Abstract
Background:
Recent experimental evidence suggests that nutritional supplementation can blunt adverse cardiopulmonary effects induced by acute air pollution exposure. However, whether usual individual dietary patterns can modify the association between long-term air pollution exposure and health outcomes have not been previously investigated. We assessed, in a large cohort with detailed diet information at the individual level, whether a Mediterranean diet modifies the association between long-term exposure to ambient air pollution and cardiovascular disease mortality risk.
Methods:
The NIH-AARP Diet and Health Study, a prospective cohort (N=548,845) across 6 states and 2 cities in the United States and with a follow-up period of 17 years (1995-2011), was linked to estimates of annual average exposures to PM2.5 and NO2 air pollution at the residential census-tract level. The alternative Mediterranean Diet Index (aMED), which uses a 9-point scale to assess conformity with a Mediterranean-style diet, was constructed for each participant from information in cohort baseline dietary questionnaires. We evaluated mortality risks for cardiovascular disease (CVD), ischemic heart disease (IHD), cerebrovascular disease (CER), or cardiac arrest (CAR) associated with long-term air pollution exposure. Effect modification of the associations between exposure and the mortality outcomes by aMED was examined via interaction terms.
Results:
For PM2.5, we observed elevated and significant associations with CVD (HR=1.13; 95% CI: 1.08-1.18), IHD (HR=1.16; 95% CI: 1.10-1.23), and CER (HR=1.15; 95% CI: 1.03-1.28). For NO2, we found significant associations with CVD (HR=1.06; 95% CI: 1.04-1.08), and IHD (HR=1.08; 95% CI: 1.05-1.11). Analyses indicated that Mediterranean diet modified these relationships, as those with a higher aMED score had significantly lower rates of air pollution-related mortality (p interaction<0.05).
Conclusions:
Mediterranean diet reduced cardiovascular disease mortality risk related to long-term exposure to air pollutants in a large prospective U.S cohort. Increased consumption of foods rich in antioxidant compounds may aid in reducing the considerable disease burden associated with ambient air pollution.
Keywords: air pollution, diet, mortality
Introduction
Cardiovascular diseases are the most important threat for population health in the 21st century.1 Ambient air pollution is a major contributor to cardiovascular disease-related mortality globally,2 with recent analysis estimating that more than 1.5 million ischemic heart disease deaths annually are attributable to ambient PM2.5 exposure.3 Both the American Heart Association and European Society of Cardiology have formally recognized ambient air pollution as a major cardiovascular risk factor.4
The overall global disease burden of ambient air pollution has been increasing over the past 25 years due to aging populations, increasing prevalence of chronic diseases, and rising air pollution levels in developing nations.3 Despite concerted abatement efforts by government and regulatory agencies across the globe, many locations continue to suffer from major pollution episodes and elevated concentration levels, with 90% of the world’s population estimated to currently live in places where air quality levels exceed World Health Organization (WHO) guidelines.5 To ameliorate the disease and economic burden imposed by ambient air pollution exposure, policy approaches complementary to air quality improvements and emissions controls may be needed.
As air pollution exerts local and systemic responses through inflammatory and oxidative stress pathways,6 dietary antioxidants could interfere with the mechanisms underlying exposure-related health effects.7 Recent results from several short-term experimental studies have demonstrated the potential of supplementation with specific foods and nutrients to blunt adverse health effects induced by acute air pollution exposure.8-10 However, whether usual long-term healthy dietary pattern can modify the association between long-term air pollution exposure and health outcomes is unclear.
A Mediterranean diet emphasizes consumption of plant-based foods, olive oil, and moderate intake of alcohol, providing a diet highly enriched in antioxidants and anti-inflammatory compounds. In this study, we investigated whether Mediterranean diet reduces the cardiovascular disease mortality risk associated with long-term exposures to fine particulate matter (PM2.5) and nitrogen dioxide (NO2). Research efforts aimed at evaluating diet as a potential effect modifier could provide insight about cardio-protective mechanisms for air pollution risk reduction.
Methods
Study Population
Detailed cohort study and participant information have been previously presented.11 Briefly, the NIH-AARP Study was initiated when members of the AARP, 50–71 years of age from six U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia; Detroit, Michigan), responded to a mailed questionnaire in 1995. The NIH-AARP cohort questionnaires elicited information on demographic and anthropometric characteristics, dietary intake, and numerous health-related factors at enrollment. Contextual environment characteristics for the census tract of each of this cohort’s participants have also been compiled, allowing us to also incorporate socioeconomic variables at the census-tract level. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. All participants provided written informed consent before completing the study. This study was approved by the Institutional Review Boards (IRBs) of the National Cancer Institute and New York University School of Medicine.
Cohort Follow-up and Mortality Ascertainment
Person-years of follow-up were considered for each participant from enrollment to the date of death, the end of follow-up (31 December 2011), or the date a participant moved out of the study state or city where s/he lived at enrollment, whichever occurred first. Vital status was ascertained through a periodic linkage of the cohort to the Social Security Administration Death Master File and follow-up searches of the National Death Index Plus for participants who matched to the Social Security Administration Death Master, cancer registry linkage, questionnaire responses, and responses to other mailings. We used the International Classification of Diseases, 9th Revision (ICD-9) and the International Statistical Classification of Diseases, 10th Revision (ICD-10) to define underlying mortality. Among 566,398 participants enrolled in the NIH-AARP cohort and available for analysis, after excluding those who responded via a proxy (N=15,760), exited the study on the study entry date (N=49), and those with missing PM2.5 (N=737) or NO2 (N=1,793) exposure data, the analytic cohort for this work includes 548,845 participants (96.9% of total cohort).
