Abstract
Introduction:
Pulmonary impairment predicts increased mortality in many settings, and respiratory viral infection (RVI) causes considerable morbidity and mortality in allogeneic hematopoietic cell transplant recipients (allo-HCT). We hypothesized that pulmonary impairment following respiratory viral infection, defined as a decline of forced expiratory volume in 1 second values by ≥10%, may identify allo-HCT recipients at high risk for mortality.
Methods:
We studied all allo-HCT recipients at our institution who had RVI with respiratory syncytial virus, parainfluenza virus, or influenza from 2004-2013 and had pre-RVI and post-RVI pulmonary function tests. We used competing risk regression models to identify risk factors for 2-year non-relapse mortality (NRM) as the primary outcome after RVI and relapse-related mortality as a competing risk.
Results:
From 223 eligible patients, pulmonary impairment following RVI was associated with over a 3-fold increase in 2-year NRM (pulmonary impairment: 25.3%; no impairment: 7.4%, univariate sub-hazard ratio [SHR] 3.9, 95% confidence interval [CI] 1.9-8.1, p<0.001). After adjusting for age and systemic steroid use, pulmonary impairment following RVI was still associated with increased 2-year NRM (SHR, 3.3 [95% CI, 1.6-6.9]; p=0.002). After adjustment for race and GVHD prophylaxis, chronic GVHD at the time of RVI (OR, 2.8 [95% CI, 1.4-5.4]; p=0.003) and lymphopenia (OR, 2.2 [95% CI, 1.1-4.2]; p=0.02) were associated with increased odds of pulmonary impairment, whereas use of non-myeloablative conditioning was associated with reduced odds of pulmonary impairment (OR, 0.4 [95% CI, 0.2-0.8]; p=0.006).
Conclusion:
In allo-HCT recipients with RVIs, pulmonary impairment following RVI is associated with high NRM at 2 years.
Keywords: Respiratory viral infection, pulmonary function, pulmonary impairment, allogeneic hematopoietic cell transplantation, hematologic malignancy
1. Introduction
Hematopoietic cell transplantation (HCT) is an important curative option for many refractory and high-risk malignancies (1). Respiratory viral infections (RVIs) are increasingly recognized as important causes of morbidity and mortality in the general population (2), and are particularly common after HCT (3, 4). Depending on the viral species, more than 30% of RVIs in HCT recipients progress to lower respiratory tract infections (LRIs) (3), resulting in high mortality (5-9). About 30% of allogeneic HCT (allo-HCT) recipients develop LRI after respiratory syncytial virus (RSV) infection, and the overall mortality rate after RSV infection is about 16% (10). Similarly, around half of all HCT recipients with parainfluenza virus (PIV) develop LRI (11), with an overall mortality rate of 7-10%, and about 30% of all HCT recipients with H1N1 influenza virus develop LRI (12), with an overall mortality rate of about 6%. Identification of HCT recipients at high risk for death after RVI is of paramount importance.
Impairments in pulmonary function have long been linked to decreased survival in the general population (13). Independent of tobacco use, declines in forced spirometric measurements are associated with increased all-cause (14-16) and cardiovascular mortality (17, 18). Pulmonary impairment is a common complication after RVIs in the general population (19, 20). In allo-HCT recipients, RVIs have been associated with bronchiolitis obliterans syndrome (BOS), a form of graft-versus-host disease (GVHD) of the lung (21, 22). BOS occurs in about 5% of HCT recipients (23), and mortality primarily occurs in patients with the most severe impairments in lung function (24-27). Outside of BOS, the association of pulmonary impairment with mortality has not been well studied in allo-HCT recipients. A previous single-center study of 442 allo-HCT recipients found that declines in forced expiratory volume (FEV1) of at least 10% were not associated with higher mortality (28). No studies have examined the impact of pulmonary impairment on survival in allo-HCT recipients who develop RVIs.
FEV1 is a highly reproducible measurement that predicts mortality in many populations (29, 30). Because allo-HCT recipients who develop RVIs are at a high risk for mortality, we sought to determine whether declines in forced expiratory volume (FEV1) of at least 10% following RVI could predict non-relapse mortality (NRM) in allo-HCT recipients. To that end, we conducted a retrospective review of all allo-HCT recipients at our institution who had RSV, PIV, or influenza virus (FLU) infection. We hypothesized that allo-HCT recipients who developed pulmonary impairment following RVI would have higher NRM than those who did not, similar to the findings in other populations.
2. Methods
2.1. Subjects
Clinical data on all allo-HCT recipients with RSV, PIV, or FLU infection at The University of Texas MD Anderson Cancer Center obtained at the time of RVI from August 2004 to July 2013 were collected from prospectively maintained institutional infection control and HCT databases. Patients who had multiple RVIs during the study period were excluded to better understand the impact of a single RVI on mortality. Those who did not complete post-RVI pulmonary function tests (PFTs) were also excluded because their declines in FEV1 could not be calculated. In addition, in order to understand the impact of new pulmonary impairment in allo-HCT recipients, we excluded allo-HCT recipients with BOS prior to RVI. The MD Anderson Institutional Review Board approved the study (PA12-0407) with a waiver of informed consent.
2.2. Definitions for RVIs
Patients were considered to have RVIs if they had acute signs and symptoms of respiratory infection (e.g., fever, rhinorrhea, cough, nasal congestion) and a nasal wash or bronchoalveolar lavage specimen positive for RSV, PIV, or FLU in viral culture and/or a direct immunofluorescent assay (DFA) as per our institutional practices during the study period. Upper respiratory tract infection (URI) was defined as viral detection in nasal specimens and no pulmonary infiltrates on thoracic images. LRI was defined as new or changing pulmonary infiltrates on thoracic images and viral detection in nasal specimens and/or bronchoalveolar lavage fluid. Patients who had resolving infiltrates or volume overload as assessed via clinical chart review were not considered to have LRIs. Respiratory co-infections were defined as the detection of any viral or non-viral pulmonary pathogen other than RSV, PIV, or FLU at the time of RVI. Antiviral therapy was defined as any therapy specifically for RVI (e.g. ribavirin for RSV or oseltamivir for FLU). We considered absolute neutrophil counts <500 ×106 cells/L to indicate neutropenia, and absolute lymphocyte counts <500 ×106 cells/L to indicate lymphopenia.
