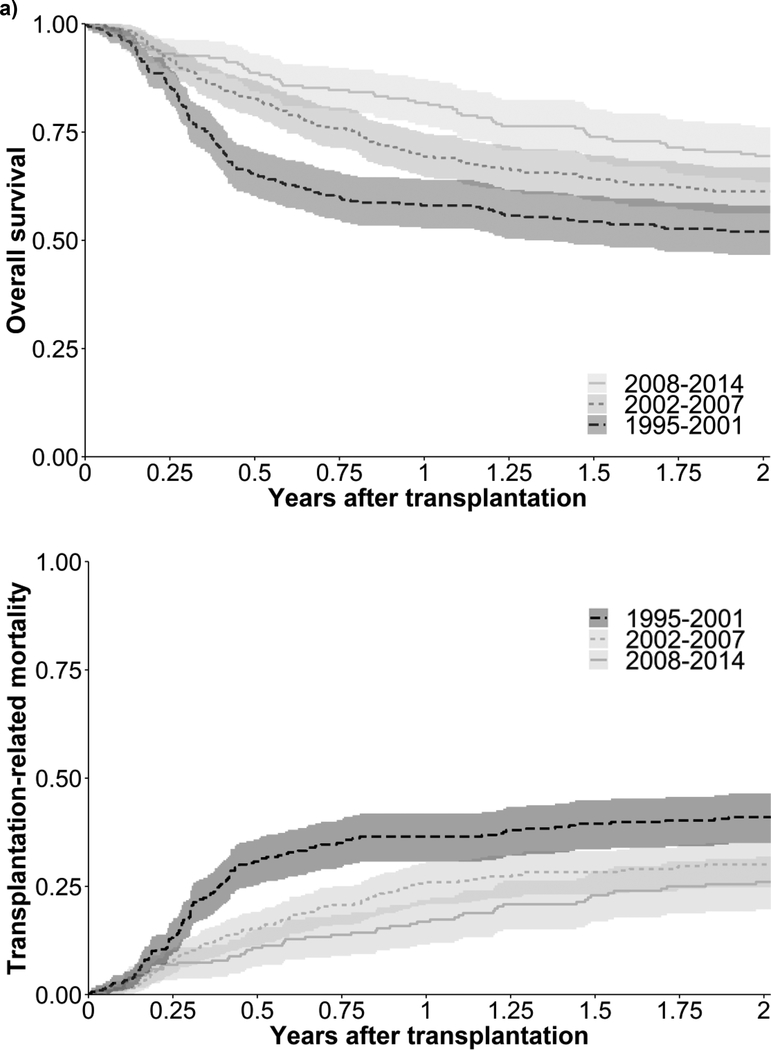
Three hundred sixty deaths (44%) occurred among the study population during the two years following UCBT. Overall survival after UCBT improved during the study period (P=0.0002; Figure 1a). Two-year all-cause mortality was 48%, 39%, and 30% in 1995–2001, 2002–2007, and 2008–2014, respectively. Two-year transplantation-related mortality was 41%, 30%, and 26% in 1995–2001, 2002–2007, and 2008–2014, respectively (P=0.001; Figure 1b). Mortality from infection without GVHD (P=0.02) and VOD (P=0.01) declined during the study period (Table 2). Comparing 2008–2014 to 1995–2001, more than half (59%) of the reduced mortality of UCBT recipients was attributable to a reduction in infectious mortality. Graft failure contributed to 31 (9%) deaths, and 43 (5%) children received a second UCBT because of graft failure.
Figure 1. Probability of a) overall survival and b) transplantation-related mortality after umbilical cord blood transplantation by year of transplantation.
The shaded areas correspond to 95% confidence intervals for the overall survival and transplantation-related mortality curves for a given time period. Overall survival (log-rank test; P=0.0002) and transplantation-related mortality (Gray’s test; P=0.001) differed by time period. Specifically, overall survival was lower in 1995–2001 than in either 2002–2007 (P=0.01) or 2008–2014 (P=0.0001). Similarly, transplantation-related mortality was higher in 1995–2001 compared with 2002–2007 (P=0.01) and 2008–2014 (P=0.002). Comparing 2002–2007 and 2008–2014, there were no significant differences in overall survival (P=0.06) and transplantation-related mortality (P=0.36).