Exposure Assessment
PM2.5 exposure estimates at the residential census tract centroids were obtained from a published spatio-temporal prediction model12 for the continental U.S, available for 1980-2010. In brief, geographic predictors and annual average PM2.5 data from 1999 through 2010 on a 25-km grid were derived from the U.S. Environmental Protection Agency (EPA) Federal Reference Method (FRM) network and the Interagency Monitoring of Protected Visual Environments (IMPROVE) network. Temporal trends before 1999 were estimated using a) extrapolation based on PM2.5 data in FRM/IMPROVE, b) PM2.5 sulfate data in the Clean Air Status and Trends Network, and c) visibility data across the Weather-Bureau-Army-Navy network. The modeling approach was validated using PM2.5 data collected before 1999 from IMPROVE, California Air Resources Board dichotomous sampler monitoring (CARB dichot), the Children’s Health Study (CHS), and the Inhalable Particulate Network (IPN). In the validation using pre-1999 data, the prediction model performed well across three trend estimation approaches when validated using IMPROVE and CHS data (R2 = 0.84–0.91).
The annual NO2 estimates at the census tract centroids was derived from a recent model13 available for years 1990-2012, which applied kriging models combining land use regression methods with satellite data to improve model performance on a 25-km grid. The satellite data consist of total tropospheric NO2 measured via satellite images from the Ozone Monitoring Instrument on the Aurora satellite, while the estimation regression covariates were dimension-reduced components of 418 geographic variables. In the kriging models, conventional cross-validated R2 averaged over all years was 0.85 for the satellite data models and 0.84 for the models without satellite data. Average spatially clustered cross-validated R2 was 0.74 for the satellite data models and 0.64 for the models without satellite data.
Dietary Intake Assessment
At study enrollment, cohort participants completed the AARP 124-item FFQ (AARP-FFQ), an early version of the Diet History Questionnaire, to assess dietary intake over the past year. The Diet History Questionnaire has been previously calibrated, and further validation was performed by using two 24-h recalls within a subset of the NIH-AARP Diet and Health Study.14 To create components for all of the scores, guidance-based food group equivalents and nutrient variables from the AARP-FFQ were utilized. The MyPyramid Equivalents Database (MPED), version 1.0, was merged with the AARP-FFQ data to derive guidance-based food group equivalents for major food groups,14 and nutrient estimates for fatty acids by using the USDA Survey Nutrient Database associated with the Continuing Survey for Food Intake by Individuals 1994–96 and the Nutrition Data System for Research.
We employed the alternative Mediterranean diet (aMED) score to assess dietary patterns. The index includes 9 components (total vegetables excluding potatoes, total fruit, nuts, legumes, fish, whole grains, MUFA to SFA ratio, alcohol, and red and processed meat) and takes into account scientific literature on effect of diet on chronic disease risk.15 The aMED is adapted for use in an American population, and scores 9 components for a total of 9 points: 1 point is scored for intake at or greater than the sex-specific median for whole grains, vegetables (excluding potatoes), fruit, nuts, legumes, fish, and FA ratio (MUFA: SFA); and 1 point is given for intakes less than the sex-specific median for red and processed meat. Alcohol was based on predetermined cutoffs (1 point is scored for within 10-25 g/day for men and 5-15 g/day for women).
Statistical Methods
We employed the extended Cox proportional hazards models to estimate hazard ratios (HRs) of mortality in relation to ambient air pollution levels (per 10 μg/m3 for PM2.5; per 10 ppb for NO2), assigning long-term exposure for the air pollutants as time-varying covariates with 1-year lagged annual average concentration levels from 1994 to 2010. Fully-adjusted multivariable models included the following covariates: age (grouped into 3-year categories), sex, region (6 U.S. states and two cities) as strata; race or ethnic group (Non-Hispanic White; Non-Hispanic Black; Hispanic; Asian, Pacific Islander, or American Indian/Alaskan Native; unknown); level of education (less than high school, some high school, high school completed, post-high school or some college, college and post graduate, unknown); marital status (married, never-married, other, unknown); body-mass index (BMI) (<18.5 kg/m2, 18.5-<25.0, 25.0-<30.0, 30-<35, 35+, unknown); alcohol (none, <1, 1-<2, 2-<3, 3-<5 and 5+ drinks per day); smoking status (never smoker, former smoker of <= 1 pack/d, former smoker of >1 pack/d, current smoker of <= 1 pack/d, current smoker of >1 pack/d, unknown), in addition to two contextual characteristics (median census tract household income and percent of census tract population with less than a high school education, based on the 2000 decennial census for the residence at study entry).
We first conducted analyses to test the associations between the air pollutants and cause-specific mortality outcomes. We then assessed potential effect modification by including multiplicative interaction terms between the pollutant and the alternative Mediterranean Diet index in the models. Likelihood ratio statistic p-values (two-sided), comparing model fit with and without interaction terms, were used to test the statistical significance of each interaction. In addition, we also examined interactions by each individual dietary component of the aMED index.