2.3. Pulmonary function testing
PFT results for all study patients were collected from our institutional PFT database. Patients who did not undergo post-infection PFTs within 1 year following RVI were excluded. The PFT immediately preceding RVI was considered the baseline PFT, and the PFT with the lowest FEV1 performed 14-365 days following RVI was considered the post-RVI PFT. We chose the earlier cutoff point of 14 days to exclude patients with very early pulmonary impairment following RVI, and we chose the later cutoff point of 365 days to allow for sufficient time for patients to undergo post-RVI PFTs, while limiting the window of risk following RVI to 1 year. Declines in FEV1 of at least 10% from pre-RVI values were considered to represent significant pulmonary impairment. All four NIH criteria for BOS was required for a diagnosis of NIH-BOS: 1) FEV1:forced vital capacity (FVC) ratio less than 0.7 or below the fifth percentile of predicted values; 2) FEV1 less than 75% of predicted values, with a greater than 10% decline over a period shorter than 2 years; 3) absence of infection in the respiratory tract documented in investigations directed by clinical symptoms; and 4) evidence of air trapping, small airway thickening, or bronchiectasis on computed tomography images, residual volume/total lung capacity (RV/TLC) ratios elevated outside the 90% confidence interval for predicted values, RV >120% of predicted values, or evidence of GVHD in a non-lung organ (31).
2.4. Statistical analysis
Descriptive statistics were calculated for demographic, clinical, and outcome data. Categorical variables were compared using chi-square or Fisher exact tests, and continuous variables were compared using Student’s t-tests. Unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) with their p-values were calculated for each independent variable and post-RVI FEV1 decline (10%) using logistic regression. Next, a final multivariable model was constructed using forward selection with Bayesian information criteria to identify risk factors associated with post-RVI FEV1 decline. The Hosmer-Lemeshow goodness-of-fit statistic was used to check the fit of the final model. Fine-Gray models (32) were constructed for each risk factor for the primary outcome of 2-year NRM, and relapse-related mortality (RRM), as defined by the Center for International Blood and Marrow Transplant Research guidelines (33), as a competing risk. Allo-HCT recipients must have died without evidence of relapsed malignancy within 2 years of RVI to fulfill the primary outcome of 2-year NRM. We chose the 2 year landmark to allow for allo-HCT recipients with pulmonary impairment late in the 1-year period after initial RVI to have at least one year of observation following the time of pulmonary impairment. Subhazard ratios (SHRs) and 95% confidence intervals (CI) with their p-values were calculated for each independent variable and 2-year NRM. All covariates found to be significant in these univariable models (p≤0.1) were included in the multivariable model. Variables were retained in the final model only if they were significant at p=0.05 or if they induced a ≥15% change in the odds ratio of another significant variable of primary interest, indicating confounding. Similar analyses were repeated in separate models with 1-year NRM and any NRM as outcomes of interest with 1-year RRM and any RRM as the competing events, respectively. Cumulative incidence failure (CIF) curves were used to compare the probability of 2-year NRM of patients in whom pulmonary impairment developed (either 10% post-RVI FEV1 decline or BOS) with that in patients who did not have this impairment, adjusted for age and use of corticosteroids. All statistical analyses were performed using the R programming language (version 3.3; R Foundation for Statistical Computing, Vienna, Austria) and the Intercooled STATA software program (version 13.1; StataCorp, College Station, TX). All tests were two-sided, with a significance and p-values of 0.05 were considered significant
3. Results
3.1. Characteristics of the study cohort
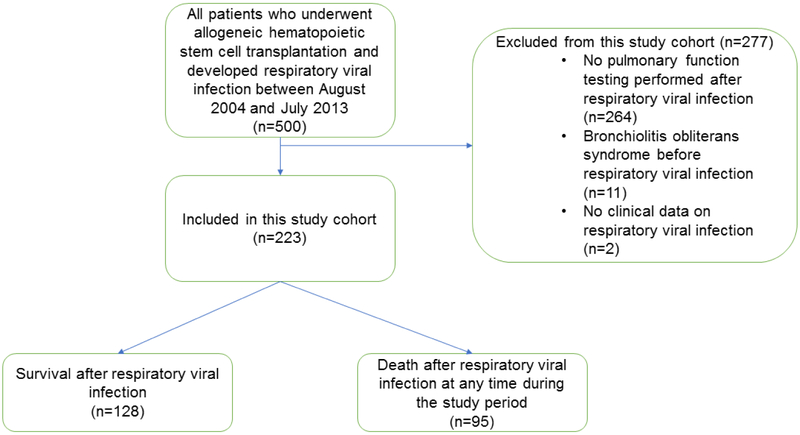
Figure 1 shows the flow chart for our cohort of patients in whom RVIs developed during the study period, after exclusion of those who did not meet our inclusion criteria. The primary reason for exclusion was absence of PFT data following RVI. We identified 223 patients who met our study criteria. In the study cohort, the median time from the PFT immediately preceding RVI to RVI was 73 days (range 1-1591 days), while the median time from RVI to the first PFT obtained following RVI was 65 days (range: 16 - 359 days). Of note, excluded patients were more likely to have RVI after day 100 post-HCT (63% vs. 51%; p=0.03) and had higher all-cause (53% vs. 32%; p<0.001) and non-relapse (31% vs. 13%; p<0.001) mortality rates at 2 years following RVI than study patients. Table 1 depicts the characteristics of the study cohort.
Figure 1.
Enrollment flow chart for the study cohort.
Table 1. Characteristics of the study cohort (n=223).
| Characteristic | N (%) |
|---|---|
| Median age (range) | 52 (19–77) |
| Gender | |
| Male | 127 (57) |
| Female | 96 (43) |
| Underlying Malignancy, n (%) | |
| Acute Leukemia | 125 (56) |
| Chronic Leukemia | 34 (15) |
| Lymphoma/Myeloma | 61 (27) |
| Other | 3 (1) |
| Days from HCT to RVI | |
| 0-30 | 27 (12) |
| 31-100 | 82 (37) |
| >100 | 114 (51) |
| HCT Type | |
| Matched related donor | 106 (48) |
| Matched unrelated donor | 91 (41) |
| Mismatched | 7 (3) |
| Haploidentical | 6 (3) |
| Cord blood | 13 (6) |
| Conditioning regimen | |
| Myeloablative | 133 (60) |
| Non-myeloablative | 90 (40) |
| T-cell depletion | |
| No | 120 (54) |
| Yes | 103 (46) |
| GVHD prophylaxis | |
| CNI/MTX | 192 (86) |
| CNI/MMF | 24 (11) |
| Other | 7 (3) |
| History of acute GVHD prior to RVI | |
| No | 114 (51) |
| Yes | 109 (49) |
| History of chronic GVHD prior to RVI | |
| No | 156 (70) |
| Yes | 67 (30) |
| CMV seropositivity* | |
| Donor − / Recipient − | 25 (11) |
| Donor + / Recipient + | 94 (42) |
| Donor − / Recipient + | 86 (39) |
| Donor + / Recipient − | 17 (8) |
| RVI type | |
| RSV | 68 (30) |
| FLU | 40 (18) |
| PIV | 115 (52) |
| All-cause mortality at 2 years post-RVI | |
| No | 152 (68) |
| Yes | 71 (32) |
| Non-relapse mortality at 2 years post-RVI | 30 (13) |
| Relapse-related mortality at 2 years post-RVI, | 41 (18) |
CMV seropositivity data were unavailable for one patient in this study.
