Abstract
The dopaminergic motive system is compromised in cocaine addiction. Abundant research has examined the roles of the dopaminergic midbrain and ventral striatum (VS) in cue-induced craving and habitual drug consumption. Interconnected with the dopaminergic circuits, the hypothalamus is widely implicated in motivated behavior, including food and drug seeking. However, very few studies have investigated how the hypothalamus responds to drug cues and whether hypothalamic responses are related to clinical features such as craving and addiction severity. Here, in 23 cocaine-dependent individuals (CD) exposed to cocaine vs neutral cues during functional magnetic resonance imaging (fMRI), we examined regional responses using established routines. At a corrected threshold, CD demonstrated increased activation to cocaine vs neutral cues in bilateral visual cortex, inferior parietal and middle frontal gyri, and the hypothalamus. The extent of hypothalamus but not other regional response was correlated with craving and cocaine addiction severity, each as assessed by the Cocaine Craving Questionnaire (CCQ) and Cocaine Selective Severity Assessment (CSSA). In contrast, subjective “acute” craving as elicited by cocaine cues during fMRI involved deactivation of bilateral orbitofrontal cortex (OFC) and angular gyri (AG), and the OFC and AG responses were not related to CCQ or CSSA score. These findings distinguished tonic craving as a critical factor in capturing cocaine addiction severity and substantiated a role of the hypothalamus in motivational dysfunction in cocaine addiction.
Keywords: cocaine addiction, fMRI, hypothalamus
1 |. INTRODUCTION
Individuals with drug addiction are characterized by motivation deficits and underresponsiveness to natural reinforcers.1 The dopaminergic (DA) pathways process reinforcing stimuli and play a critical role in motivated behavior.2 Dopamine establishes the motivational value of extrinsic stimuli and links incentives to action through conditioning and instrumental learning.2 Repeated administration of psychostimulants alters DA signaling and motivational behaviors.3 Substantial research has focused on the role of the DA motive system in conditioned responses to drug cues.4,5 In humans, drug-conditioned cues trigger craving and, along with altered saliency attribution and impulse control, precipitate relapse to drug use.6–10
The hypothalamus also receives extensive projections from the DA midbrain and is implicated in functions from those essential to survival to cognitive and affective processes in support of goal-directed behavior.11,12 Previous animal studies have implicated the hypothalamus in drug addiction. For instance, reinstatement of cocaine seeking was associated with increased c-Fos expression in the hypothalamus.13 Compulsive cocaine use altered an array of dopamine gene expression, leading to functional reorganization of the hypothalamus.14 On the other hand, whereas many human imaging studies have elucidated ventral striatal (VS) responses to drug cues and craving, it remains unclear how the hypothalamus partakes in these processes.
Drug cues precipitate craving and drug seeking. Craving is a subjective experience of wanting to use a drug.15 Numerous studies have examined cerebral responses to cue-induced craving.16,17 However, craving measure may reflect tonic or phasic shifts in desire for drug,18 and extant research has not distinguished these processes. In the current study, we examined the neural correlates of cue-induced cocaine craving in cocaine-dependent individuals (CD). We posited that motivation dysfunction would manifest as higher hypothalamus activation to drug cues in CD and that cue-evoked hypothalamic activation would be associated with tonic craving, as evaluated by the Cocaine Craving Questionnaire (CCQ) over a period of 2 to 3 weeks of inpatient stay. In contrast, craving rating during cue exposures would reflect acute desire for the drug and engage neural processes not necessarily related to tonic craving or addiction severity.
2 |. MATERIALS AND METHODS
2.1 |. Subjects, informed consent, and assessment
Twenty-three recently abstinent, treatment-seeking individuals (17 men) with cocaine dependence (CD) participated in this study (Table 1). CD met criteria for current cocaine dependence, as diagnosed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.19 Recent cocaine use was confirmed by urine toxicology screens. They were drug free while staying in the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center prior to the current functional magnetic resonance imaging (fMRI) study. The CNRU was a locked inpatient unit where alcohol or drug (except coffee) use was strictly prohibited and (for smokers) all smoking breaks were supervised. Daily activities in the CNRU were highly regimented with 85% of day time occupied. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None reported having a history of head injury or neurological illness. Other exclusion criteria included dependence on another psychoactive substance (except nicotine) and current or past history of Axis I disorders. Individuals with current depressive or anxiety symptoms requiring treatment or currently being treated for these symptoms were excluded as well. The Human Investigation Committee at Yale University School of Medicine approved all study procedures, and all subjects signed an informed consent prior to study participation.
