Abstract
In humans, circulating levels of the hormone melatonin and the initiation of spontaneous labor are both higher at night than during the day. Since activation of uterine melatonin receptors can stimulate human in vitro uterine contractions and these receptors are only expressed on the uterine tissue of women in labor, we hypothesized that circulating melatonin concentrations would affect uterine contractions in vivo. We evaluated the impact of light-induced modulation of melatonin secretion on uterine contractions in women during late third-trimester (~36–39 weeks) of pregnancy in two inpatient protocols. We found a significant (p<0.05) positive linear association between circulating melatonin concentrations and the number of uterine contractions under both protocols. On average, uterine contractions increased between 1.4 to 2.1 contractions per 30 minutes for every 10 pg/ml*h increase in melatonin concentration. These findings have both basic science and clinical implications for pregnant women, since endogenous melatonin levels and melatonin receptor activity can be altered by light and/or pharmaceutical agents.
Keywords: Melatonin, uterus, pregnancy, contractions, light, circadian, human
Introduction
In humans, spontaneous labor in term pregnancies is more often initiated, and more babies are born at night [1–4], a time when the pineal gland secretes the hormone melatonin into the circulation. Melatonin receptor expression on the human pregnant uterus has been reported only during labor [5, 6]. We tested the association between melatonin concentrations and uterine contractions in late-term pregnant women by modulating circulating melatonin concentrations using ocular light exposure.
Melatonin levels follow a circadian rhythm, with high levels during the night and low baseline levels during the day in normally entrained individuals [7]. The same pattern is observed in both nocturnal and diurnal animals, suggesting that melatonin is the biological signal of darkness. Melatonin also has anti-oncotic [8], anti-nociceptive [9, 10], anti-oxidant [11, 12], and other physiological properties. Light exposure at night suppresses melatonin production at the pineal gland and lowers circulating melatonin concentrations [13]. Light-induced melatonin suppression is wavelength dependent with the highest response being induced by short wavelengths (i.e., blue or short-wavelength green light) and less by long wavelengths (i.e., red light) [14–17]. The response begins at the retina, mediated primarily by melanopsin containing intrinsically photosensitive retinal ganglion cells (ipRGCs), and modulated by the rods and cones that are responsible for vision [18–20]. Light exposure-dependent changes in circulating melatonin levels play a critical role in seasonally-reproducing animals, because the duration of nocturnal melatonin secretion reflects the photoperiod length that lengthens or shortens across the season and thereby conveys this seasonal information [21–23].
Melatonin-specific binding sites have been documented in the human and rodent uterus [24–26]. There is increased melatonin receptor mRNA expression, melatonin receptor protein expression, and melatonin-binding within the same in vitro samples of myometrial tissue from pregnant women in labor compared to myometrial tissue from pregnant women who are not in labor [5, 6]. Additionally, melatonin and oxytocin receptors in the human myometrium increase in parallel as a function of gestational age [5]. Melatonin and oxytocin have an additive effect on human myometrial cell contractions: 1nM melatonin plus 1nM oxytocin produce the same level of the contractility of myometrial cells in vitro as 100 nM oxytocin (100x the concentration) alone [27, 28]. Since circulating melatonin levels are high at night, such synergistic action between melatonin and known labor-inducing agents would be restricted to the night and may partially explain the higher incidence of spontaneous labor induction at night.
Interestingly, the modulatory role of melatonin on uterine contractions differs between nocturnal and diurnal animals. In both nocturnal and diurnal animals, melatonin secretion occurs at night [29, 30], but, in nocturnal rodents, birthing occurs when melatonin levels are low during the day. Consistent with this observation, pharmacological doses of melatonin directly inhibit both spontaneous and oxytocin-induced uterine contractility in rodents [31–34]. Female rats whose endogenous melatonin was eliminated by pinealectomy have no disturbances in estrous cyclicity or in their ability to become pregnant, but birthing is no longer exclusively during the daytime [35].