Table 2.
Causes of death among the study population
| Time Period | P | ||||||
|---|---|---|---|---|---|---|---|
| 1995–2001 (n=298) | 2002–2007 (n=323) | 2008–2014 (n=203) | |||||
| n | % | n | % | n | % | ||
| GVHD | 3 | 1 | 2 | 1 | 3 | 1 | 0.67 |
| Hemorrhage | 10 | 3 | 8 | 2 | 4 | 2 | 0.33 |
| Infection with GVHD | 27 | 9 | 28 | 9 | 16 | 8 | 0.65 |
| Infection without GVHD | 55 | 18 | 45 | 14 | 22 | 11 | 0.02 |
| Organ failure | 9 | 3 | 12 | 4 | 5 | 2 | 0.80 |
| Primary disease | 26 | 8 | 33 | 10 | 15 | 7 | 0.70 |
| Veno-occlusive disease | 12 | 4 | 5 | 2 | 1 | <1 | 0.01 |
| Other | 8 | 3 | 5 | 2 | 6 | 3 | 0.95 |
GVHD, graft-versus-host disease
In multivariable analyses, race and UCBT indication were associated with two-year all-cause mortality (Table 3). The hazard of death was higher for children of black race [hazard ratio (HR): 1.61; 95% confidence interval (CI): 1.20–2.16] and other racial minority groups (HR: 1.80; 95% CI: 1.35–2.40) than for white race. Black children tended to have a higher risk of death for which graft-versus-host disease (18% vs 11%; P=0.06) or graft failure (9% vs 3%; P=0.01) were contributory compared with white children. Children from other racial minority groups were more likely to die of organ failure (9% vs 2%; P=0.001) and VOD (6% vs 1%; P=0.01) than white children. With regard to UCBT indication, the hazard of death was lower for non-malignant conditions (HR: 0.74; 95% CI: 0.57–0.97) than for malignancies. Supplemental Table 1 presents predictors of transplantation-related mortality in the study population. UCBT indication was not associated with transplantation-related mortality.
Table 3.
Multivariable analyses evaluating associations between demographic and UCBT practices and two-year all-cause mortality
| HR | (95% CI) | P | |
|---|---|---|---|
| Time Period | |||
| 1995–2001 | 1.00 | Ref | - |
| 2002–2007 | 0.46 | (0.30, 0.70) | 0.0004 |
| 2008–2014 | 0.27 | (0.15, 0.49) | <0.0001 |
| Age | |||
| <1 year | 1.00 | Ref | - |
| 1 to 4 years | 0.84 | (0.60, 1.19) | 0.32 |
| 5 to 11 years | 0.72 | (0.49, 1.06) | 0.09 |
| ≥12 years | 1.11 | (0.72, 1.71) | 0.63 |
| Race | |||
| White | 1.00 | Ref | - |
| Black | 1.61 | (1.20, 2.16) | 0.002 |
| Other | 1.80 | (1.35, 2.40) | <0.0001 |
| UCBT indication | |||
| Malignancy | 1.00 | Ref | - |
| Non-malignancy | 0.74 | (0.57, 0.97) | 0.03 |
| Conditioning intensity | |||
| Myeloablative | 1.00 | Ref | - |
| Reduced-intensity or non-myeloablative | 0.80 | (0.45, 1.43) | 0.43 |
| GVHD prophylaxis | |||
| Regimen with corticosteroids | 1.00 | Ref | - |
| Regimen without corticosteroids | 0.83 | (0.59, 1.17) | 0.29 |
| Antifungal prophylaxis | |||
| Amphotericin B lipid complex | 1.00 | Ref | - |
| Voriconazole | 1.24 | (0.84, 1.85) | 0.28 |
HR, hazard ratio; CI, confidence interval; Ref, reference group; UCBT, umbilical cord blood transplantation; GVHD, graft-versus-host disease
Sex and UCBT graft type were not associated with all-cause mortality in univariable analyses.
This multivariable model also adjusted for an interaction term between time period and days after UCBT.