TABLE 1.
Demographics and clinical measures of the subjects
| Subject Characteristic | CD (n = 23) |
|---|---|
| Age (y) | 42.2 ± 7.6 |
| Gender (M/F) | 17/6 |
| Race (EA/AA/others) | 7/15/1 |
| Years of drinking | 20.3 ± 13.7 |
| Years of smoking | 14.2 ± 12.7 |
| TPQ HA score | 9.3 ± 6.5 |
| TPQ RD score | 4.9 ± 2.2 |
| TPQ NS score | 5.7 ± 2.9 |
| Averaged CCQ score | 41.0 ± 12.6 |
| CCQ score on the day of the MR scan | 41.2 ± 12.4 |
| CSSA score | 32.9 ± 15.8 |
| Amount of average monthly cocaine use (gm) in the prior year |
17.5 ± 15.5 |
| Amount per use in grams | 1.1 ± 0.7 |
| Days of cocaine use in the prior month | 15.4 ± 8.7 |
| Years of cocaine use | 16.0 ± 9.7 |
| Days between last cocaine use and fMRI | 12.1 ± 5.8 |
Abbreviations: AA, African American; CCQ, Cocaine Craving Questionnaire; CSSA, Cocaine Selective Severity Assessment; EA, European American; fMRI, functional magnetic resonance imaging; HA, harm avoidance; NS, novelty seeking; RD, reward dependence; TPQ, Tridimensional Personality Questionnaire. Values are mean ± SD.
CD participants were interviewed with the 18-item Cocaine Selective Severity Assessment (CSSA) Scale20 to evaluate cocaine withdrawal signs and symptoms. CSSA scores were highly correlated with recent cocaine use and with severity measures from the Addiction Severity Index (ASI) including the interviewer severity rating and composite score in the drug section. A previous study suggested that initial CSSA scores were higher for those who failed to achieve abstinence or who subsequently dropped out of treatment.20 Cocaine craving was assessed with the CCQ, Brief version (CCQ-Brief), for all CDs every 2 to 3 days.21 The CCQ-Brief is a 10-item questionnaire, abbreviated from the CCQ-Now22 and highly congruent with the CCQ-Now and other cocaine craving measures.21 Each item was rated on a scale from 1 to 7, with a higher total score (ranging from 10 to 70) indicating greater craving. We used CCQ score averaged across all assessments during the 2- to 3-week inpatient stay to index tonic level of craving. All participants were assessed with the Cloninger’sTridimensional Personality Questionnaire—Short Form (TPQ-Short)23 for personality traits that may be related to substance misuse. Derived from the 100-item long form of the TPQ,24 the TPQ-Short demonstrated reliability and validity.23 It consists of 44 yes/no questions covering novelty seeking (NS; 13 items), harm avoidance (HA; 22 items), and reward dependence (RD; 9 items). Each personality subscale score was calculated by summing the item scores, with reverse scoring where necessary. A higher subscore each represents a higher level of NS, HA, and RD. All these assessments were obtained on the admission day of the inpatient stay.
2.2 |. Behavioral tasks
We employed a cue-induced cocaine craving task (CCT). In a block design, participants were exposed to different cocaine/neutral pictures and reported cocaine craving on a visual analog scale. Briefly, a fixation cross appeared on the screen to engage attention at the beginning of each block. The task started with a 10-second instruction (“Look at the pictures and think about how you may relate to the scenes”). Each block contained a fixation period of 2 s, six pictures (with cocaine or neutral cues, in alternating blocks, Figure S1) each presented for 6 seconds, a 6-second window for craving rating. There were a total of 12 blocks. Thus, the total duration of a “run” was 10s + (2 s + 6 × 6 s + 6 s)× 12 = 538 s or approximately 9 minutes. Each subject participated in two runs, with the order of cocaine and neutral blocks counterbalanced across subjects. Participants were asked to look at the pictures and think about how they may relate to the scenes. At the end of each block, participants were asked to report how much they craved for cocaine on a visual analog scale from 0 (no craving) to 10 (highest craving ever) by pressing a button on the handheld box in the scanner within a 6-second window. Cocaine and neutral pictures were obtained from the Internet. Cocaine pictures included those displaying people preparing and snorting/smoking cocaine whereas neutral pictures comprising scenes of people performing various acts with similar color and complexity as inspected visually.