Since ocular light exposure modulates melatonin concentrations, and melatonin modulates uterine contractions, it is possible that light exposure can modulate uterine contractions by altering melatonin levels. A previous study reported decreased uterine contractions in humans during a brief 1 hour bright light exposure administered at midnight [5]. That study did not, however, control for inter-individual differences in habitual sleep-wake timing, which in turn influences endogenous circadian phase and therefore melatonin secretion rhythms. Additionally, all participants received the same bright light pulse, which was visibly different than the dimmer background ambient lighting that individuals received prior to the start of the bright light exposure, raising the possibility of a placebo response or some other non-melatonin related pathway mediating the observed changes in uterine contractions induced by the bright light exposure. Therefore, in the current study we used two different single-blind block-randomized design protocols (Figure 1A and Figure 2A); in both, individuals received either short-wavelength (λmax 507 nm) or long-wavelength (λmax 630 nm) light for 4 hours centered around each individual’s habitual bedtime, at a time when melatonin levels were expected to be high. In the first protocol (n=7), individuals were randomized to either spectral condition for one night (Figure 1A). In the second protocol (n=20), all individuals received long-wavelength light on the first night and were then randomized to either short- or long-wavelength light on the second night (Figure 2A). Circulating melatonin levels were assayed in saliva and uterine contractions were measured objectively using tocometry (Figure 1B-E, Figure 2B-E).
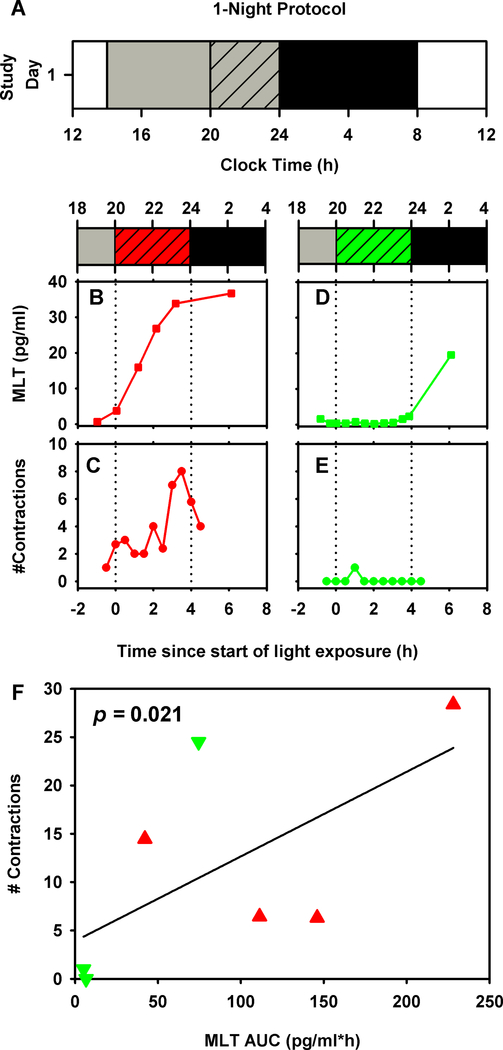
Figure 1:
Full term pregnant women were studied in a 1-night (n=8, panel A) inpatient protocol. White bars represent wake episodes under typical indoor light intensity (~90 lux), gray bars represent wake episodes under dim lighting (<15 lux), gray hashed bars represent 4-h experimental light exposure with either short- or long-wavelength visible light, and black bars represent sleep in dim lighting (<3 lux). Time-course of salivary melatonin levels under long- ( ) and short-wavelength light (
) and short-wavelength light ( ) and uterine contractions under long- (
) and uterine contractions under long- ( ) and short-wavelength light (
) and short-wavelength light ( ) are shown for one individual in each light exposure condition (panels B-E). Unadjusted linear regression results testing the association between uterine contractions and salivary melatonin AUC are shown (panel F). Corresponding uterine contractions and melatonin AUC values from each individual are shown under long- (
) are shown for one individual in each light exposure condition (panels B-E). Unadjusted linear regression results testing the association between uterine contractions and salivary melatonin AUC are shown (panel F). Corresponding uterine contractions and melatonin AUC values from each individual are shown under long- ( ) and short-wavelength light (
) and short-wavelength light ( ) conditions.
) conditions.