White children were more likely to have a 5/6 or 6/6-matched donor than black children (56% vs. 27%; P<0.0001) or children from other racial minority groups (56% vs. 42%; P=0.01). White children were also more likely to receive a race-matched cord blood unit than black children (85% vs. 56%; P<0.0001) or children from other racial minorities (85% vs. 32%; P<0.0001). In univariable analyses, 3/6 and 4/6 HLA matching (HR: 1.47; 95% CI: 1.17–1.86) and race mismatching (HR: 1.41; 95% CI: 1.11–1.81) were associated with higher two-year all-cause mortality, while a cryopreserved total nucleated cell dose of ≥10.0 × 107/kg was associated with lower mortality than a total nucleated cell dose of <5.0 × 107/kg (HR: 0.69; 95% CI: 0.52–0.92). However, in multivariable analyses, we did not observe any associations between these donor selection factors and two-year all-cause mortality (Table 4).
Table 4.
Multivariable analyses of factors for demographic and donor selection characteristics and two-year all-cause mortality in 667 children who underwent single UCBT
| HR | (95% CI) | P | |
|---|---|---|---|
| Time Period | |||
| 1995–2001 | 1.00 | Ref | - |
| 2002–2007 | 0.51 | (0.35, 0.74) | 0.0003 |
| 2008–2014 | 0.38 | (0.21, 0.69) | 0.001 |
| Age | |||
| <1 year | 1.00 | Ref | - |
| 1 to 4 years | 0.90 | (0.61, 1.31) | 0.57 |
| 5 to 11 years | 0.72 | (0.45, 1.18) | 0.19 |
| ≥12 years | 0.99 | (0.56, 1.74) | 0.97 |
| Race | |||
| White | 1.00 | Ref | - |
| Black | 1.49 | (1.08, 2.07) | 0.02 |
| Other | 1.74 | (1.23, 2.45) | 0.002 |
| UCBT indication | |||
| Malignancy | 1.00 | Ref | - |
| Non-malignancy | 0.70 | (0.52, 0.92) | 0.01 |
| Cryopreserved TNC | |||
| <5.0 × 107 TNC/kg | 1.00 | Ref | - |
| 5.0–9.9 × 107 TNC/kg | 1.03 | (0.74, 1.42) | 0.88 |
| ≥10.0 × 107 TNC/kg | 0.89 | (0.59, 1.36) | 0.59 |
| HLA matching | |||
| 5/6 or 6/6 | 1.00 | Ref | - |
| 3/6 or 4/6 | 1.21 | (0.95, 1.56) | 0.13 |
| Race matchinga | |||
| Matched | 1.00 | Ref | - |
| Mismatched | 1.14 | (0.87, 1.49) | 0.35 |
HR, hazard ratio; CI, confidence interval; Ref, reference group; UCBT, umbilical cord blood transplantation; TNC, total nucleated cells; HLA, human leukocyte antigen
Racial categories considered for these analyses were white, black, Hispanic, Asian, Middle Eastern, and Native American.
Sex and sex matching were not associated with all-cause mortality in univariable analyses.
This multivariable model also adjusted for an interaction term between time period and days after UCBT.
Infectious Causes of Death
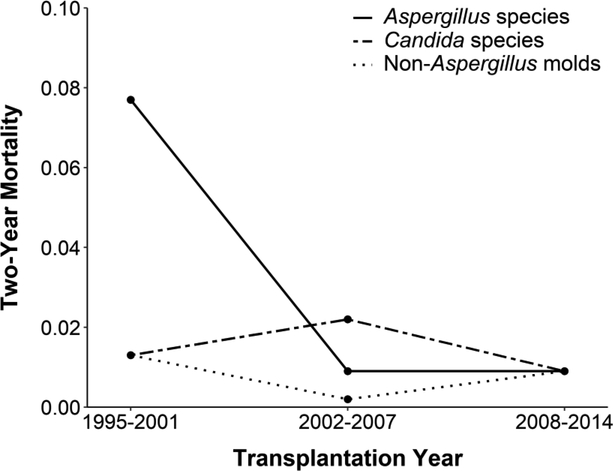
Table 5 shows infectious causes of death in the two years following UCBT. Fungi and viruses were the most frequent causes of infectious mortality. Most of the deaths from viral infections were from adenovirus (n=50), CMV (n=27), and parainfluenza viruses (n=14). Mortality from viral infections did not change during the study period (P=0.70). In contrast, fungal mortality declined during the study period (P=0.0001). Figure 2 shows mortality from invasive fungal infections by year of UCBT. Two-year mortality from invasive aspergillosis declined from 8% in 1995–2001 to <1% in 2002–2007 and 2008–2014 (P<0.0001). Reduced mortality from invasive aspergillosis accounted for 45% of the reduction in mortality of children after UCBT between 1995–2001 and 2008–2014. In contrast, the two-year mortality from Candida spp. and non-Aspergillus molds did not change over time (P=0.86 and P=0.56, respectively). In multivariable analyses (Table 6), antifungal prophylaxis with voriconazole was associated with lower mortality from invasive aspergillosis than prophylaxis with low-dose amphotericin B lipid complex (HR: 0.09; 95% CI: 0.01–0.76). This association between antifungal prophylaxis and death from invasive aspergillosis was independent of UCBT indication and GVHD prophylaxis regimen.