Abbreviations: CNI, calcineurin inhibitor; MTX, methotrexate; MMF, mycophenolate mofetil; CMV, cytomegalovirus.
3.2. Predictors of pulmonary impairment following RVI
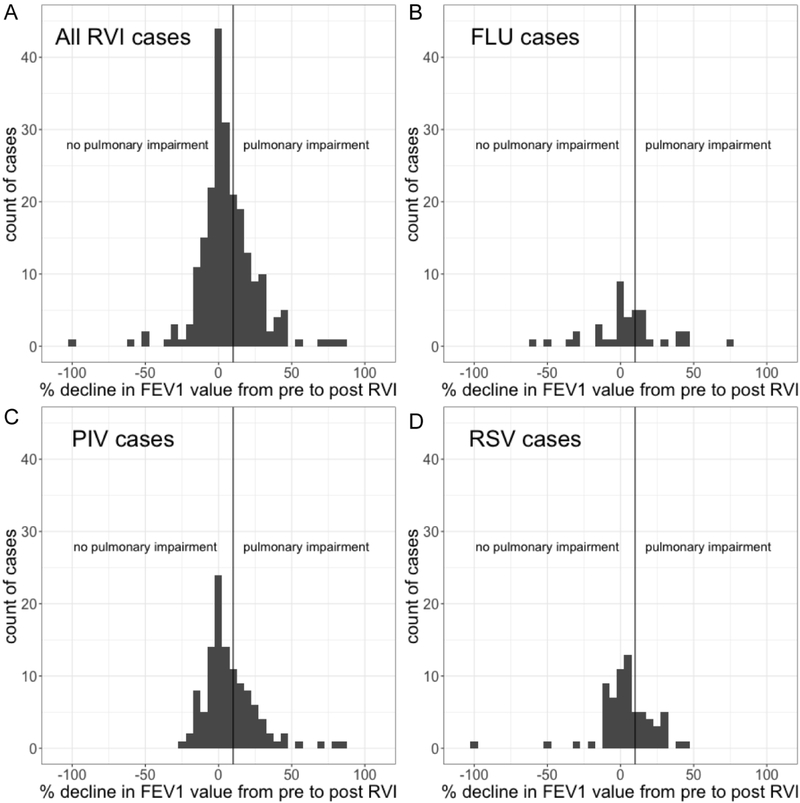
Within one year following RVI, 75 patients developed significant pulmonary impairment (34%). Of these 75 patients, 12 eventually developed BOS, of whom 11 developed BOS within one year of RVI. The median time to the diagnosis of BOS was 136 days after RVI (interquartile range: 22-708 days). Table 2 summarizes the bivariable and multivariable logistic regression models for the prediction of pulmonary impairment within 1 year after RVI. In the multivariable model, after adjustment for race and type of GVHD prophylaxis, chronic GVHD at the time of RVI (OR, 2.8 [95% CI, 1.4-5.4]; p=0.003) and lymphopenia (OR, 2.2 [95% CI, 1.1-4.2]; p=0.02) were associated with increased odds of pulmonary impairment, whereas use of non-myeloablative conditioning was associated with reduced odds of pulmonary impairment (OR, 0.4 [95% CI, 0.2-0.8]; p=0.006). We observed no significant differences in the odds of pulmonary impairment among patients who had RSV, PIV, or FLU infections (Table 2). Figure 2 shows a histogram of changes in FEV1 from pre-RVI to post-RVI values in all patients and in those with RSV, PIV, or FLU infection. Figure 3 shows a histogram of time to pulmonary impairment among decliners who had RVI. There was no significant difference in time to the diagnosis of pulmonary impairment among patients with RSV, PIV or FLU infection (p=0.35).
Table 2. Bivariable and multivariable risk factors for pulmonary impairment after RVI.
| Characteristic | Non- decliners (n=148) |
Decliners (n=75) |
Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p- value |
OR (95% CI) | p- value |
|||
| Median age at time of RVI*, median (range) | 51 (19-72) | 55 (19-77) | 1.1 (0.9-1.4) | 0.34 | ||
| Gender, n (%) | ||||||
| Male | 87 (59) | 40 (53) | 1.0 | |||
| Female | 61 (41) | 35 (47) | 1.3 (0.4-1.5) | 0.44 | ||
| Race**, n (%) | ||||||
| White | 112 (78) | 61 (82) | 1.0 | 1.0 | ||
| Non-white | 32 (22) | 13 (18) | 0.8 (0.4-1.5) | 0.42 | 0.7 (0.3-1.5) | 0.36 |
| Underlying Malignancy, n (%) | ||||||
| Acute Leukemia | 78 (53) | 47 (63) | 1.0 | |||
| Chronic Leukemia | 24 (16) | 10 (13) | 0.7 (0.3-1.6) | 0.38 | ||
| Lymphoma/Myeloma | 44 (30) | 17 (23) | 0.6 (0.3-1.3) | 0.19 | ||
| Other | 2 (1) | 1 (1) | 0.8 (0.1-9.4) | 0.88 | ||
| Days between HCT and RVI | ||||||
| ≤100 | 73 (49) | 36 (48) | 1.0 | |||
| >100 | 75 (51) | 39 (52) | 1.1 (0.6-1.8) | 0.85 | ||
| HCT Type, n (%) | ||||||
| Matched related donor | 73 (49) | 33 (44) | 1.0 | |||
| Matched unrelated donor | 58 (39) | 33 (44) | 1.3 (0.7-2.3) | 0.45 | ||
| Mismatched | 5 (3) | 2 (3) | 0.9 (0.2-4.8) | 0.89 | ||
| Haploidentical | 6 (4) | 0 | - | - | ||
| Cord blood | 6 (4) | 7 (9) | 2.6 (0.8-8.3) | 0.11 | ||
| Steroids 30 days prior to RVI, n (%) | ||||||
| No | 81 (55) | 32 (43) | 1.0 | |||
| Yes | 67 (45) | 43 (57) | 1.4 (0.6-2.9) | 0.09 | ||
| GVHD Prophylaxis, n (%) | ||||||
| CNI/MTX | 127 (86) | 65 (87) | 1.0 | 1.0 | ||
| CNI/MMF | 14 (9) | 10 (13) | 1.4 (0.6-3.3) | 0.45 | 2.2 (0.9-5.7) | 0.10 |
| Other | 7 (5) | 0 | - | - | ||
| History of acute GVHD prior to RVI, n (%) | ||||||
| No | 79 (53) | 35 (47) | 1.0 | |||