As a sensitivity analysis, we additionally adjusted for pre-existing diseases (heart disease, stroke history, and diabetes) and evaluated random effects at the metropolitan statistical area (MSA) level. Given the observable differences across aMED quintile for education and BMI levels, we also tested for their potentially confounding effects by stratifying the cohort by these covariates and assessing if the effect modification by aMED remained present in the stratified results. In addition, we compared the results after stratifying the population according to population density to test for potential urban/rural differences, and we also performed penalized ridge analyses to simulataneously adjust for both PM2.5 and NO2 in the same model.
To validate our results, we tested whether the Cox proportional hazards assumption was satisfied through examination of the Schoenfeld plots, and we evaluated the degree to which unmeasured confounding potentially influence our results by calculating the E-values.16 We also assessed whether the linearity assumption of the exposure-response relationship is appropriate by calculating and comparing the Bayesian Information Criterion (BIC) values for natural spline models with different degrees of freedom.
Packages “survival” and “coxme” in R (version 3.4.4) were employed for analysis.
Results
During the follow-up period considered in this study (1995 through 2011), 126,817 (23.1%) of cohort participants died, of which: 39,532 were due to all cardiovascular diseases (CVD; ICD-9: 390-459, ICD-10: I00-I99); 22,329 were due to ischemic heart disease (IHD; ICD-9: 410-414, ICD-10: I20-I25); 5,592 were from cerebrovascular diseases (CER; ICD-9: 430-438, ICD-10: I60-I69); and 6,811 were from dysrhythmias, heart failures, and cardiac arrests (CAR; ICD-9: 420-429, ICD-10: I30-I51). The cohort (N=548,845), with an average age of 62.2 (SD=5.4) at entry, is characterized as mostly white (91.2% of the cohort), male (59.1%), either never (34.8%) or former (49.4%) smokers, with college or post-college education level (38.3%), overweight (41.3%), and married (68.1%). Summary statistics are provided in Table 1; air pollution concentration levels and covariates were generally consistent across the aMED quintiles, except that those in higher quintiles tended to be never smokers and have higher levels of education. The overall average concentration for PM2.5 during the study period was 12.9 (ranging from 3.4 to 23.0) μg/m3, with a SD of 3.2 μg/m3; for NO2, the average was 13.3 (ranging from 2.0 to 37.7) ppb, with SD of 7.6 ppb. The concentration levels for both of the pollutants decreased substantially during the study period across all locations (Supplemental Figure 1); the overall annual average levels were 15.3 μg/m3 and 18.6 ppb in 1994, and 9.2 μg/m3 and 9.8 ppb in 2010, for PM2.5 and NO2, respectively. The R2 between PM2.5 and NO2 average annual concentrations was high (0.63), suggesting shared emission sources.
Table 1.
Descriptive characteristics of the NIH-AARP Diet and Health Study based on quintiles of alternative Mediterranean Diet Index (aMED) scores
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-val | |
|---|---|---|---|---|---|---|
| Range of Index Scores | 0-2 | 3 | 4 | 5 | 6-9 | |
| N | 102099 | 97566 | 112578 | 105761 | 130841 | |
| PM2.5 (SD) | 12.3 (2.8) | 12.3 (2.9) | 12.4 (2.9) | 12.4 (2.9) | 12.3 (3.0) | 0.88 |
| NO2 (SD) | 12.9 (6.5) | 13.0 (6.6) | 13.1 (6.6) | 13.2 (6.7) | 13.3 (6.7) | 0.88 |
| Entry Age (SD) | 61.5 (5.5) | 61.9 (5.4) | 62.2 (5.3) | 62.4 (5.3) | 62.7 (5.2) | 0.54 |
| Sex (%) | 0.95 | |||||
| Male | 61532 (60.3%) | 55776 (57.2%) | 64364 (57.2%) | 61307 (58%) | 81139 (62%) | |
| Female | 40567 (39.7%) | 41790 (42.8%) | 48214 (42.8%) | 44454 (42%) | 49702 (38%) | |
| Race (%) | 0.99 | |||||
| White | 93825 (91.9%) | 88662 (90.9%) | 101675 (90.3%) | 95904 (90.7%) | 120505 (92.1%) | |
| Black | 3964 (3.9%) | 4029 (4.1%) | 4876 (4.3%) | 4330 (4.1%) | 4383 (3.3%) | |
| Hispanic | 1695 (1.7%) | 1933 (2%) | 2307 (2%) | 2088 (2%) | 2174 (1.7%) | |
| Asian/Pacific Islander/Native American | 1176 (1.2%) | 1492 (1.5%) | 2009 (1.8%) | 1956 (1.8%) | 2246 (1.7%) | |
| Education (%) | 0.60 | |||||