Table 5.
Infectious causes of death following UCBT by time period
| Time Period | P | ||||||
|---|---|---|---|---|---|---|---|
| 1995–2001 | 2002–2007 | 2008–2014 | |||||
| n | % | n | % | n | % | ||
| Bacteria | 18 | 6 | 15 | 5 | 9 | 4 | 0.39 |
| Gram-negative | 9 | 3 | 10 | 3 | 3 | 1 | |
| Gram-positive | 8 | 3 | 3 | 1 | 6 | 3 | |
| Polymicrobial | 1 | <1 | 2 | 1 | 0 | 0 | |
| Fungi | 32 | 11 | 11 | 3 | 6 | 3 | 0.0001 |
| Aspergillus spp. | 23 | 8 | 3 | 1 | 2 | 1 | |
| Candida spp. | 4 | 1 | 7 | 2 | 2 | 1 | |
| Non-Aspergillus molds | 4 | 1 | 1 | <1 | 2 | 1 | |
| Parasites | 3 | 1 | 1 | <1 | 1 | <1 | 0.41 |
| Virusesa | 29 | 10 | 43 | 13 | 21 | 10 | 0.70 |
| Adenovirus | 12 | 4 | 27 | 8 | 11 | 5 | |
| Cytomegalovirus | 7 | 2 | 12 | 4 | 8 | 4 | |
| Herpes simplex | 2 | 1 | 4 | 1 | 0 | 0 | |
| Parainfluenza | 5 | 2 | 6 | 2 | 3 | 1 | |
| Other | 4 | 1 | 0 | 0 | 5 | 2 | |
| Suspected infection, no organism identified | 10 | 3 | 7 | 2 | 7 | 3 | 0.95 |
| Pneumonia | 5 | 2 | 5 | 2 | 3 | 1 | |
| Sepsis | 5 | 2 | 2 | 1 | 4 | 2 | |
UCBT, umbilical cord blood transplantation
Number of deaths may not sum to the deaths from infection in Table 2 because multiple infections were identified for some subjects.
Deaths from specific viruses may not sum to category heading because some children had more than one virus identified as a primary cause of death
Figure 2. Two-year mortality from invasive fungal infections among children undergoing umbilical cord blood transplantation by year of transplantation.
Mortality from invasive aspergillosis declined during the study period (P<0.0001), while the mortalities from Candida species and non-Aspergillus molds did not differ by year of transplantation.
Table 6.
Multivariable analyses evaluating associations between demographic and UCBT characteristics and two-year mortality from invasive aspergillosis
| HR | (95% CI) | P | |
|---|---|---|---|
| Age | |||
| <12 years | 1.00 | Ref | - |
| ≥12 years | 4.88 | (2.09, 11.39) | 0.0003 |
| UCBT indication | |||
| Malignancy | 1.00 | Ref | - |
| Non-malignancy | 0.52 | (0.18, 1.49) | 0.22 |
| GVHD prophylaxis | |||
| Regimen with corticosteroids | 1.00 | Ref | - |
| Regimen without corticosteroids | 1.00 | (0.11, 9.46) | 0.99 |
| Antifungal prophylaxis | |||
| Amphotericin B lipid complex | 1.00 | Ref | - |
| Voriconazole | 0.08 | (0.01, 0.76) | 0.03 |
HR, hazard ratio; CI, confidence interval; Ref, reference group; UCBT, umbilical cord blood transplantation; GVHD, graft-versus-host disease
Sex, race, UCBT graft type, and conditioning intensity were not associated with mortality from invasive aspergillosis in univariable analyses.