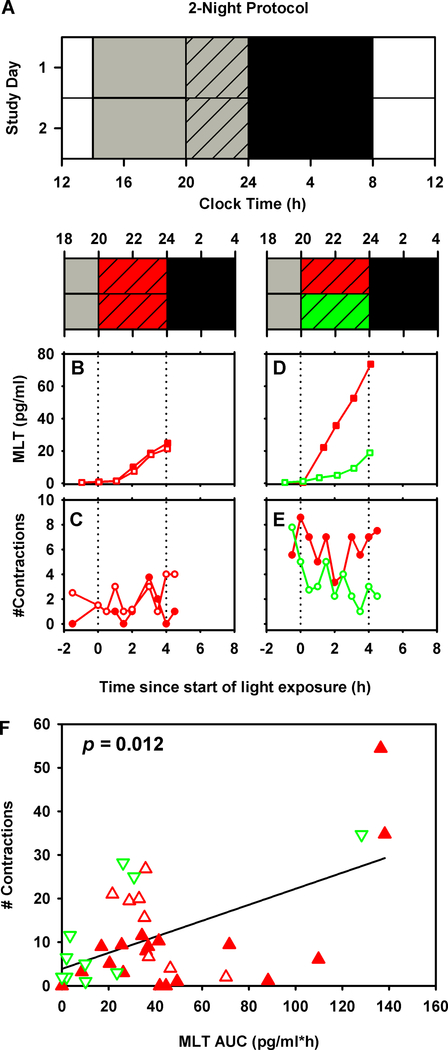
Figure 2:
Full term pregnant women were studied in a 2-night (n=17, panel A) inpatient protocol. White bars represent wake episodes under typical indoor light intensity (~90 lux), gray bars represent wake episodes under dim lighting (<15 lux), gray hashed bars represent 4-h experimental light exposure with either short- or long-wavelength visible light, and black bars represent sleep in dim lighting (<3 lux). Time-course of salivary melatonin levels under long- ( ) and short-wavelength light (
) and short-wavelength light ( ) and uterine contractions under long- (
) and uterine contractions under long- ( ) and short-wavelength light (
) and short-wavelength light ( ) are shown for one individual (panels B-E). Unadjusted linear regression results testing the association between uterine contractions and salivary melatonin AUC are shown (panel F). Corresponding uterine contractions and melatonin AUC values from each individual are shown under long- (
) are shown for one individual (panels B-E). Unadjusted linear regression results testing the association between uterine contractions and salivary melatonin AUC are shown (panel F). Corresponding uterine contractions and melatonin AUC values from each individual are shown under long- ( ) and short-wavelength light (
) and short-wavelength light ( ) conditions. Values from the first night are shown as filled symbols and values from the second night are shown as unfilled symbols.
) conditions. Values from the first night are shown as filled symbols and values from the second night are shown as unfilled symbols.
Materials and Methods:
We studied 33 pregnant women ages 18–35 [mean age (± SD): 27.0 ± 4.9 years] and weeks 35.7–39.1 of pregnancy [mean gestational age (± SD): 37.8 ± 1.0 weeks] across 2 different protocols (Figure 1A and B). The study was powered to detect a difference of 3 contractions between light conditions (2-night protocol) with 15 people per group (90% power, alpha=0.05) using a two-sided two-sample t-test. Data from eight women were not included in the final analysis (participants choosing not to complete the study n=4; missing tocometer data n=3; missing tocometer and salivary melatonin data n=1). This was the first singleton full-term pregnancy for all participants. They were not using medications that affect sleep or circadian rhythms. Additional predefined exclusion criteria included clinical or histological chorioamnionitis, rupture of membranes >12 h, abnormal vaginal discharge, or positive uterine cultures for β-strep, gonorrhea, trichomonas or syphilis, and pre-pregnancy BMI >35 kg/m2. Women provided their estimated sleep and wake times approximately one week prior to admission.