| Yes | 69 (47) | 40 (53) | 1.3 (0.8-2.3) | 0.34 | ||
| History of chronic GVHD prior to RVI, n (%) | ||||||
| No | 112 (76) | 44 (59) | 1.0 | 1.0 | ||
| Yes | 36 (24) | 31 (41) | 2.2 (1.2-4.0) | 0.01 | 2.8 (1.4-5.4) | 0.003 |
| CMV seropositivity***, n (%) | ||||||
| Donor − / Recipient − | 16 (11) | 9 (12) | 1.0 | |||
| Donor or Recipient + | 131 (89) | 66 (88) | 0.9 (0.4-2.1) | 0.80 | ||
| RVI species, n (%) | ||||||
| RSV | 48 (32) | 20 (27) | 1.0 | |||
| FLU | 26 (18) | 14 (19) | 1.3 (0.6-3.0) | 0.55 | ||
| PIV | 74 (50) | 41 (55) | 1.3 (0.7-2.5) | 0.39 | ||
| Non-myeloablative conditioning, n (%) | ||||||
| No | 80 (54) | 43 (57) | 1.0 | 1.0 | ||
| Yes | 68 (46) | 32 (43) | 0.5 (0.3-0.9) | 0.02 | 0.4 (0.2-0.8) | 0.006 |
| T-cell depletion | ||||||
| No | 83 (56) | 53 (71) | 1.0 | |||
| Yes | 65 (44) | 22 (29) | 1.3 (0.7-2.3) | 0.34 | ||
| ALC at time of RVI | ||||||
| ≥500 ×106 cells/L | 99 (67) | 42 (56) | 1.0 | 1.0 | ||
| <500 ×106 cells/L | 49 (33) | 33 (44) | 1.2 (0.9-1.4) | 0.11 | 2.2 (1.1-4.2) | 0.02 |
| ANC at time of RVI | ||||||
| ≥500 ×106 cells/L | 140 (95) | 71 (95) | 1.0 | |||
| <500 ×106 cells/L | 8 (5) | 4 (5) | 1.0 (0.7-1.5) | 0.98 | ||
| Site of infection at time of RVI | ||||||
| URI | 121 (82) | 50 (67) | 1.0 | |||
| LRI | 27 (18) | 25 (33) | 2.2 (1.2-4.2) | 0.01 | ||
| Co-infection at time of LRI | ||||||
| No | 127 (86) | 57 (76) | 1.0 | |||
| Yes | 27 (14) | 18 (24)_ | 1.1 (0.7-2.0) | 0.64 | ||
| Anti-viral therapy at time of LRI | ||||||
| No | 60 (41) | 28 (37) | 1.0 | |||
| Yes | 88 (59) | 47 (63) | 1.1 (0.7-2.0) | 0.64 | ||
Analyzed in increments of 10 years
Data for race were unavailable for 5 patients
CMV seropositivity data was unavailable for one patient.
Abbreviations: CNI, calcineurin inhibitor; MTX, methotrexate; MMF, mycophenolate mofetil; CMV, cytomegalovirus; ANC, absolute neutrophil count.
Figure 2.
Histogram showing changes in FEV1 from pre-RVI to post-RVI values in (a) the entire study cohort and in patients with (b) FLU infection, (c) PIV infection, and (d) RSV infection. The magnitude of FEV1 decline did not differ significantly between viral species.
Figure 3.
Histogram showing days to decline among patients with significant pulmonary impairment after RVI in (a) the entire study cohort and in patients with (b) FLU infection, (c) PIV infection, and (d) RSV infection. Each bin represents one week of time. There was no significant difference in time to decline after RVI between viral species.
3.3. Predictors of 2-year NRM following RVI
During the study period, 71 patients (30%) had a fatal outcome by 2 years following RVI. Of these 71 patients, 40 developed RRM, and 31 developed NRM. Table 3 shows unadjusted and adjusted competing risk models in which the primary outcome was 2-year NRM and the competing risk was 2-year RRM. In the multivariable competing risk model, increased age (SHR, 1.4 per 10-year interval [95% CI, 1.0-1.8]; p=0.03), systemic steroid use in the 30 days prior to RVI (SHR, 5.3 [95% CI, 2.0-13.7]; p=0.001), and significant pulmonary impairment following RVI (SHR, 3.3 [95% CI, 1.6-6.9]; p=0.002) remained significant predictors of death. The proportional hazards assumption was met, and there was no loss to follow up at 2 years.
Table 3. Competing risk model for NRM at 2 years after RVI with RRM as a competing risk.