Discussion
In this retrospective review of 824 children who underwent UCBT at a single center, overall survival improved substantially over the past 20 years. More than half of the improved survival was related to reduced mortality from infections, particularly invasive aspergillosis. Race was associated with lower mortality even after accounting for donor selection characteristics.
Several prior studies demonstrated improved survival over time after allogeneic hematopoietic stem cell transplantation. Gooley et al. reported that mortality among children and adults after allogeneic stem cell transplantation at the Fred Hutchinson Cancer Research Center fell 52% from 1993–1997 to 2003–2007.12 Similarly, five-year overall survival among children who underwent allogeneic hematopoietic stem cell transplantation at a single institution improved from 52% to 64% between 1983–1999 and 2000–2010.13 Finally, using data reported to the Center for International Blood and Marrow Transplant Research, Horan et al. found that the two-year overall survival of children after unrelated donor bone marrow transplantation improved from 35% in 1987–1995 to 58% in 2003–2006.14 These studies consisted mostly of adults, were limited to patients with hematological malignancies, included relatively few UCBT recipients, or did not include data from the past 10 years.12-16 In this study, we present detailed data on causes of death in the largest single-center cohort of pediatric UCBT recipients reported to date. While broadly consistent with the findings from these other studies, our results indicate that overall survival among children after UCBT improved substantially at our institution over the past two decades.
Mortality from invasive aspergillosis among children receiving UCBT fell markedly during the 20-year study period. Between 1995–2001 and 2008–2014, two-year mortality from aspergillosis declined from 8% to <1%. This reduction in mortality was strongly associated with use of voriconazole for antifungal prophylaxis in the study population. Importantly, we found that this effect of voriconazole prophylaxis on mortality from invasive aspergillosis was independent of UCBT indication and other major changes in clinical practice, including a shift away from use of steroids for GVHD prophylaxis. The efficacy of voriconazole for antifungal prophylaxis was previously reported in observational studies.17,18 However, voriconazole did not lower the incidence of invasive fungal infection during the first 180 days after allogeneic stem cell transplantation compared with fluconazole in a randomized controlled trial, which could be related to sub-therapeutic voriconazole dosing or an insufficient duration of follow-up in this trial.19 Our results suggest that voriconazole effectively prevents invasive aspergillosis mortality among children after UCBT.
With the decline in mortality from invasive aspergillosis, adenovirus and CMV have become the dominant infectious causes of death in children after UCBT. These two viruses accounted for 19 of 72 (26%) deaths occurring in the most recent time period (2008–2014). There has been little progress in the prevention and treatment of these viral infections over the past several decades. There is no Food and Drug Administration (FDA)-approved therapy for adenovirus infection. Cidofovir has been available for off-label use since 1996; however, adenovirus-related mortality remains high in allogeneic stem cell transplant recipients despite cidofovir, and this antiviral medication is associated with severe renal toxicity.20,21 The standard medications for the prevention and treatment of CMV disease in allogeneic stem cell transplant recipients are ganciclovir and foscarnet – antiviral medications that were available throughout the study period. However, neither of these medications have proved effective for CMV prevention after stem cell transplantation, and both are associated with substantial toxicity. Several novel therapeutics have the potential to lower mortality from adenovirus and CMV infections. Brincidofovir, a derivative of cidofovir without the associated renal toxicity, has potent activity against adenovirus, although further study is needed before it can be recommended routinely for these infections.22 Letermovir, a novel anti-CMV agent, was recently approved by the FDA for prevention of CMV infection and disease in adult HSCT patients, but data on dosing and efficacy are not yet available for children.23 Finally, virus-specific T lymphocytes have been used for the prevention and treatment of adenovirus and CMV infections in allogeneic stem cell transplant recipients, and evaluation of these products is ongoing.24