| Less than high school | 8981 (8.8%) | 7112 (7.3%) | 7130 (6.3%) | 5356 (5.1%) | 4676 (3.6%) | |
| Some high school | 26079 (25.5%) | 21940 (22.5%) | 22704 (20.2%) | 18769 (17.7%) | 17885 (13.7%) | |
| 12 years or high school completed | 11425 (11.2%) | 10310 (10.6%) | 11265 (10%) | 9903 (9.4%) | 10845 (8.3%) | |
| Post-high school or some college | 23437 (23%) | 22787 (23.4%) | 26320 (23.4%) | 24706 (23.4%) | 29910 (22.9%) | |
| College and post graduate | 28845 (28.3%) | 32197 (33%) | 41568 (36.9%) | 43831 (41.4%) | 64304 (49.1%) | |
| BMI (%) | 0.98 | |||||
| <18.5 | 1045 (1%) | 811 (0.8%) | 890 (0.8%) | 839 (0.8%) | 1068 (0.8%) | |
| 18.5-25 | 30068 (29.4%) | 30182 (30.9%) | 36022 (32%) | 36347 (34.4%) | 51775 (39.6%) | |
| 25-30 | 41501 (40.6%) | 39890 (40.9%) | 46961 (41.7%) | 44343 (41.9%) | 54159 (41.4%) | |
| 30-35 | 18029 (17.7%) | 16463 (16.9%) | 18223 (16.2%) | 15701 (14.8%) | 15874 (12.1%) | |
| >35 | 8396 (8.2%) | 7344 (7.5%) | 7440 (6.6%) | 5775 (5.5%) | 4904 (3.7%) | |
| Marital (%) | 0.99 | |||||
| Married | 68928 (67.5%) | 65228 (66.9%) | 75627 (67.2%) | 71929 (68%) | 91864 (70.2%) | |
| Never Married | 27197 (26.6%) | 26565 (27.2%) | 30325 (26.9%) | 27810 (26.3%) | 32166 (24.6%) | |
| Other | 5065 (5%) | 4824 (4.9%) | 5564 (4.9%) | 5125 (4.8%) | 5995 (4.6%) | |
| Smoking (%) | 0.33 | |||||
| Never | 30107 (29.5%) | 32807 (33.6%) | 40077 (35.6%) | 38871 (36.8%) | 49177 (37.6%) | |
| former smoker of <=1 pack/day | 21436 (21%) | 23880 (24.5%) | 29804 (26.5%) | 30031 (28.4%) | 40750 (31.1%) | |
| former smoker of >1 pack/day | 21943 (21.5%) | 20071 (20.6%) | 23409 (20.8%) | 22222 (21.0%) | 27687 (21.2%) | |
| current smoker of <=1 pack/day | 13927 (13.6%) | 10729 (11%) | 10079 (9%) | 7570 (7.2%) | 6538 (5%) | |
| current smoker of >1 pack/day | 10669 (10.4%) | 6194 (6.3%) | 4789 (4.3%) | 3008 (2.8%) | 2108 (1.6%) |
Long-term exposures to the air pollutants were significantly associated with the cause-specific mortality outcomes evaluated (Table 2). For PM2.5, we observed elevated and statistically significant associations with CVD (HR=1.13; 95% CI: 1.08-1.18), IHD (HR=1.16; 95% CI: 1.10-1.23) and CER (HR=1.15; 95% CI: 1.03-1.28). For NO2, we found significant associations with CVD (HR=1.06; 95% CI: 1.04-1.08), and IHD (HR=1.08; 95% CI: 1.05-1.11). We did not observe significant associations between these air pollutants and cardiac arrest mortality. The aMED variable remained significantly and negatively associated with mortality risks in these models. Adjusting for pre-existing diseases and entering a random effects variable at the MSA-level did not significantly alter our findings. In the co-pollutant penalized ridge regression analysis, CVD and IHD mortality risks associated with both PM2.5 and NO2 remained significant, but not CER (Supplemental Table 1).
Table 2.
Cause-specific mortality hazard ratios with 95% confidence intervals in relation to air pollution concentrations (per 10 μg/m3 for PM2.5; per 10 ppb for NO2)
| Base Model | Base Model + Adjustment for pre-existing diseases |
Base Model + Adjustment for MSA-level random effects |
||||||
|---|---|---|---|---|---|---|---|---|
| PM2.5 | NO2 | PM2.5 | NO2 | PM2.5 | NO2 | |||
| Cause of Death | Deaths (n) |
ICD-9 & ICD-10 Codes |
HR (95% CI)* | HR (95% CI)* | HR (95% CI)* | HR (95% CI)* | HR (95% CI)* | HR (95% CI)* |
| Cardiovascular Disease | 39,532 | 390-459; I00-I99 | 1.13 (1.08-1.18) | 1.06 (1.04-1.08) | 1.13 (1.09-1.18) | 1.08 (1.05-1.09) | 1.11 (1.05-1.16) | 1.06 (1.04-1.09) |
| Ischemic Heart Disease | 22,329 | 410-414; I20-I25 | 1.16 (1.10-1.23) | 1.08 (1.05-1.11) | 1.17 (1.10-1.23) | 1.08 (1.05-1.11) | 1.17 (1.10-1.23) | 1.06 (1.03-1.09) |
| Cerebrovascular Disease | 5,592 | 430-438; I60-I69 | 1.15 (1.03-1.28) | 1.04 (0.99-1.09) | 1.16 (1.04-1.30) | 1.05 (0.99-1.10) | 1.16 (1.04-1.30) | 1.05 (0.99-1.10) |
| Cardiac Arrest | 6,811 | 420-429; I30-I51 | 0.98 (0.88-1.09) | 1.02 (0.97-1.07) | 1.00 (0.91-1.12) | 1.03 (0.98-1.08) | 1.04 (0.92-1.18) | 1.04 (0.98-1.10) |
Adjusted for sex and location as strata; and age (time-varying), race, BMI, education, smoking, diet (aMED), and marriage at individual-level; and median income and % with high school education at census-tract level.