In the 1-night protocol, eight pregnant women ages 18–30 years and weeks 36.0–38.7 of pregnancy were studied in our inpatient facility at the Intensive Physiological Monitoring Unit of the Center for Clinical Investigations at the Brigham and Women’s Hospital in a single overnight visit (Figure 1A). To measure uterine contractions, a uterine tocometer (Corometrics® 170 Series Monitor and Corometrics® Nautilus Transducer, GE Medical Systems, MA, USA) was placed 4 hours prior to reported bedtime and was worn for 8 hours; ocular light exposure [randomized to short-wavelength (λmax 507 nm, target intensity: ~75 μW/cm2, ~250 lux, measured in the vertical plane) or long-wavelength (λmax 630 nm, target intensity: ~1.5 μW/cm2, ~3 lux, measured in the vertical plane), (Twin Tower Lo-Light, Sunnex Biotechnologies, Winnipeg, MB, Canada)] began at 2 hours prior to reported bedtime and lasted for 4 hours. Participants sat in a semi-recumbent posture ~1 m from the light source during light exposure times. Illuminance and radiometric measures were taken with illuminance meters and spectroradiometers [IL1400 radiometer/powermeter with an SEL-033/Y/W or SEL-033/F/W detector, respectively (International Light, Inc., Newburyport, MA)] next to the participant’s eye to monitor corneal exposure at 30 min intervals throughout the light exposure. Illuminance levels were adjusted to maintain target intensity by changing the distance of the participant from the light source. Participants were not informed that short-wavelength light was expected to be the active condition. Starting 6 h before the start of the monochromatic light exposure and ending at the completion of the 4-h light exposure interval, room lighting was a dim background ambient white light (<4 lux) (against which the monochromatic light was delivered) At all other times when the participant was awake, ambient lighting remained at typical indoor levels [23 μW/cm2 or (~88 lux) when measured in the vertical plane]. Ambient room lighting was generated using ceiling-mounted 4100K fluorescent lamps (F96T12/41U/ HO/EW, 95W; F32T8/ADV841/A, 32W; F25T8/TL841, 25W; Philips Lighting, The Netherlands) with digital ballasts (Hi-Lume 1% and Eco-10 ballasts, Lutron Electronics Co., Inc., Coopersburg, PA) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, Pittsfield, MA). Participants were asked every 30 minutes when they were awake to document the number of contractions they had felt. Participants went to sleep 2 hours after their reported bedtime. Saliva was collected every hour when the participant was awake and was assayed for melatonin. Melatonin assays were carried out by a laboratory blind to the study conditions using standard RIA (Specialty Assay Research Core, BWH, Boston, MA). One nurse blinded to the study conditions reviewed all tocometer strips and indicated time of onset and offset of contractions.
In the 2-night protocol, 25 pregnant women ages 18.8–35 and weeks 35.7–39.1 of pregnancy were studied in the same inpatient facility for two consecutive overnights (Figure 1B). All procedures were identical except the tocometry starting 6 h prior to reported bedtime and was worn for 16 h. All women received long-wavelength light exposure on the first night; women were randomized to long-wavelength or short-wavelength light exposure on the second night.
Contraction data were summed across the 4-h light exposure interval. Area under the curve (AUC) was calculated for the melatonin time-course across the 4-h light exposure interval. Contraction data missed due to movement of the uterine tocometer was adjusted for the percentage of duration missed within each 30-min data collection block across the 4-h interval. If 60% or more of the tocometer data were missing then the participant was not included in the final analysis. Summed contraction and melatonin AUC were correlated using univariate and multivariate linear regression in the 1-night protocol. The same outcome measures were correlated using repeated measures mixed-model linear regression in the 2-night protocol. Change in contraction and melatonin AUC induced by light exposure and study day were assessed using mixed model regression analysis. Residuals from regression analyses were confirmed to be normally distributed (Shapiro-Wilkinson test) and data were tested for homogeneity of variance before comparing means. Non-normal melatonin AUC data were square-root transformed prior to analysis. All statistical analyses were carried out using SAS 9.4 (SAS, Carry, NC). Investigators conducting the analysis or randomization of the subjects were not blinded to the study conditions.