A key finding in this study is that black children and children from other racial minority groups had higher all-cause mortality than white children. Numerous prior studies described racial disparities in allogeneic HSCT.27–30 Multiple contributing factors have been proposed including a lower likelihood of finding an unrelated HLA-matched donor, lower socioeconomic status, provider bias, and structural factors within the health care system.31 We found that black children and children from other racial minorities were more likely to receive 3/6 or 4/6 HLA-matched and race-mismatched cord blood units than white children, and that receipt of these units was associated with higher mortality in univariable analyses. However, these associations did not persist in multivariable analyses adjusting for race and other patient characteristics. Two prior studies also did not find an association between donor race matching and mortality after hematopoietic stem cell transplantation.25,26 However, in these studies, race matching data was missing for a large portion (about 30%) of the analytic sample25 or only racial minorities only comprised of a small part of the analytic cohort (13%).26 Similarly, less than 30% of our sample included non-white children. To allow to a more detailed examination of the effect of race mismatching on mortality, future research should include a greater representation of racial minorities. These findings lend further support to ongoing initiatives to improve the representation of racial minority groups in umbilical cord blood registries.32
Our study has several limitations. First, it was conducted at a single academic hospital, the transplant practices of which may not be reflective of those at other transplant centers. Furthermore, the indications for UCBT changed over time at our center, with a declining proportion of transplants being performed for malignancy. This trend could have contributed to the improved survival of patients over time and could reflect both the increasing use of UCBT for non-malignant conditions and the growing number of centers performing UCBT.33 Moreover, although we reviewed autopsy reports, provider notes, and the results of microbiological testing for all deceased subjects, misclassification of the cause of death remains possible. In addition, analyses of the infectious causes of death did not account for changes in the diagnostic testing available for specific infections, particularly invasive fungal and viral infections. The availability of these assays in the later years of this study could have led to the improved diagnosis of fatal invasive fungal or viral infections or, alternatively, earlier diagnosis and improved survival from these infections.34–37 We did not have data on the incidence of invasive aspergillosis in this population and are thus unable to determine the extent to which the decline in mortality is attributable to prevention of Aspergillus infections rather than improved treatment outcomes. Although the dose of voriconazole used for antifungal prophylaxis was standardized, plasma trough voriconazole levels were not routinely followed. Given the substantial intraindividual variability in voriconazole metabolism, we are unable to more precisely determine associations between voriconazole exposures and invasive aspergillosis.38 Moreover, because the overwhelming majority of children in this cohort received amphotericin B lipid complex or voriconazole for antifungal prophylaxis, we are unable to compare the effectiveness of these medications to other antifungal agents. Finally, for consistency across the 20-year study period, we determined degree of HLA matching based only on allele-level typing of HLA-A, -B, and -DRB1. Typing of HLA-C and -DQB1 is currently being performed at our center and many other institutions, and it is unclear if the lack of association between HLA matching and mortality after cord blood transplantation would persist after accounting for matching at these additional HLA loci.
Clinical advances over the past 20 years have substantially improved survival of children after UCBT, further supporting its use as a stem cell source for children who do not have a matched sibling or unrelated donor. Routine use of voriconazole prophylaxis was associated with a dramatic reduction in mortality from invasive aspergillosis. However, there is still an urgent need for improved strategies for the prevention and treatment of adenovirus, CMV, and GVHD. Future research should focus on whether the development of new commercial molecular assays for adenovirus and CMV has led to improved survival in this high-risk patient population.
Supplementary Material
Acknowledgements
LPS was supported by a National Research Service Award Post-Doctoral Traineeship (5T32 HS000032-28) from the Agency for Healthcare Research and Quality, sponsored by the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. CS is a Stanford Child Health Research Institute Pete and Arline Harman Fellow, and received funding from the National Institutes of Health (T32-DK098132). ADS was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (5KL2 TR001115).
Footnotes
Disclosure of Conflicts of Interest
We declare no conflicts of interest.
References
- 1.D’Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2017. Available at: http://www.cibmtr.org. Accessed June 24, 2018.