2.3 |. Imaging protocol
Brain images were collected using multiband imaging with a 3-Tesla Siemens Trio TIM system equipped with a 32-channel head coil. Conventional T1-weighted spin-echo sagittal anatomical images were acquired for slice localization. Anatomical 3D MP RAGE image was next obtained with spin-echo imaging in the axial plane parallel to the AC-PC line with TR = 1900 milliseconds, TE = 2.52 milliseconds, inversion time = 900 milliseconds, bandwidth = 170 Hz/pixel, field of view = 250 × 250 mm, matrix = 256 × 256, and 176 slices with slice thickness = 1 mm and no gap. Functional, blood oxygenation level- dependent (BOLD) signals were then acquired with a single-shot gradient-echo echo-planar imaging (EPI) sequence. Fifty-one axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 1000 milliseconds, TE = 30 milliseconds, band-width = 2290 Hz/pixel, flip angle = 62°, field of view = 210 × 210 mm, matrix = 84 × 84, 51 slices with slice thickness = 2.5 mm and no gap, and acceleration factor = 3. Images from the first 10 TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between radio frequency (RF) pulsing and relaxation.
2.4 |. Imaging data preprocessing
Data were analyzed with Statistical Parametric Mapping (SPM8). Standard image preprocessing was performed. Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were coregistered with the high-resolution structural image and then segmented for normalization with affine registration followed by non-linear transformation. The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. The images were normalized to Montreal Neurological Institute (MNI) space with voxel size of 3×3×3 mm3. Finally, the images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum.
2.5 |. Imaging data modeling
Data blocks were first distinguished of “cocaine picture” and “neutral picture.” A statistical analytical block design was constructed for each individual subject using general linear model (GLM). Because each block was associated with a craving rating, we included a column of block onset parametrically modulated by its corresponding craving score as a regressor in the model. Realignment parameters in all six dimensions were also entered in the model. Serial autocorrelation caused by aliased cardiovascular and respiratory effects was corrected by a first-degree autoregressive or AR(1) model. The GLM estimated the component of variance that could be explained by each of the regressors.
In the first-level analysis, we constructed for each individual subject statistical contrasts of “cocaine picture” versus “neutral picture.” These contrasts allowed us to evaluate brain regions that responded differently to viewing of cocaine pictures, as compared with viewing of neutral pictures, in CD. The contrast images of the first-level analysis were then used for the second-level group statistics. Following current reporting standards, all imaging results were evaluated with at voxel P < 0.05 corrected for familywise error (FWE) of multiple comparisons or at voxel P < 0.001, uncorrected, in combination with cluster P < 0.05, FWE corrected, on the basis of Gaussian random field theory as implemented in SPM.
In region of interest (ROI) analysis, we used MarsBaR (http:// marsbar.sourceforge.net/) to derive for each individual subject the effect size of activity difference for the ROIs. Functional ROIs were defined based on clusters as obtained from whole brain analysis. All voxel activations were presented in MNI coordinates.
3 |. RESULTS
3.1 |. Cue-induced craving
CD reported higher craving when viewing cocaine pictures as compared with viewing neutral pictures (P = 0.0006, t = 3.85, two-tailed paired sample t test; cocaine picture: mean ± SD = 4.2 ± 3.1, neutral picture: 1.6 ± 1.2). The differences (cocaine-neutral) in visual analog rating of craving did not correlate with either CCQ score (P = 0.82, r = −0.05, averaged across days; P = 0.59, r = −0.12, current CCQ) or CSSA score (P = 0.54, r = 0.13). The CSSA score was positively correlated with days of cocaine use in the prior month (P = 0.004, r = 0.58) and amount of average monthly cocaine use in the prior year (P = 0.01, r = 0.51).