MSA indicates metropolitan statistical level.
Estimates of the modification of the air pollution-mortality associations by aMED scores are shown in Figure 1 (and in Supplemental Table 2). We observed decreasing associations between PM2.5 and NO2 with both CVD (p-interaction<0.01) and IHD (p-interaction<0.01) mortality risks with higher aMED quintiles, with these associations becoming statistically non-significant in the highest aMED quintile. Association between PM2.5 with CER mortality (p-interaction<0.01) was also significantly reduced among participants with higher aMED scores.
Figure 1.
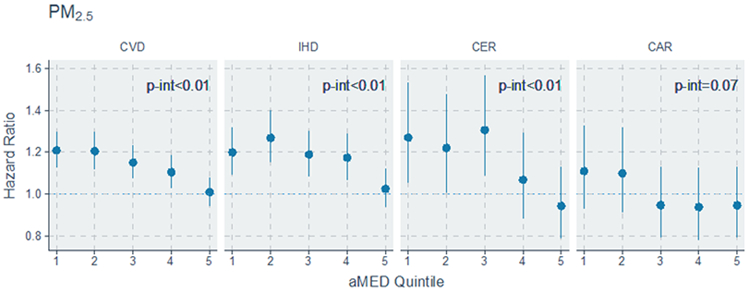
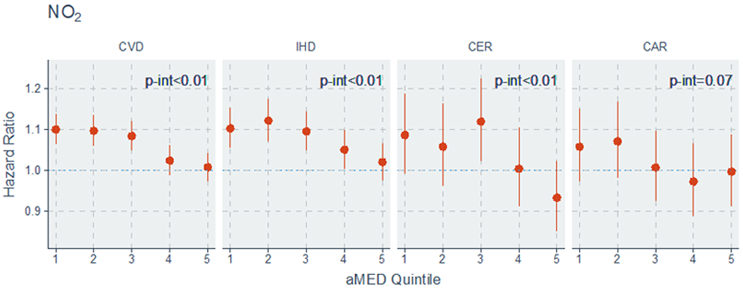
Hazard Ratio and 95% CI associated with air pollutants by quintiles of aMED (alternative Mediterranean Diet Index) score
CVD indicates cardiovascular disease; IHD, ischemic heart disease; CER, cerebrovascular disease; and CAR, cardiac arrests
We also evaluated effect modification by the individual dietary components of a Mediterranean diet (Supplemental Figure 2). The PM2.5-CVD, PM2.5-IHD, NO2-CVD and NO2-IHD mortality associations were significantly reduced in subjects who consumed higher amounts of vegetables, while the PM2.5-CVD and PM2.5-IHD mortality associations were significantly lower among those who consumed higher amounts of wholegrains. We also observed that NO2-IHD association was significantly reduced in subjects who consumed higher amounts of fruits, while the NO2-CVD association was significantly lower among those consuming foods with a higher MUFA:SFA ratio.
The effect modification by aMED of the associations between PM2.5 and NO2 with CVD mortality risk remained statistically significant across different groups of education level (Supplemental Table 3), while for IHD mortality the effect modification was only observed for those with education levels at high school level or less. We also found significant effect modification by aMED among those who are normal or overweight, but not among thoese who are obese; however, among the obese the air pollution mortality risks were reduced and generally not statistically significant. Across different smoking history groups, we found significant effect modification by aMED among those who are never or ever smokers but not among who are current smokers, although among this group the air pollution mortality risks were also not statistically significant. We also observed that the air pollution-associated mortality risks and modification by aMED were present among participants residing in locations with greater population density, where there were higher air pollution concentration levels (Supplemental Table 4).
Examination of the Schoenfeld plots revealed that the Cox proportal hazards assumption was satisfied (results not shown), and the E-values ranged from 1.51 to 1.57 for mortality outcomes associated with PM2.5 and 1.24 to 1.37 for mortality outcomes associated with NO2 (Supplemental Table 5), suggesting that the level of unmeasured confounding needed to explain away the effect estimates is likely to be large. Assessment of natural spline models with different degrees of freedom (df) suggested that assumption of linearity of the exposure-response relationship was appropriate, with df=1 having the lowest BIC.
Discussion
In this large, well-characterized prospective U.S cohort, long-term exposures to PM2.5 and NO2 were significantly associated with cardiovascular disease and ischemic heart disease mortality, and exposure to PM2.5 was also significantly associated with cerebrovascular disease mortality. Results showed that Mediterranean diet significantly modified these relationships, as those with a higher aMED score had significantly lower rates of CVD mortality adjusting for usual CVD contributors.