| Characteristic | Survivors at 2 years after RVI (n=152) |
NRM at 2 years after RVI (n=30) |
Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|
| SHR for NRM (95% CI) |
p- value |
SHR for NRM (95% CI) |
p- value |
|||
| Median age at time of RVI*, (range) | 51 (19-72) | 54 (26-77) | 1.4 (1.1-1.8) | 0.02 | 1.4 (1.0-1.8) | 0.03 |
| Gender, n (%) | ||||||
| Male | 83 (55) | 20 (67) | 1.0 | |||
| Female | 69 (45) | 10 (33) | 0.7 (0.3-1.4) | 0.27 | ||
| Race**, n (%) | ||||||
| White | 117 (80) | 27 (90) | 1.0 | |||
| Non-white | 30 (20) | 3 (10) | 0.4 (0.1-1.3) | 0.13 | ||
| Underlying Malignancy, n (%) | ||||||
| Acute Leukemia | 82 (54) | 18 (60) | 1.0 | |||
| Chronic Leukemia | 28 (18) | 2 (2) | 0.4 (0.1-1.8) | 0.23 | ||
| Lymphoma/Myeloma | 41 (27) | 10 (33) | 1.1 (0.5-2.5) | 0.71 | ||
| Other | 1 (1) | 0 | - | - | ||
| Days between HCT and RVI | ||||||
| ≤100 | 64 (42) | 17 (57) | 1.0 | |||
| >100 | 88 (58) | 13 (43) | 0.9 (0.6-1.2) | 0.37 | ||
| HCT Type, n (%) | ||||||
| Matched related donor | 76 (50) | 14 (47) | 1.0 | |||
| Matched unrelated donor | 60 (39) | 14 (47) | 1.2 (0.6-2.5) | 0.65 | ||
| Mismatched | 5 (3) | 0 | - | - | ||
| Haploidentical | 4 (3) | 0 | - | - | ||
| Cord blood | 7 (5) | 2 (7) | 1.2 (0.3-5.1) | 0.83 | ||
| Steroids 30 days prior to RVI, n (%) | ||||||
| No | 83 (55) | 5 (17) | 1.0 | 1.0 | ||
| Yes | 69 (45) | 25 (83) | 5.7 (2.2-14.9) | <0.001 | 5.3 (2.0-13.7) | 0.001 |
| GVHD Prophylaxis, n (%) | ||||||
| CNI/MTX | 131 (86) | 27 (90) | 1.0 | |||
| CNI/MMF | 15 (10) | 3 (10) | 0.9 (0.3-2.9) | 0.83 | ||
| Other | 6 (4) | 0 | - | |||
| History of acute GVHD prior to RVI, n (%) | ||||||
| No | 78 (51) | 12 (40) | 1.0 | |||
| Yes | 74 (49) | 18 (60) | 1.6 (0.8-3.3) | 0.20 | ||
| History of chronic GVHD prior to RVI, n (%) | ||||||
| No | 104 (68) | 20 (67) | 1.0 | |||
| Yes | 48 (32) | 10 (33) | 1.2 (0.6-2.6) | 0.65 | ||
| Decline in FEV1 of 10% or greater, n (%) | ||||||
| No | 109 (72) | 11 (37) | 1.0 | 1.0 | ||
| Yes | 43 (28) | 19 (63) | 3.9 (1.9-8.1) | <0.001 | 3.3 (1.6-6.9) | 0.002 |
| CMV seropositivity***, n (%) | ||||||
| Donor − / Recipient − | 15 (10) | 5 (17) | 1.0 | |||
| Donor or Recipient + | 136 (90) | 25 (83) | 0.6 (0.2-1.6) | 0.29 | ||
| RVI species, n (%) | ||||||
| RSV | 47 (31) | 5 (17) | 1.0 | |||
| FLU | 29 (19) | 3 (10) | 1.0 (0.3-4.4) | 0.96 | ||
| PIV | 76 (50) | 22 (73) | 2.8 (1.1-7.3) | 0.04 | ||
| Site of infection at time of RVI | ||||||
| URI | 120 (79) | 22 (73) | 1.0 | |||
| LRI | 32 (21) | 8 (27) | 1.2 (0.6-2.8) | 0.60 | ||
| Co-infection at time of LRI | ||||||
| No | 128 (84) | 24 (80) | 1.0 | |||
| Yes | 24 (16) | 6 (20) | 1.3 (0.5-3.1) | 0.63 | ||
| Anti-viral therapy at time of LRI | ||||||
| No | 62 (41) | 14 (47) | 1.0 | |||
| Yes | 90 (59) | 16 (53) | 0.7 (0.4-1.5) | 0.40 | ||
| Non-myeloablative conditioning, n (%) | ||||||
| No | 93 (61) | 18 (60) | 1.0 | |||
| Yes | 59 (39) | 12 (40) | 1.0 (0.5-2.0) | 0.95 | ||
| T-cell depletion | ||||||
| No | 85 (56) | 16 (53) | 1.0 | |||
| Yes | 67 (44) | 14 (47) | 1.0 (0.5-2.1) | 0.93 | ||
| ALC at time of RVI | ||||||
| ≥500 ×106 cells/L | 111 (73) | 16 (53) | 1.0 | |||
| <500 ×106 cells/L | 41 (27) | 14 (47) | 1.3 (1.0-1.6) | 0.04 | ||
| ANC at time of RVI | ||||||
| ≥500 ×106 cells/L | 146 (96) | 28 (93) | 1.0 | |||
| <500 ×106 cells/L | 6 (4) | 2 (7) | 1.1 (0.7-1.8) | 0.74 | ||
Analyzed in increments of 10 years.
Data for race were unavailable for 5 patients
CMV seropositivity data were unavailable for one patient.
Abbreviations: CNI, calcineurin inhibitor; MTX, methotrexate; MMF, mycophenolate mofetil; CMV, cytomegalovirus; ANC, absolute neutrophil count.
Figure 4 shows cumulative incidence function curves for mortality among patients with and without significant pulmonary impairment. Survival was significantly lower among allo-HCT recipients with significant pulmonary impairment. 2-year NRM was 25.3% in patients with pulmonary impairment following RVI, as compared to 7.4% among those without impairment. 2-year NRM was similar among patients who developed pulmonary impairment within 90 days of RVI (33% 2-year NRM [10/33], SHR 4.9 [95% CI 2.1-11.5]; p<0.001) and those who developed pulmonary impairment more than 90 days after RVI (21% 2-year NRM [9/42], SHR 3.2 [95% CI 1.3-7.6], p=0.01). There was no difference in 2-year NRM in allo-HCT recipients with post-RVI pulmonary impairment who developed airflow obstruction as compared to those with post-RVI pulmonary impairment but without airflow obstruction (obstruction: 2-year NRM 29% [5/17]; no obstruction: 2-year NRM 24% [14/58]; SHR 1.2, 95%CI 0.5-3.7, p=0.68).
Figure 4.
Cumulative incidence function curves for mortality in the first two years after RVI among those with pulmonary impairment (solid line) and without pulmonary impairment (dashed line) after adjustment for age and systemic steroid use within 30 days.
4. Discussion
In this study of allo-HCT recipients who had RSV, PIV, or FLU infections, we observed that pulmonary impairment, defined as at least 10% decline in FEV1 from pre-RVI values, was an independent predictor of 2-year NRM following RVI. Myeloablative conditioning regimens during HCT and LRI at the time of RVI were predictive of pulmonary impairment following RVI. Among those with pulmonary impairment, NRM was high regardless of whether or not impairment was diagnosed within 90 days of RVI or was associated with airflow obstruction. Our findings suggest that screening for pulmonary impairment following RVI may identify allo-HCT recipients at high risk for NRM.