Results
There was a significant positive linear relationship between the tocometer-recorded number of contractions and Area Under the Curve (AUC) of salivary melatonin during the 4-hour experimental intervention in both protocols (1-night protocol: p=0.021; 2-night protocol: p=0.012). In the 1-night protocol, we found that contractions increased by 2.3 contractions for every 10 pg/ml*h change in melatonin (Figure 1F). Similarly, in the 2-night protocol we found that contractions increased by 1.4 contractions for every 10 pg/ml*h change in melatonin (Figure 2F). Other potential covariates tested were maternal age and gestational age. In the 1-night protocol, the relationship between melatonin and contractions was still significant after adjustment for maternal age (p=0.022, adjusted R-squared=0.49). Since only maternal age was significant in the 1-night protocol, this was tested as a potential covariate in the 2-night protocol, but no significant effect was observed and therefore it was removed from the final model. There was no relationship between either AUC of melatonin and self-reported number of contractions, or between tocometer-measured contractions and self-reported number of contractions.
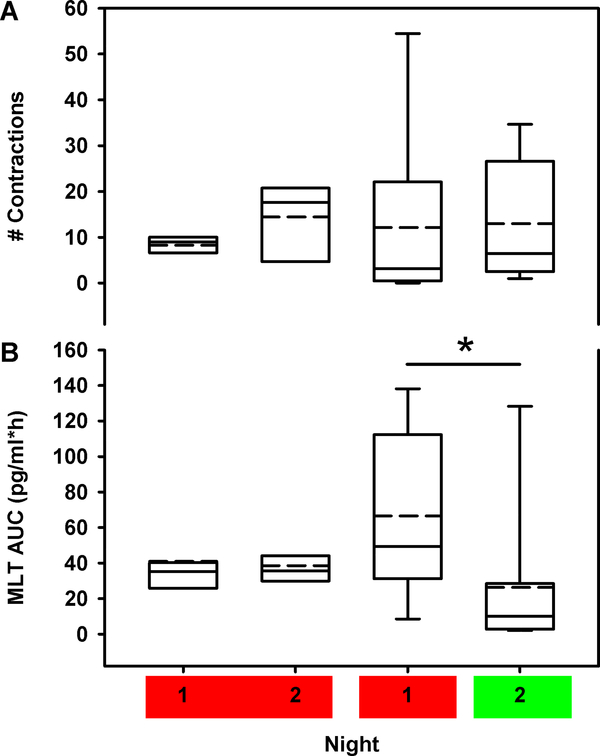
The 2-night protocol allowed both within-subject testing of the daily variability in both the number of uterine contractions and melatonin concentration, and comparison of the effects of short-wavelength and long-wavelength light uterine contractions and melatonin. The number of uterine contractions was the same on the first night in the groups that received short-wavelength or long-wavelength light in night 2 (p=0.54) (Figure 3A), demonstrating that the primary outcome measure was similar at baseline between the two groups. The change in the number of uterine contractions across the two nights did not differ for either light-exposure condition (p=0.30) (Figure 3A). Given the large inter-individual variability of melatonin concentration and the limited number of participants in this trial, we only had the statistical power to detect correlations between melatonin concentration and uterine contractions; we did not have statistical power to investigate the direct effects of light on uterine contractions. Likewise, circulating melatonin levels on the first night did not differ between the two groups (p=0.16) (Figure 3B). As expected, melatonin levels were unchanged when individuals received long-wavelength red light again on the second night (p=0.80) compared to the levels under long-wavelength light exposure on the first night, but decreased significantly (p=0.0003) when individuals received short-wavelength green light on the second night (Figure 3B). Therefore, while we did not find a difference in uterine contractions by lighting condition, lighting condition could affect melatonin concentrations and melatonin concentrations were correlated with uterine contractions.
Figure 3:
The box and whisker plots show the median (solid horizontal line), 25th and 75th percentile (box limits), the 10th and 90th percentiles (whiskers), and the mean (dashed horizontal line) for uterine contractions (A) and melatonin AUC (B) for the each of the two nights in the 2-night protocol under the long- (left two box plots) and short-wavelength (right two box plots) light exposure conditions. Data were compared between light exposure conditions and study days using repeated measures mixed-model regression. * Signifies p<0.0001 between the first and second night within a light exposure condition.