3.2 |. Cue-induced brain activations
CD showed increased activations during viewing of cocaine vs neutral pictures in bilateral visual cortex, bilateral inferior parietal cortex (IPC), bilateral middle frontal gyri (MFG), and hypothalamus at voxel P < 0.05 corrected for FWE of multiple comparisons (Figure 1 and Table 2). No brain regions showed higher activation during viewing of neutral vs cocaine pictures.
FIGURE 1.
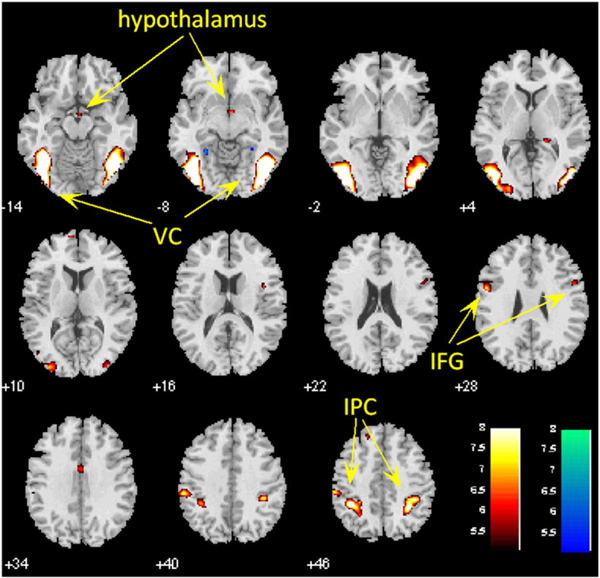
Brain regions showing activations during cocaine vs neutral cue exposure in CD at voxel P < 0.05, corrected for familywise error of multiple comparisons. Hot color represents clusters showing higher responses to cocaine vs neutral pictures. VC, visual cortex; IFG, inferior frontal gyrus; IPC, inferior parietal cortex
TABLE 2.
Cocaine vs neutral cue-induced brain activations; voxel P < 0.05 corrected for FWE of multiple comparisons
| MNI Coordinates, mm |
||||||
|---|---|---|---|---|---|---|
| Volume, mm3 | Peak Voxel (z) | x | y | z | Side | Identified Brain Region |
| Cocaine > neutral | ||||||
| 19 737 | 6.96 | −39 | −58 | −14 | L | Visual cortex |
| 18 036 | 6.80 | 42 | −82 | −8 | R | Visual cortex |
| 7452 | 6.32 | −30 | −61 | 55 | L | Inferior parietal cortex |
| 4914 | 6.26 | 33 | −46 | 49 | R | Inferior parietal cortex |
| 729 | 5.39 | −54 | 2 | 28 | L | Middle frontal gyrus |
| 540 | 5.14 | 51 | 8 | 25 | R | Middle frontal gyrus |
| 675 | 5.14 | 3 | −4 | −14 | L/R | Hypothalamus |
| Neutral > cocaine | ||||||
| None | ||||||
Abbreviations: FWE, familywise error; MNI, Montreal Neurological Institute. R, left; L, left.
3.3 |. Brain activations to acute cue craving rating
We performed whole brain linear regression analysis on contrast images of cocaine greater than neutral against differences (cocaine-neutral) in visual analog rating of craving. Bilateral lateral orbital frontal gyri and angular gyrus (AG) showed negative correlation with the differences in craving in CD at voxel P < 0.001 uncorrected and cluster-level P < 0.05, FWE corrected (Figure 2 and Table 3).
FIGURE 2.