A Mediterranean diet has shown beneficial effects on cardiovascular health, improving blood pressure, endothelial function, and lipid profiles while reducing inflammatory responses and oxidative stress.17,18 These benefits have been consistently observed in meta-analyses, cohort studies, and randomized control trials.19-21 Previous investigations within the NIH-AARP cohort also reported reduction in all-cause and cause-specific mortality risk with conformity to a Mediterranean diet14,22. Attenuation of the cardiovascular disease mortality risk associated with air pollution exposure by dietary patterns, as observed here, is therefore consistent with past evidence of oxidative stress as underlying mechanism for air pollution-induced health effects, and suggest that a healthful dietary pattern enriched in antioxidant and anti-inflammatory compounds could interact with mechanisms underlying air pollution-induced health effects. For example, dietary antioxidants may scavenge the reactive oxygen species and free radicals generated from exposure to air pollution before they can activate pathways in pathogenesis of air pollution-induced cardiovascular health effects.23 Attenuation of air pollution-CVD and IHD mortality associations by unsaturated fats, fruits, vegetables, and wholegrains, as observed in our analysis supports this hypothesis.
Multiple intervention studies to date have demonstrated the beneficial capacity of dietary supplementation to mitigate adverse cardiopulmonary effects of air pollution exposure. In a randomized controlled trial in Mexico City, supplementation with fish oil reduced the negative impact of PM2.5 on HRV and biomarkers of response to oxidative stimuli among the elderly.24,25 Supplementation with vitamins C and E for six months decreased several biomarkers of oxidative stress among electric-power plant workers and provided protection against the oxidative insult associated with air pollutants derived from coal burning.26 In an experimental study of healthy middle-aged adults exposed to concentrated air pollution (CAP), dietary supplementation with olive oil attenuated CAP-induced impaired vascular endothelial function while altering blood markers associated with fibrinolysis and vasoconstriction.27 In a 12-week randomized intervention trial in Chinese adults, broccoli sprouts also facilitated the urinary excretion of mercapturic acids of air pollutants – the glutathione-derived conjugates of benzene and acrolein – suggesting that supplementation with broccoli sprouts enhanced detoxification of traffic-related pollutants.28 Broccoli sprout extracts also suppressed the nasal inflammatory response, measured by total white blood cell counts in nasal lavage fluid induced by diesel exhaust particles.29
Recent mechanistic studies have also identified several potential biological pathways by which diet could modulate the association between air pollution exposure and health responses. In a single-blind placebo-controlled crossover study of 10 adults, PM2.5 exposure induced methylation changes in genes involved in mitochondrial oxidative energy metabolism, while B vitamins supplementation prevented these changes.7 Increased blood levels of fish-based omega-3 fatty acids attenuated increases in fibrinogen associated with short-term increases in ambient PM among 135 patients.30 Vitamin E and omega-3 fatty acids mitigated PM2.5-induced inflammation and oxidative stress, decreasing inflammatory cytokines interleukin 6 (L-6) and tumor necrosis factor a (TNF-a) levels in human umbilical vein vascular endothelial cells.31 In animal studies, vitamin E and omega-3 fatty acids also protected against cardiac tissue injury,32 while blueberry anthocyanin-enriched extracts improved electrocardiogram abnormalities, decreased cytokine levels, and inhibited cardiomyocyte apoptosis in PM2.5-exposed rats.33
Although such past studies have sought to identify specific foods or nutrients capable of mitigating acute responses to air pollution exposures, whether a healthful dietary pattern can modify the association between long-term air pollution exposure and health outcomes has not yet been assessed. Similar large cohort epidemiological evidence supporting our findings is very limited, mainly due to a lack of available detailed dietary information in comparable cohort studies on long-term effects of air pollution. Our results here are, however, consistent with findings from the Danish Cancer and Diet cohort (N=52,061), which found that participants with higher consumption of total of fruits and vegetables had significantly lowered risks of cardiovascular and ischemic heart disease mortality associated with long-term NO2 exposure.23 In an earlier analysis of this current cohort with a limited analysis of three dietary variables (fruit, vegetables, and total fat), we found that higher fruit and vegetable consumption lowered diabetes mortality associated with PM2.5 and NO2 exposures.34 Evaluation of the PM2.5-CVD associations in the comparable American Cancer Society CPS-II Cohort (N=669,046), on the other hand, did not find that fat, fruit, vegetable, and fiber consumptions modified these relationships.35
The high spatial correlation between PM2.5 and NO2 and similar findings of effect modification of cardiovascular mortality outcomes by diet suggest that these pollutants likely originate from fossil fuel combustion from a similar source, most likely traffic. NO2 is an indicator of vehicle engine exhaust emissions, a complex mixture of including transition metals and polycyclic aromatic hydrocarbons.36 Recent results37 implicate fossil fuel combustion-derived air pollution as most responsible for the associations that have been observed between long-term air pollution exposure and cardiovascular disease mortality. Exposures to PM2.5 and NO2 (and correlated substances) can activate pathways mediating oxidative stress and inflammation, thereby potentially promoting cardiovascular disease mechanisms including systemic endothelial dysfunction, thrombosis, autonomic imbalance, atherosclerosis progression, insulin resistance, and dyslipidemia.38
This study has the major advantage of drawing upon a large prospective cohort specifically designed to assess participant diet and its relationship to chronic diseases. This study also has the strength of utilizing the latest air pollution prediction models to provide spatiotemporally detailed exposure estimates for the air pollutants for the entire follow-up period. We used a commonly employed index score to measure diet quality, which allows for findings to be more readily translated to public health guidelines. However, several potential weaknesses also exist in this study. With measures of diet collected only at baseline, we could not account for possible changes in intake and trends in patterns over time. Similarly, this analysis only included personal covariates recorded at baseline, so that any follow-up changes in these factors could not be accounted for. Other than knowing if and when participants leave the NIH-AARP cohort study areas, we presently lack information on residence location after those participants moved out of the study region, which may result in potential exposure misclassification. Moreover, a Mediterranean dietary pattern may also reflect other overall healthy behaviors that were not fully adjusted for in this analysis. Another limitation is that the NIH-AARP cohort has a limited number of participants in races other than white and black non-Hispanic, and therefore our findings may not be generalizable to others in the U.S. general population, though a reason that this would not be so is not obvious.