Discussion
These data document a positive relationship between melatonin concentrations and uterine contractions in women after ~35 weeks of pregnancy. Agents that change melatonin levels, including light exposure, may be used to modulate uterine contractions. There are several limitations to our study that merit future work. (i) Our limited data set do not support a direct effect of light exposure on uterine contractions, additional studies of sufficient statistical power are required to test this. (ii) Our current study does not allow us to differentiate between Braxton Hicks contractions and other pre-labor contractions that may mediate uterine priming for delivery. (iii) The impact of changing contraction frequency by modulating melatonin levels cannot be assessed based on our data. (iv) Although we collected labor and delivery information as part of the safety monitoring protocol for the study, they were not included as analytic endpoints. Therefore, additional studies are required to elucidate the clinical relevance of the change in contractions assessed in the current study.
There are several important implications of these findings. Given the ubiquity and increasing intensity of nocturnal exposure to artificial light, pregnant women may be inadvertently adversely affecting labor progression and childbirth via exposure to light or common pharmacologic agents that suppress melatonin secretion. Moreover, this discovery may provide a new mechanism for therapeutically influencing the timing of labor and childbirth, since endogenous melatonin levels can be suppressed by both light and pharmacologic agents, and melatonin receptors can be activated by melatonin and melatonin agonists.
There are many potential applications of such physiology in humans and other animals, including suppressing uterine contractions or pre-term labor using light exposure or melatonin antagonists; reducing melatonin-suppressing light exposure during normal labor; and/or inducing labor with or without oxytocin using melatonin or its agonists. In Western societies, preterm labor occurs in more than 12% of all pregnancies [36]. It remains the major cause of perinatal morbidity and is associated with 70% of neonatal mortality [36]. According to the Institute of Medicine in 2007, the economic burden of preterm births in the United States is over $26 billion per year (>$50,000 per infant) [37]. Most preterm births occur in women with no known significant risk factors; all pre-term etiologies, however, result in premature contractions [37]. If uterine melatonin receptors are expressed and/or activated during pregnancy, they may provide a key hormonal event in the initiation of labor; blocking these receptors may be an effective treatment in pre-term or late-term labor. Additionally, preterm neonates undergo extensive clinical intervention during intensive care that exposes them to nociceptive stimuli at a time when it is developmentally unexpected [9]. A recent meta-analysis [10] documents the anti-nociceptive properties of melatonin suggesting a potential role of exogenous melatonin in reducing pain in preterm neonates. Moreover, prophylactic use of exogenous melatonin, which has been well documented as an antioxidant, may potentially ameliorate oxidative-stress related complications during pregnancy and delivery (reviewed in [11, 12]). Therefore, tuning the intensity and spectral characteristics of light to prevent melatonin suppression induced by ambient light exposure at-home and/or in the clinical setting could be beneficial both to the mother and child around the time of labor and delivery.
In addition, duration of labor in pregnant women has become longer in modern obstetrical cohorts [38]. Data from pregnancies at term, in spontaneous labor with cephalic, singleton fetuses were compared between the Collaborative Perinatal Project (CPP, n=39,491 delivering 1959–1966) and the Consortium on Safe Labor (CSL; n=98,359 delivering 2002–2008): that the first stage of labor in the ‘02–08 CSL cohort was 2.6 hours longer in nulliparas and 2.0 hours in multiparas, even after adjusting for maternal and pregnancy characteristics, suggesting that the prolonged labor is mostly due to changes in clinical practice patterns. While various biological factors may contribute to this difference (e.g., BMI, age), other environmental changes may also be involved. Specifically, the orders of magnitude increase in per capita exposure to artificial light [39], which mostly occurs after dusk during this same ~50-year interval may contribute to lengthening labor, since light suppresses melatonin secretion. The converse may also be beneficial: giving melatonin or its agonists alone, before or with oxytocin to stimulate or shorten labor.
Acknowledgements:
Harvard Milton Fund #520.45414.624473.730001.0000.67800, NIH K24-HL105664, NIH-NHLBI T32- HL07901, NIH R21-HD086392, NIH UL1-TR001102, NIH P01-AG009975.