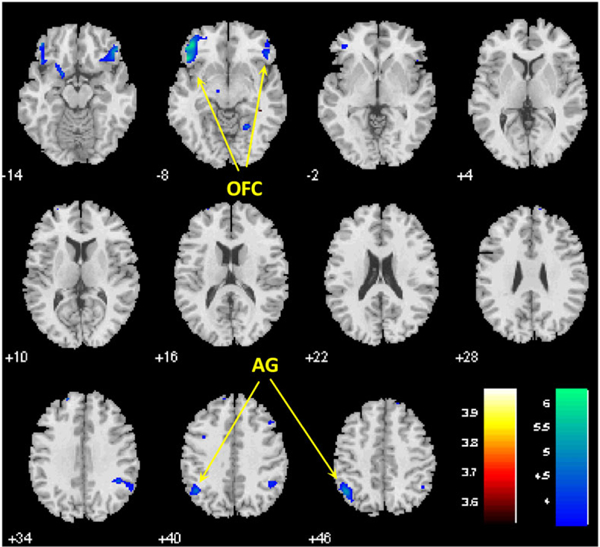
Bilateral orbital frontal cortex (OFC) and angular gyri (AG) showed negative correlation with the differences in visual analog ratings of craving during viewing of cocaine versus neutral pictures in CD; P < 0.001 uncorrected and cluster-level P < 0.05, FWE corrected
TABLE 3.
Brain activations to cocaine vs neutral cues in correlation with acute craving rating; P < 0.001 uncorrected and cluster‐level P < 0.05 corrected for FWE of multiple comparisons
| MNI Coordinates, mm |
||||||
|---|---|---|---|---|---|---|
| Volume, mm3 | Peak Voxel (z) | x | y | z | Side | Identified Brain Region |
| Positive correlation | ||||||
| None | ||||||
| Negative correlation | ||||||
| 3753 | 4.67 | −48 | 26 | −8 | L | Lateral orbital frontal gyrus |
| 2646 | 4.18 | 48 | 35 | −14 | R | Lateral orbital frontal gyrus |
| 2754 | 4.22 | −45 | −58 | 46 | L | Angular gyrus |
Abbreviations: FWE, familywise error; MNI, Montreal Neurological Institute. R, left; L, left.
There are more men than women in CD. Thus, in an additional analysis, we examined brain activations with gender as a covariate. The results were nearly identical (Figure S2). Further, because the duration between last cocaine use and fMRI scan varied across CDs, we examined brain activations with days of abstinence as a covariate. The results again showed that the brain activations were nearly identical with or without days of abstinence as a covariate (Figure S3).
3.4 |. Relationship to clinical characteristics
We examined whether regional responses to cocaine vs neutral cues were related to clinical characteristics with a linear regression of the effect size of each ROI against years of cocaine use, tonic craving (averaged CCQ score), current craving (CCQ score on the scan day), addiction severity (CSSA score), and TPQ NS, RD, and HA subscores. With the four ROIs and seven clinical measures, we evaluated the results at a corrected P = 0.05/(4 × 7) = 0.0018 and considered an arbitrary P = 0.01 as showing a trend toward significance.
Across CD, the hypothalamus activation was correlated positively with averaged (P = 0.0009, r = 0.64) and current (P = 0.0012, r = 0.63) CCQ score and with CSSA score (P = 0.0017, r = 0.62) (Figure 3). The hypothalamus activation was not correlated with years of cocaine use (P = 0.36, r = −0.2), NS (P = 0.76, r = 0.07), HA (P = 0.13, r = −0.33), or RD (P = 0.15, r = −0.31) subscore. Visual cortical activation was negatively correlated with the NS subscore (P = 0.006, r = −0.55) at a trend level, but not with other clinical measures (all P’s > 0.41). Inferior parietal and middle frontal cortical activation was not correlated with any clinical measures (all P’s > 0.02 and > 0.17, respectively).
FIGURE 3.
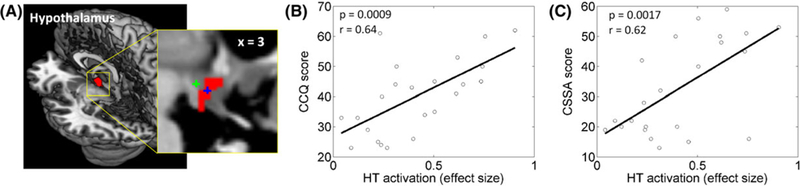
A, Hypothalamus (HT) cluster shown in a 3D structural image. The inset shows the cluster along with the center coordinates of medial (blue +) and lateral (green +) hypothalamus as characterized in a previous study.25 HT activation was positively correlated with B, Cocaine Craving Questionnaire (CCQ) score and with C, Cocaine Selective Severity Assessment (CSSA) score across CD subjects (P = 0.009, r = −0.53). None of the other correlations were significant (all P’s > 0.03).