The global public health and associated economic burden of ambient air pollution is immense. According to the WHO, exposure to ambient air pollution is the fifth leading mortality risk factor in the world, estimated to cause more than 4.2 million deaths annually.5 The findings here suggest that a healthful dietary pattern can modify air pollution-induced adverse cardiovascular health effects, and thereby reduce mortality risk associated with long-term air pollution exposures. As it is unknown whether aMED has similar associations with attenuating other adverse effects of air pollution, such as respiratory events or lung cancer, or whether the attenuation of CVD events would extend to higher levels of long-term air pollution exposure, additional studies are needed to address these gaps of knowledge. Overall, our results have the potential to inform affected individuals exposed to high ambient air pollution levels, as well as both policymakers and those developing dietary guidelines, as to the potential role of dietary patterns in protecting public health from adverse effects of air pollution. Thus, in concert with air quality standards and emissions controls policies to protect health against the most harmful effects of air pollution, individual-level prevention strategies and population-wide policy efforts to promote healthier diets, aimed at countering the oxidative stress induced by air pollution exposure, may provide complementary approaches.
Conclusion
In this prospective cohort of older U.S. adults, long-term ambient air pollution exposure was significantly associated with an increase in cardiovascular mortality. Those reporting eating a Mediterranean diet had significantly lower rates of cardiovascular disease mortality associated with long-term air pollution exposure, suggesting that dietary patterns enriched in antioxidant foods and compounds could potentially provide protection against the adverse health effects induced by long-term exposure to ambient air pollution. These findings are also consistent with past evidence of oxidative stress as underlying mechanism for air pollution-induced health effects. Confirmatory studies exploring other health outcomes, combinations of certain foods and nutrients, and randomized clinical trials would further strengthen our findings.
Supplementary Material
Clinical Perspective.
What Is New?
Whether usual individual dietary patterns can modify the association between long-term air pollution exposure and health outcomes have not been previously examined.
We linked a large (N=548,845) and well-characterized prospective cohort study with spatiotemporally resolved exposure estimates for annual average fine particulate matter (PM2.5) and nitrogen dioxide (NO2) concentration levels between 1995 and 2011.
Those reporting more of a Mediterranean diet (higher aMED score) had lower rates of death due to CVD, IHD, and CER associated with long-term exposure to PM2.5, and CVD and IHD associated with long-term exposure to NO2.
What Are the Clinical Implications?
A Mediterranean diet is indicated to provide protection against adverse cardiovascular effects induced by long-term air pollution exposure.
These results add to a growing body of literature suggesting dietary patterns may help reduce cardiovascular events due to air pollution exposure, potentially through augmenting antioxidants and reducing oxidative stress.
Confirmatory independent studies, such as in other prospective cohorts, examinations of clinical outcomes, and/or long-term randomized interventions, would further strengthen our findings.
Acknowledgement
C.L designed the study, analyzed the data, and wrote the manuscript. D.S, R.J, J.A, and Y.S edited the manuscript and contributed to discussion. R.H and G.T are the main investigators for the study and edited the manuscript.
Funding
The study is supported by the NIH National Insitute of Environmental Health Sciences (1R01ES019584-01A1, R21 ES021194), New York University National Insitute of Environmental Health Sciences Center of Excellence (ES00260), and the American Lung Association (Dissertation Grant).
Footnotes
Disclosures
None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman J, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino M, Nasir K, Neumar R, Palaniappan L, Pandey D, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Munter P. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, Aryee M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012:380;2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet: 2017:389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook RD, Newby DE, Rajagopalan S. The global threat of outdoor ambient air pollution to cardiovascular health: time for intervention. JAMA Cardiol 2017:2;353–354. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (May 2, 2018). 9 out of 10 people worldwide breathe polluted air, but more countries are taking action. Available at https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action. Accessed September 16, 2018.