While risk factors for post-HCT BOS have been well described (23, 26, 34-40), there is a lack of data on the risk of pulmonary impairment following RVI in allo-HCT recipients. RVIs are common in allo-HCT recipients, though incidence rates are likely to be underestimated by a lack of routine screening (4, 41). Short-term pulmonary impairment after RVI has been well described in the general population, though longterm data is lacking (19, 20). Impairments after certain RVIs, such as influenza, are associated with increases in airway resistance in normal individuals that may last for weeks (42-44). In our study, we found that 34% of allo-HCT recipients who developed RVI had a significant pulmonary impairment within one year of RVI, with about half of impairments occurring within 90 days of RVI.
Similar to the study by Erard et al. (21), which examined risk factors for BOS in allo-HCT recipients, in our univariate analyses we found that LRI at the time of RVI was a strong predictor of pulmonary impairment. However, in the multivariate model, LRI was no longer predictive of pulmonary impairment. Instead, we observed that patients who received myeloablative conditioning regimens were at higher risk for pulmonary impairment following RVI than those who received non-myeloablative regimens, similar to the known increase in the risk for BOS with myeloablative conditioning in allo-HCT recipients (45). In allo-HCT recipients, myeloablative conditioning and non-myeloablative conditioning regimens are associated with similar rates of RVI, but progression to LRI and mortality is higher among those receiving myeloablative conditioning (46-48). Additionally, we found that the presence of chronic GVHD prior to RVI predicted post-RVI pulmonary impairment. In allo-HCT recipients with chronic GVHD who develop RVI, it is possible that the requirement for systemic immunosuppression mediates much of the risk of pulmonary impairment. Although, we did not find an association between steroid use prior to RVI and subsequent pulmonary impairment, we did find an association between lymphopenia at the time of RVI and subsequent pulmonary impairment. Allo-HCT recipients are commonly on immunosuppressive agents which work by suppressing T-lymphocyte function and reducing lymphocyte counts. Immunosuppression in allo-HCT recipients can thereby result in lymphopenia and predispose to more severe infections (49). Therefore, it is possible that much of the variance in outcome with LRI as a predictor is also explained by the type of conditioning regimen, chronic GVHD and lymphopenia, but the precise contribution of each of these variables to the risk for pulmonary decline needs further study.
To our knowledge, this is the first study to show that pulmonary impairment following RVI is strongly associated with increased 2-year NRM in allo-HCT recipients. Development of BOS is associated with increased mortality in allo-HCT recipients (23, 28), but allo-HCT recipients with BOS typically have a substantial decline in FEV1 at the time of clinical evaluation (24, 50). We found that a 10% decline in FEV1 following RVIs in allo-HCT recipients was a strong predictor for 2-year NRM, suggesting that even small decrements in pulmonary function are associated with worse outcomes after RVI. RVIs induce inflammatory responses in the lung which can injure the airways and lung parenchyma, and these injuries can manifest as declines in FEV1 (51-53). In this scenario, impairment in forced spirometry serves as a biomarker for mortality risk in allo-HCT recipients with RVI, analogous to findings in the general population (14-18). Because most allo-HCT recipients do not die immediately after an RVI episode, these individuals would likely benefit from screening PFTs either administered in a PFT laboratory or through the use of home spirometric monitoring programs (54).
In addition to pulmonary impairment, we found that advanced age and systemic steroid use at the time of RVI were risk factors for 2-year NRM. Advanced age is a well-known marker of adverse outcomes after viral LRI (55) and may be explained in part by the shift from lymphoid to myeloid cell proliferation (56), impaired B- and T-cell lymphopoiesis (57, 58), and impaired T-cell co-stimulation (59). Lymphopenia at the time of RVI is a well-known risk factor for adverse outcomes (3, 60), and in a univariate analysis, we found that an ALC less than 500 × 106 cells/L at the time of RVI predicted 2-year NRM. However, in our multivariate competing risk model, increased age predicted 2-year NRM, but an ALC less than 500 × 106 cells/L did not. Other studies have shown that advanced age and lymphopenia predict mortality in allo-HCT recipients following RSV infection (61) and H1N1 influenza infection (12). Further work is necessary to identify the relative and cumulative impacts of age and lymphopenia on mortality risk in allo-HCT recipients. We also found that systemic steroid use prior to RVI was a strong predictor of mortality. The immunosuppressive effects of systemic steroids are well-known (62), and the primary reason for the use of systemic steroids in our cohort was the treatment of GVHD. In allo-HCT recipients, systemic steroid use in the setting of RVI is associated with increased rates of progression from URI to LRI in RSV (63) and PIV infections (64), though not influenza (65, 66). In the general population, however, systemic steroid use during influenza infection has been associated with higher mortality (67). Both acute and chronic GVHD are associated with increased mortality in proportion to severity (68-70). Systemic steroid therapy may often be necessary for GVHD treatment, and efforts to minimize immunosuppression must be weighed against the risk of worsening GVHD.
Our study has some strengths and limitations. The strengths included consecutive enrollment of all allo-HCT recipients at a high-volume transplant center, the use of comprehensive infection control, HCT and PFT databases, and complete follow-up of subjects. However, during the study period, molecular assays, including multiplex polymerase chain reaction assays, which are sensitive, specific, and comprehensive, were not available at our institution, and therefore we restricted the analysis to allo-HCT recipients with RVI diagnosed by DFA or viral culture (71). In addition, our cohort consisted of allo-HCT recipients who underwent follow-up PFTs to document FEV1 decline. We may have excluded some patients who did not complete post-RVI PFTs because of debility. This would imply that our cohort could be affected by a survival bias in that patients must be healthy enough to survive to undergo post-RVI PFTs. Because PFTs were not clinically mandated following RVI, an alternative explanation could be that only patients who remained symptomatic underwent post-RVI PFTs, suggesting that these patients had probably persistent post-viral inflammation than did those who did not complete post-RVI PFTs. Similarly, we assumed that pulmonary function remained stable from pre-RVI PFTs to the time RVI. Because pulmonary function tends to decline over time (72), we may have overestimated the decline at the time of RVI in subjects with a long delay between pre-RVI PFTs and time of RVI, which would bias our results towards the null hypothesis. Overall, our cohort is representative of those in typical clinical practice, in which symptomatic patients are evaluated for RVIs, and patients who are able to undergo PFTs following RVI do so. In this representative population, pulmonary impairment was highly predictive of post-RVI NRM. PFTs obtained shortly after RVI may incorrectly identify patients who subsequently recover lung function as “decliners.” However, classification of these patients as decliners would bias our results towards the null hypothesis. Furthermore, if PFTs were used to screen for allo-HCT recipients at high risk for NRM after RVI, some false positive findings may be acceptable in order to detect all patients at risk for NRM. Few patients in our study developed BOS; therefore, we could not analyze risk factors for post-RVI BOS in allo-HCT recipients. Finally, due to the lack of allo-HCT recipients who had lymphocyte counts below 300×106 cells/L, our cutoff for defining lymphopenia (500×106 cells/L) was higher than has been used in other studies (12, 61), which could overestimate the severity of immunosuppression in allo-HCT recipients we considered to be lymphopenic.