We also explored potential relations between acute craving rating-related activations to cue exposure and the same set of clinical variables. Across CD, activations of bilateral lateral orbital frontal cortex were negatively correlated with RD subscore at a trend level
4 |. DISCUSSION
The current findings showed increased activation of the hypothalamus in recently abstinent cocaine-dependent individuals (CD) viewing cocaine versus neutral pictures at a stringent, corrected threshold. Importantly, the effect size of hypothalamic activation was significantly correlated with CCQ and CSSA scores, with greater hypothalamic activation reflecting stronger daily cocaine craving and addiction severity. These findings provided new evidence of DA circuit dysfunction in cocaine addiction. Higher hypothalamic response to cocaine cue is related to tonic level of craving and addiction severity.
The hypothalamus regulates arousal, food intake, sexual drive, reward, and affective responses.26,27 Numerous animal studies have implicated the hypothalamus in drug addiction, and the cellular and molecular mechanisms of feeding and drug addiction are interlinked in the hypothalamus.26 Cocaine administration induced activation of the hypothalamic-pituitary-adrenal axis28 and altered the levels of neurohypophyseal hormones oxytocin and vasopressin in the hypothalamus.29 In rodents, the transition from controlled to compulsive cocaine self-administration was associated with substantial remodeling of hypothalamic circuitry.14 Despite preclinical evidence implicating the hypothalamus in drug addiction, few human imaging studies have examined the role of the hypothalamus in the etiological processes of addiction. In an earlier work, increased hypothalamic activation was observed after intravenous injections of nicotine in current cigarette smokers.30 Another study reported decreased activation in a cluster encompassing the hypothalamus in CD as compared with healthy controls (HC) during viewing erotic vs neutral pictures.31 Hypothalamic response to monetary reward vs nonreward was increased in CD as compared with HC32 and was associated with the duration of abstinence in CD.33 The current findings add to this literature by specifying hypothalamic activation to cocaine cues and the relationship of hypothalamic cue response with tonic cocaine craving and addiction severity.
Increased activations were also observed in bilateral visual cortex, MFG, and IPC during viewing of cocaine vs neutral pictures. Although not typically a focus of the addiction literature, the visual cortex was often reported to be activated during exposure to drug cues.34–36 A recent meta-analysis showed that 86% of published functional imaging studies reported significant drug cue-induced activity in the visual cortex.35 Notably, treatment-seeking participants as well as participants with strong motivation to quit demonstrated decreased cocaine cue-induced visual cortical activation, as compared with non-treatment-seeking and less-motivated participants.37 The current finding of increased activation in bilateral visual cortex is consistent with these earlier studies. The MFG and IPC are part of the saliency circuit and cocaine-induced craving correlated positively with MFG activity during cocaine self-administration in non-treatment-seeking CD.38 On the other hand, visual, frontal, and parietal cortical response to cocaine cues did not correlate with tonic craving or addiction severity. Together, these findings distinguished a specific role of the hypothalamus in registering tonic cocaine craving and cocaine addiction severity and add to the literature in characterizing clinically relevant craving and addiction severity measures in cocaine dependence.39,40
Bilateral orbitofrontal cortex (OFC) and AG responded negatively to higher craving rating during exposure to cocaine than to neutral cues. Interconnected with mesolimbic brain regions, the OFC integrates reward values and sensory and interoceptive signals to guide motivated behavior.41,42 In particular, the lateral OFC is involved in inhibition of actions.43 Activation of the lateral OFC is associated with resisting highly tasty food in weight-concerned women.44,45 In rats, inactivation of the lateral OFC impaired behavioral reversals in a probabilistic learning task.46 Together, this body of work supports a role of the lateral OFC in goal-directed impulse control. Decreased lateral OFC activity in response to cue-elicited craving may reflect diminished control of the desire to use drugs. The AG is part of the default mode network, which deactivates in response to cognitive and affective challenges. AG response to drug cues may reflect disruption of internal mental states as a result of directed attention to environmental influences. Notably, lateral OFC and AG responses to cue exposures were not correlated with CCQ or CSSA score, suggesting that these regional activities were effects of acute craving and may not be related to addiction severity.