- 2.Ballen KK, Gluckman E, Broxmeyer HE.Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman E, Rocha V, Boyer-Chammard A, et al. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337(6):373–381. [DOI] [PubMed] [Google Scholar]
- 4.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97(10):2962–2971. [DOI] [PubMed] [Google Scholar]
- 5.Hwang WY, Samuel M, Tan D, Koh LP, Lim W, Linn YC. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13(4):444–453. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri A, Eapen M, Scaravadou A, et al. Eurocord Registry; Center for International Blood and Marrow Transplant Research; New York Blood Center. Umbilical cord blood transplantation for children with thalassemia and sickle cell disease. Biol Blood Marrow Transplant. 2011;17(9):1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman E, Rocha V, Ionescu I, et al. Eurocord-Netcord and EBMT. Results of unrelated cord blood transplant in fanconi anemia patients: risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant. 2007;13(9):1073–1082. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Soiffer RJ, Antin JH, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16(7):1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44(2):266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46(12):1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradburn M, Clark T, Love S, Altman D. Survival analysis part III: multivariate data analysis – choosing a model and assessing its adequacy and fit. Br J Cancer. 2003;89(4):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brissot E, Rialland F, Cahu X, et al. Improvement of overall survival after allogeneic hematopoietic stem cell transplantation for children and adolescents: a three-decade experience of a single institution. Bone Marrow Transplant. 2016;51(2):267–72. [DOI] [PubMed] [Google Scholar]
- 14.Horan JT, Logan BR, Agovi-Johnson M-A, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29(7):805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn T, McCarthy TL Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013; 31(19): 2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMillan ML, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):16–22. [DOI] [PubMed] [Google Scholar]
- 17.Siwek GT, Pfaller MA, Polgreen PM, et al. Incidence of invasive aspergillosis among allogeneic hematopoietic stem cell transplant patients receiving voriconazole prophylaxis. Diagn Microbiol Infect Dis. 2006;55(3):209–212. [DOI] [PubMed] [Google Scholar]
- 18.Martin T, Sharma M, Damon L, et al. Voriconazole is safe and effective as prophylaxis for early and late fungal infections following allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2010;12(1):45–50. [DOI] [PubMed] [Google Scholar]
- 19.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Symeonidis N, Jakubowski A, Pierre‐Louis S, et al. Invasive adenoviral infections in T‐cell‐depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transpl Infect Dis. 2007;9(2):108–113. [DOI] [PubMed] [Google Scholar]
- 21.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31(6):481–486. [DOI] [PubMed] [Google Scholar]
- 22.Grimley MS, Chemaly RF, Englund JA, et al. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant. 2017;23(3):512–521. [DOI] [PubMed] [Google Scholar]
- 23.Chemaly RF, Ullmann AJ, Stoelben S, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370(19):1781–1789. [DOI] [PubMed] [Google Scholar]
- 24.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third party virus-specific T-cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballen KK, Klein JP, Pedersen TL, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18(6):903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ustun C, Bachanova V, Shanley R, et al. Importance of donor ethnicity/race matching in unrelated adult and cord blood allogeneic hematopoietic cell transplant. Leuk Lymphoma. 2014;55(2):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballen KK, Klein JP, Pedersen TL, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18(6):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16(11):1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the US registry. N Engl J Med. 2014;371(4):339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballen KK, Klein JP, Pedersen TL, Bhatla D, Duerst R, Kurtzberg J, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18(6):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majhail N, Nayyar S, Santibanez MB, Murphy E, Denzen E. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47(11):1385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Marrow Donor Program. Donor registry data. https://bloodcell.transplant.hrsa.gov/research/registry_donor_data/index.html. Accessed 22 August 2017.
- 33.D’Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017;23(9):1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after heamopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jha AK, Bansal D, Chakrabarti A, Shivaprakash M, Trehan A, Marwaha RK. Serum galactomannan assay for the diagnosis of invasive aspergillosis in children with haematological malignancies. Mycoses. 2013;56(4):442–448. [DOI] [PubMed] [Google Scholar]
- 36.Feghoul L, Chevret S, Cuinet A, et al. Adenovirus infection and disease in paediatric haematopoietic stem cell transplant patients: clues for antiviral pre-emptive treatment. Clin Microbiol Infect. 2015;21(7):701–709. [DOI] [PubMed] [Google Scholar]
- 37.Guitard J, Tabone M-D, Senghor Y, et al. Detection of β-D-glucan for the diagnosis of invasive fungal infection in children with hematological malignancy. J Infect. 2016;73(6):607–615. [DOI] [PubMed] [Google Scholar]
- 38. Gautier-Veyret, X Fonrose, Tonini J, et al. Variability of voriconazole plasma concentrations after allogeneic hematopoietic stem cell transplantation: impact of cytochrome p450 polymorphisms and comedications on initial and subsequent trough levels. Antimicrob Agents Chemother. 2015;59(4):2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.