In conclusion, we report that allo-HCT recipients who have pulmonary impairment following RVI are at increased risk for 2-year NRM. Furthermore, increased age and systemic steroid use prior to RVI were associated with higher 2-year NRM. Increased surveillance with PFTs may identify allo-HCT recipients with early pulmonary impairments following RVI, but the value or benefit of screening will depend upon the identification of effective post-RVI interventions to mitigate non-relapse mortality.
Highlights.
Allo-HCT recipients are at high risk for death after respiratory viral infection (RVI)
1/3 of allo-HCT recipients with RVI develop lung impairment (FEV1 decline ≥10%)
Chronic GVHD, lymphopenia and myeloablative conditioning predict lung impairment
Non-relapse mortality in those with lung impairment increases from 7.4% to 25.3%
Screening for lung impairment may identify recipients at high risk for death
Acknowledgments
Funding: This work was supported by the NIH/NIAID (K23 AI117024; to A.S.), the American Cancer Society (Mentored Research Scholar Grants in Applied and Clinical Research [MRSG-16-152-01-CCE] to D.P.S.), the Duncan Family Institute Cancer Survivorship Grant (to D.P.S.) and the NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group.
Abbreviations
- Allo-HCT
allogeneic hematopoietic cell transplantation
- BOS
bronchiolitis obliterans
- BOS 0p
bronchiolitis obliterans syndrome stage 0p
- CI
confidence interval
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- FLU
influenza virus
- GVHD
graft-versus-host disease
- HCT
hematopoietic cell transplantation
- NIH
National Institutes of Health
- OR
odds ratio
- PIV
parainfluenza virus
- LRI
lower respiratory tract infection
- RSV
respiratory syncytial virus
- RV
residual volume
- RVI
respiratory viral infection
- SHR
subhazard ratio
- TLC
total lung capacity
- URI
upper respiratory tract infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Milojkovic D, Mufti GJ. Extending the role of allogeneic stem-cell transplantation. Lancet 2001;357(9257):652–654. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med 2015;373(5):415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemaly RF, Ghosh S, Bodey GP, Rohatgi N, Safdar A, Keating MJ et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85(5):278–287. [DOI] [PubMed] [Google Scholar]
- 4.Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014;59 Suppl 5:S344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaly RF, Hanmod SS, Rathod DB, Ghantoji SS, Jiang Y, Doshi A et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood 2012;119(12):2738–2745; quiz 2969. [DOI] [PubMed] [Google Scholar]
- 6.Renaud C, Xie H, Seo S, Kuypers J, Cent A, Corey L et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 2013;19(8):1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waghmare A, Campbell AP, Xie H, Seo S, Kuypers J, Leisenring W et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis 2013;57(12):1731–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo S, Xie H, Campbell AP, Kuypers JM, Leisenring WM, Englund JA et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis 2014;58(10):1357–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo S, Waghmare A, Scott EM, Xie H, Kuypers JM, Hackman RC et al. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica 2017;102(6):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah DP, Ghantoji SS, Shah JN, El Taoum KK, Jiang Y, Popat U et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013;68(8):1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah DP, Shah PK, Azzi JM, Chemaly RF. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: A systematic review. Cancer Lett 2016;370(2):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljungman P, de la Camara R, Perez-Bercoff L, Abecasis M, Nieto Campuzano JB, Cannata-Ortiz MJ et al. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica 2011;96(8):1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson J On the capacity of the lungs, and on the respiratory functions, with a view of establishing a precise and easy method of detecting disease by the spirometer. Med Chir Trans 1846;29:137–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashley F, Kannel WB, Sorlie PD, Masson R. Pulmonary function: relation to aging, cigarette habit, and mortality. Ann Intern Med 1975;82(6):739–745. [DOI] [PubMed] [Google Scholar]
- 15.Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Spirometric findings and mortality in never-smokers. J Clin Epidemiol 1990;43(9):867–873. [DOI] [PubMed] [Google Scholar]
- 16.Lee HM, Le H, Lee BT, Lopez VA, Wong ND. Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. Eur Respir J 2010;36(5):1002–1006. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Hubert H, Lew EA. Vital capacity as a predictor of cardiovascular disease: the Framingham study. Am Heart J 1983;105(2):311–315. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med 1976;294(20):1071–1075. [DOI] [PubMed] [Google Scholar]
- 19.Hall WJ, Hall CB. Clinical significance of pulmonary function tests. Alterations in pulmonary function following respiratory viral infection. Chest 1979;76(4):458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picken JJ, Niewoehner DE, Chester EH. Prolonged effects of viral infections of the upper respiratory tract upon small airways. Am J Med 1972;52(6):738–746. [DOI] [PubMed] [Google Scholar]
- 21.Erard V, Chien JW, Kim HW, Nichols WG, Flowers ME, Martin PJ et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis 2006;193(12):1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant 2010;16(6):782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168(2):208–214. [DOI] [PubMed] [Google Scholar]
- 24.Cheng GS, Storer B, Chien JW, Jagasia M, Hubbard JJ, Burns L et al. Lung Function Trajectory in Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Cell Transplant. Ann Am Thorac Soc 2016;13(11):1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn JH, Jo KW, Song JW, Shim TS, Lee SW, Lee JS et al. Prognostic role of FEV1 for survival in bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Clin Transplant 2015;29(12):1133–1139. [DOI] [PubMed] [Google Scholar]
- 26.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 2003;9(10):657–666. [DOI] [PubMed] [Google Scholar]
- 27.Bergeron A, Godet C, Chevret S, Lorillon G, Peffault de Latour R, de Revel T et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant 2013;48(6):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abedin S, Yanik GA, Braun T, Pawarode A, Magenau J, Goldstein SC et al. Predictive value of bronchiolitis obliterans syndrome stage 0p in chronic graft-versus-host disease of the lung. Biol Blood Marrow Transplant 2015;21(6):1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003;58(5):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schunemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 2000;118(3):656–664. [DOI] [PubMed] [Google Scholar]