Examining brain activations to food and cocaine cues in 20 cocaine- dependent individuals (CD) using both fMRI and positron emission tomography (PET) imaging, a recent work reported that cocaine and food cues elicited similar circuit responses and that striatal dopamine D2/D3 receptor binding potentials modulated these responses.36 Specifically, the authors observed “deactivations” of the VS and hypothalamus to food and cocaine as compared with neutral cues, with less deactivation in the hypothalamus to cocaine as compared with food cues. The study highlighted hypothalamic responses to cue exposure; on the other hand, as acknowledged by the authors, the findings of decreased hypothalamus and VS activations were surprising and incontrast to a literature of enhanced DA circuit activation to drug cues.3,47 The authors suggested that the findings might reflect the use of videos rather than pictures in their cue reactivity paradigms. More research is needed to determine how video and picture cues may engage regional brain activations differently in cue craving studies.
In summary, we observed robust activation of the hypothalamus to cocaine cues in cocaine addicted individuals in spite of a relatively small sample size in the current study. Hypothalamic response to drug cues was correlated with daily cocaine craving, as assessed by the CCQ and cocaine addiction severity, as evaluated by the CSSA. These findings together substantiate a role of the hypothalamus in cue-induced tonic craving and addiction severity in cocaine dependence, and add to the literature of biomarkers of this chronic illness.48–50
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH grants DA040032, R21 DA044749 and DA023248 as well as the Peter McManus Charitable Trust and Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut. The funding agencies otherwise have no roles in the conceptualization of the study, data collection and analysis, or the decision to publish these results.
Funding information
Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut; National Institute on Drug Abuse, Grant/Award Number: DA023248, DA044749 DA040032; Peter McManus Charitable Trust
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
We declare no financial interests in the current work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Kopetz CE, Lejuez CW, Wiers RW, Kruglanski AW. Motivation and self-regulation in addiction: a call for convergence. Perspectives on Psychological Science: A Journal of the Association for Psychological Science. 2013;8(1):3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Wise RA, Baler R. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci. 2017;18(12):741–752. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol. 2013;48(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein RZ, Tomasi D, Rajaram S, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein RZ, Tomasi D, Alia-Klein N, et al. Dopaminergic response to drug words in cocaine addiction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(18):6001–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konova AB, Parvaz MA, Bernstein V, et al. Neural mechanisms of extinguishing drug and pleasant cue associations in human addiction: role of the VMPFC. Addict Biol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. [DOI] [PubMed] [Google Scholar]
- 10.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98(5):886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Wang W, Zhornitsky S, Li CR. Resting state functional connectivity of the lateral and medial hypothalamus in cocaine dependence: An exploratory study. Front Psychiatry. 2018;9:344 10.3389/fpsyt.2018.00344. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamlin AS, Clemens KJ, Mcnally GP. Renewal of extinguished cocaine- seeking. Neuroscience. 2008;151(3):659–670. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed SH, Lutjens R, van der Stap LD, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102(32):11533–11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96(1):33–46. [DOI] [PubMed] [Google Scholar]
- 16.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regier PS, Monge ZA, Franklin TR, et al. Emotional, physical and sexual abuse are associated with a heightened limbic response to cocaine cues. Addict Biol. 2017;22(6):1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiffany ST, Warthen MW, Goedeker KC. The functional significance of craving in nicotine dependence In: Bevins R, Caggiula A, eds. Nebraska Symposium on Motivation: The Motivational Impact of Nicotine and its Role in Tobacco Use. Lincoln, NE: The University of Nebraska Press; 2008:171–197. [DOI] [PubMed] [Google Scholar]
- 19.First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV (SCID). Washington DC: American Psychiatric Association; 1995. [Google Scholar]