- 6.Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010:121;2331–2378. [DOI] [PubMed] [Google Scholar]
- 7.Zhong J, Karlsson O, Wang G, Li J, Guo Y, Lin X, Zemplenyi M, Sanchez-Guerra M, Trevisi L, Urch B, Speck M, Liang L, Coull BA, Koutrakis P, Silveramn F, Gold DR, Wu T, Baccarelli AA. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc. Natl. Acad. Sci. U.S.A 2017:114;3503–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Péter S, Holguin F, Wood LG, Clougherty JE, Raederstorff D, Antal M, Weber P, Eggersdorfer M. Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients. 2015:7;10398–10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong H. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta Gen Subj 2016:1860;2891–2898. [DOI] [PubMed] [Google Scholar]
- 10.Hooper LG, Kaufman JD. Ambient Air Pollution and Clinical Implications for Susceptible Populations. Ann Am Thorac Soc 2018:15;S64–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, Midthune D, Kipnis V. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001:154:1119–1125. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Olives C, Sheppard L, Sampson PD, Larson TV, Keller JP, Kaufman JD. Historical prediction modeling approach for estimating long-term concentrations of PM2.5 in cohort studies before the 1999 implementation of widespread monitoring. Environ Health Perspect 2016:125;38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young MT, Bechle MJ, Sampson PD, Szpiro AA, Marshall JD, Sheppard L, Kaufman JD. Satellite-Based NO2 and Model Validation in a National Prediction Model Based on Universal Kriging and Land-Use Regression. Environ Sci Technol 2016:50;3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher Diet Quality Is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J Nutr 2014:144;881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005:82;163–73. [DOI] [PubMed] [Google Scholar]
- 16.VanderWeele TJ, Peng D. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017:167;268–274 [DOI] [PubMed] [Google Scholar]
- 17.Galland L. Diet and inflammation. Nutr Clin Pract 2010:25;634–40. [DOI] [PubMed] [Google Scholar]
- 18.Salas-Salvadó J, Guasch-Ferré M, Lee CH, Estruch R, Clish CB, Ros E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J Nutr 2015:146;920S–927S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013:368;1279–1290. [DOI] [PubMed] [Google Scholar]
- 20.Ros E, Martínez-González MA, Estruch R, Salas-Salvadó J, Fitó M, Martínez JA, Corella D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv Nutr 2014:5;330S–336S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med 2015:128;229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitrou PN. Mediterranean Dietary Pattern and Prediction of All-Cause Mortality in a US Population. Arch Intern Med 2007:167;2461. [DOI] [PubMed] [Google Scholar]
- 23.Raaschou-Nielsen O, Andersen ZJ, Jensen SS, Ketzel M, Sørensen M, Hansen J, Loft S, Tjønneland A, Overvad K. Traffic air pollution and mortality from cardiovascular disease and all causes: a Danish cohort study. Environ Health. 2010:11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romieu I, Tellez-Rojo MM, Lazo M, Manzano-Patino A, Cortez- Lugo M, Julien P, Belanger M, Hernandez-Avila M, Holguin F. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med 2005:172;1534–1540. [DOI] [PubMed] [Google Scholar]
- 25.Romieu I, Garcia-Esteban R, Sunyer J, Rios C, Alcaraz-Zubeldia M, Velasco SR, Holguin F. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM2.5. Environ Health Perspect 2008:116;1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Possamai FP, Júnior SÁ, Parisotto EB, Moratelli AM, Inácio DB, Garlet TR, Dal-Pizzol F, Wilhelm Filho D. Antioxidant intervention compensates oxidative stress in blood of subjects exposed to emissions from a coal electric-power plant in South Brazil. Environ Toxicol Pharmacol 2010:30;175–180. [DOI] [PubMed] [Google Scholar]
- 27.Tong H, Rappold AG, Caughey M, Hinderliter AL, Bassett M, Montilla T, Case MW, Berntsen J, Bromberg PA, Cascio WE, Diaz-Sanchez D. Dietary supplementation with olive oil or fish oil and vascular effects of concentrated ambient particulate matter exposure in human volunteers. Environ Health Perspect 2015123;1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egner PA, Chen JG, Zarth AT, Ng D, Wang J, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prev Res (Phila). 2014:7;813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TYA., Thames G, Krak M, Zhang Y, Nel A. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct 2014:5;35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croft D, Block R, Cameron SJ, Evans K, Lowenstein CJ, Ling F, Zareba W, Hopke PK, Utell MJ, Thurston SW, Thevenet-Morrison K. Do elevated blood levels of omega-3 fatty acids modify effects of particulate air pollutants on fibrinogen? Air Qual Atmos Health. 2018:11;791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bo L, Jiang S, Xie Y, Kan H, Song W, Zhao J. Effect of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS One, 2016;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du X, Jiang S, Bo L, Liu J, Zeng X, Xie Y, He Q, Ye X, Song W, and Zhao J. Combined effects of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced cardiovascular injury in rats. Chemosphere. 2018:173;14–21. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Pang W, He C, Li Y, Jiang Y, Guo C. Blueberry anthocyanin-enriched extracts attenuate fine particulate matter (PM2.5)-induced cardiovascular dysfunction. J Agric Food Chem 2017:65;87–94. [DOI] [PubMed] [Google Scholar]
- 34.Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR, Garcia C, Thurston GD. Association between long-term exposure to ambient air pollution and diabetes mortality in the US. Environ Res 2018:165;330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope CA III, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships Between Fine Particulate Air Pollution, Cardiometabolic Disorders, and Cardiovascular Mortality. Circ Res 2015:116:108–115. [DOI] [PubMed] [Google Scholar]
- 36.Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential- A workshop report and consensus statement. Inhal Toxicol 2008;20:75–99. [DOI] [PubMed] [Google Scholar]
- 37.Thurston GD, Burnett RT, Turner MC, Shi Y, Krewski D, Lall R, Ito K, Jerrett M, Gapstur SM, Diver WR, Pope CA III. Ischemic Heart Disease Mortality and Long-Term Exposure to Source-Related Components of U.S. Fine Particle Air Pollution. Environ Health Perspect 2015:124;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: Part II–mechanistic insights. Eur Heart J. 2016:38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