- 31.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389–401 e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 33.Copelan E, Casper JT, Carter SL, van Burik JA, Hurd D, Mendizabal AM et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant 2007;13(12):1469–1476. [DOI] [PubMed] [Google Scholar]
- 34.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011;17(7):1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2010;16(1 Suppl):S106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med 1989;111(5):368–376. [DOI] [PubMed] [Google Scholar]
- 37.Clark JG, Schwartz DA, Flournoy N, Sullivan KM, Crawford SW, Thomas ED. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med 1987;107(5):648–656. [DOI] [PubMed] [Google Scholar]
- 38.Gazourian L, Rogers AJ, Ibanga R, Weinhouse GL, Pinto-Plata V, Ritz J et al. Factors associated with bronchiolitis obliterans syndrome and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Am J Hematol 2014;89(4):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakaseko C, Ozawa S, Sakaida E, Sakai M, Kanda Y, Oshima K et al. Incidence, risk factors and outcomes of bronchiolitis obliterans after allogeneic stem cell transplantation. Int J Hematol 2011;93(3):375–382. [DOI] [PubMed] [Google Scholar]
- 40.Vieira AG, Funke VA, Nunes EC, Frare R, Pasquini R. Bronchiolitis obliterans in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant 2014;49(6):812–817. [DOI] [PubMed] [Google Scholar]
- 41.Wolfromm A, Porcher R, Legoff J, Peffault de Latour R, Xhaard A, de Fontbrune FS et al. Viral respiratory infections diagnosed by multiplex PCR after allogeneic hematopoietic stem cell transplantation: long-term incidence and outcome. Biol Blood Marrow Transplant 2014;20(8):1238–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall WJ, Douglas RG Jr., Hyde RW, Roth FK, Cross AS, Speers DM. Pulmonary mechanics after uncomplicated influenza A infection. Am Rev Respir Dis 1976;113(2):141–148. [DOI] [PubMed] [Google Scholar]
- 43.Little JW, Hall WJ, Douglas RG Jr., Hyde RW, Speers DM. Amantadine effect on peripheral airways abnormalities in influenza. A study in 15 students with natural influenza A infection. Ann Intern Med 1976;85(2):177–182. [DOI] [PubMed] [Google Scholar]
- 44.Little JW, Hall WJ, Douglas RG Jr., Mudholkar GS, Speers DM, Patel K. Airway hyperreactivity and peripheral airway dysfunction in influenza A infection. Am Rev Respir Dis 1978;118(2):295–303. [DOI] [PubMed] [Google Scholar]
- 45.Yoshihara S, Tateishi U, Ando T, Kunitoh H, Suyama H, Onishi Y et al. Lower incidence of Bronchiolitis obliterans in allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning compared with myeloablative conditioning. Bone Marrow Transplant 2005;35(12):1195–1200. [DOI] [PubMed] [Google Scholar]
- 46.Boeckh M The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol 2008;143(4):455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakrabarti S, Avivi I, Mackinnon S, Ward K, Kottaridis PD, Osman H et al. Respiratory virus infections in transplant recipients after reduced-intensity conditioning with Campath-1H: high incidence but low mortality. Br J Haematol 2002;119(4):1125–1132. [DOI] [PubMed] [Google Scholar]
- 48.Schiffer JT, Kirby K, Sandmaier B, Storb R, Corey L, Boeckh M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica 2009;94(8):1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan MD, Wilkes DS. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc 2005;2(5):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bashoura L, Gupta S, Jain A, Couriel DR, Komanduri KV, Eapen GA et al. Inhaled corticosteroids stabilize constrictive bronchiolitis after hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41(1):63–67. [DOI] [PubMed] [Google Scholar]
- 51.Hansel TT, Tunstall T, Trujillo-Torralbo MB, Shamji B, Del-Rosario A, Dhariwal J et al. A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-gamma and IFN-lambda) and Type 2 Inflammation (IL-5 and IL-13). EBioMedicine 2017;19:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 2014;190(12):1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng S, Wu J, Liu J, Qi F, Liu B. IL-33 Receptor (ST2) Signalling is Important for Regulation of Th2-Mediated Airway Inflammation in a Murine Model of Acute Respiratory Syncytial Virus Infection. Scand J Immunol 2015;81(6):494–501. [DOI] [PubMed] [Google Scholar]
- 54.Williams KM, Cheng GS, Pusic I, Jagasia M, Burns L, Ho VT et al. Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2016;22(4):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289(2):179–186. [DOI] [PubMed] [Google Scholar]
- 56.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 2005;102(26):9194–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley RL. Impaired B lymphopoiesis in old age: a role for inflammatory B cells? Immunol Res 2013;57(1-3):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol 2004;173(1):245–250. [DOI] [PubMed] [Google Scholar]
- 59.Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol 1997;21(6):471–478. [DOI] [PubMed] [Google Scholar]
- 60.Kim YJ, Guthrie KA, Waghmare A, Walsh EE, Falsey AR, Kuypers J et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis 2014;209(8):1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah DP, Ghantoji SS, Ariza-Heredia EJ, Shah JN, El Taoum KK, Shah PK et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood 2014;123(21):3263–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 2011;335(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damlaj M, Bartoo G, Cartin-Ceba R, Gijima D, Alkhateeb HB, Merten J et al. Corticosteroid use as adjunct therapy for respiratory syncytial virus infection in adult allogeneic stem cell transplant recipients. Transpl Infect Dis 2016;18(2):216–226. [DOI] [PubMed] [Google Scholar]
- 64.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001;98(3):573–578. [DOI] [PubMed] [Google Scholar]
- 65.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 2004;39(9):1300–1306. [DOI] [PubMed] [Google Scholar]
- 66.Boudreault AA, Xie H, Leisenring W, Englund J, Corey L, Boeckh M. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol Blood Marrow Transplant 2011;17(7):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang JW, Fan LC, Miao XY, Mao B, Li MH, Lu HW et al. Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis. Clin Microbiol Infect 2015;21(10):956–963. [DOI] [PubMed] [Google Scholar]
- 68.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood 2002;100(2):406–414. [DOI] [PubMed] [Google Scholar]
- 69.Arora M, Hemmer MT, Ahn KW, Klein JP, Cutler CS, Urbano-Ispizua A et al. Center for International Blood and Marrow Transplant Research chronic graft-versus-host disease risk score predicts mortality in an independent validation cohort. Biol Blood Marrow Transplant 2015;21(4):640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood 2015;126(1):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ginocchio CC, McAdam AJ. Current Best Practices for Respiratory Virus Testing. Journal of Clinical Microbiology 2011;49(9 Suppl):S44–48. [Google Scholar]
- 72.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159(1):179–187. [DOI] [PubMed] [Google Scholar]