- 20.Kampman KM, Volpicelli JR, McGinnis DE, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23(4):449–461. [DOI] [PubMed] [Google Scholar]
- 21.Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83(3):233–237. [DOI] [PubMed] [Google Scholar]
- 22.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. [DOI] [PubMed] [Google Scholar]
- 23.Sher KJ, Wood MD, Crews TM, Vandiver PA. The Tridimensional Personality Questionnaire—reliability and validity studies and derivation of a short form. Psychol Assessment. 1995;7(2):195–208. [Google Scholar]
- 24.Cloninger CR. A systematic method for clinical description and classification of personality variants: a proposal. Arch Gen Psychiatry. 1987;44(6):573–588. [DOI] [PubMed] [Google Scholar]
- 25.Kullmann S, Heni M, Linder K, et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp. 2014;35(12):6088–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73(6):759–768. [DOI] [PubMed] [Google Scholar]
- 27.Poeppl TB, Langguth B, Rupprecht R, et al. The neural basis of sex differences in sexual behavior: a quantitative meta-analysis. Front Neuroendocrinol. 2016;43:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manetti L, Cavagnini F, Martino E, Ambrogio A. Effects of cocaine on the hypothalamic-pituitary-adrenal axis. J Endocrinol Invest. 2014;37(8):701–708. [DOI] [PubMed] [Google Scholar]
- 29.Sarnyai Z, Vecsernyes M, Laczi F, Biro E, Szabo G, Kovacs GL. Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides. 1992;23(1):27–31. [DOI] [PubMed] [Google Scholar]
- 30.Stein EA, Pankiewicz J, Harsch HH, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155(8):1009–1015. [DOI] [PubMed] [Google Scholar]
- 31.Asensio S, Romero MJ, Palau C, et al. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI study. Addict Biol. 2010;15(4):504–516. [DOI] [PubMed] [Google Scholar]
- 32.Vaquero L, Camara E, Sampedro F, et al. Cocaine addiction is associated with abnormal prefrontal function, increased striatal connectivity and sensitivity to monetary incentives, and decreased connectivity outside the human reward circuit. Addict Biol. 2017;22(3):844–856. [DOI] [PubMed] [Google Scholar]
- 33.Bustamante JC, Barros-Loscertales A, Costumero V, et al. Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict Biol. 2014;19(5):885–894. [DOI] [PubMed] [Google Scholar]
- 34.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70(8):785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend. 2014;143:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum Brain Mapp. 2015;36(1):120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict Biol. 2014;19(2):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risinger RC, Salmeron BJ, Ross TJ, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26(4):1097–1108. [DOI] [PubMed] [Google Scholar]
- 39.Moeller SJ, Konova AB, Goldstein RZ. Multiple ambiguities in the measurement of drug craving. Addiction. 2015;110(2):205–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiat. 2016;73(11):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15(3):358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stalnaker TA. A new perspective on the role of orbitofrontal cortex in decision-making, judgment and adaptive behavior. Alcohol Clin Exp Res. 2009;33:302a–302a. [Google Scholar]
- 43.Deng WL, Rolls ET, Ji XX, et al. Separate neural systems for behavioral change and for emotional responses to failure during behavioral inhibition. Hum Brain Mapp. 2017;38:3527–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. Activation in inhibitory brain regions during food choice correlates with temptation strength and self-regulatory success in weight-concerned women. Front Neurosci. 2014;8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollmann M, Hellrung L, Pleger B, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond). 2012;36(5):648–655. [DOI] [PubMed] [Google Scholar]
- 46.Dalton GL, Wang NY, Phillips AG, Floresco SB. Multifaceted contributions by different regions of the orbitofrontal and medial prefrontal cortex to probabilistic reversal learning. J Neurosci. 2016;36(6):1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91(5):486–498. [DOI] [PubMed] [Google Scholar]
- 48.Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: Duration of use and gender difference. Drug Alcohol Depend. 2014;134:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Zhang S, Ide JS, et al. Dynamic network dysfunction in cocaine dependence: Graph theoretical metrics and stop signal reaction time. Neuroimage Clin. 2018;18:793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S, Li CR. Ventral striatal dysfunction in cocaine dependence - difference mapping for subregional resting state functional connectivity. Transl Psychiatry. 2018;8(1):119 10.1038/s41398-018-0164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