Footnotes
Conflicts of Interest:
JNR and CB: no competing interests. SAR: Potential conflicts of interest include patents for prevention of circadian rhythm disruption by using optical filters and improving sleep performance in subject exposed to light at night; SAR owns equity in Melcort Inc.; SAR is a co-investigator on studies sponsored by Biological Illuminations, LLC; Vanda Pharmaceuticals Inc. SAR has provided paid consulting services to Sultan & Knight Limited; Bambu Vault, LLC. JO has patents in the area of photic control of uterine contractions and is a pro bono member of the Board of KynderMed, Inc. (a Women’s Health Company). CAC has received consulting fees from or served as a paid member of scientific advisory boards for: Columbia River Bar Pilots; Ganésco Inc.; Institute of Digital Media and Child Development; Klarman Family Foundation; Samsung Electronics; Vanda Pharmaceuticals; and Washington State Board of Pilotage Commissioners and Zurich Insurance Company, Ltd. Dr. Czeisler has also received education/research support from Optum, Philips Respironics, Inc., San Francisco Bar Pilots, Schneider Inc., Sysco, and Vanda Pharmaceuticals. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine, and the Sleep Matters Initiative (which Dr. Czeisler directs) have received funding for educational activities from Cephalon, Inc., Jazz Pharmaceuticals, ResMed, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries Ltd., Sanofi-Aventis, Inc., Sepracor, Inc., Wake Up Narcolepsy, and Mary Ann & Stanley Snider via Combined Jewish Philanthropies. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, South Carolina Central Railroad Co., Steel Warehouse Inc., Stric-Lan Companies LLC and United Parcel Service (UPS). Dr. Czeisler owns or owned an equity interest in Vanda Pharmaceuticals. He received royalties from Houghton Mifflin Harcourt/Penguin, McGraw Hill and Koninklijke Philips Electronics, N.V. for the Actiwatch-2 and Actiwatch-Spectrum devices. EBK: Potential conflicts of interest include consulting for Pfizer and travel support from the Sleep Research Society and the National Sleep Foundation. SAR, CB, CAC, JNR and EBK interests are reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
All participants gave informed consent. All protocols were reviewed and approved by Partners Human Research Committee. This protocol was registered on ClinicalTrials.org: NCT01863446.
Data Availability. The data reported in this study are available on request from the corresponding author [SAR].
References:
- 1.Glattre E, Bjerkedal T (1983). The 24-hour rhythmicity of birth. A populational study. Acta Obstet Gynecol Scand, 62(1), 31–36. [DOI] [PubMed] [Google Scholar]
- 2.Cooperstock M, England JE, Wolfe RA (1987). Circadian incidence of labor onset hour in preterm birth and chorioamnionitis. Obstet Gynecol, 70(6), 852–5. [PubMed] [Google Scholar]
- 3.Panduro-Baron G, Gonzalez-Moreno J, Hernandez-Figueroa E (1994). The biorhythm of birth. Int J Gynaecol Obstet, 45(3), 283–4. [DOI] [PubMed] [Google Scholar]
- 4.Lindow SW, Jha RR, Thompson JW (2000). 24 hour rhythm to the onset of preterm labour. BJOG, 107(9), 1145–8. [DOI] [PubMed] [Google Scholar]
- 5.Olcese J, Lozier S, Paradise C (2012). Melatonin and the circadian timing of human parturition. Reprod Sci, 20(2):168–174. [DOI] [PubMed] [Google Scholar]
- 6.Olcese J, Beesley S (2014). Clinical significance of melatonin receptors in the human myometrium. Fertil Steril, 102(2), 329–35. [DOI] [PubMed] [Google Scholar]
- 7.Czeisler CA, Buxton OM Human circadian timing system and sleep-wake regulation In: Principles and practice of sleep medicine. Kryger MH, Roth T, Dement WC eds., W.B. Saunders Company, Philadelphia, 2017; pp. 362–376. [Google Scholar]
- 8.Li Y, Li S, Zhou Y et al. (2017). Melatonin for the prevention and treatment of cancer. Oncotarget, 8(24), 39896–39921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gitto E, Pellegrino S, Manfrida M et al. (2012). Stress response and procedural pain in the preterm newborn: The role of pharmacological and non-pharmacological treatments. Eur J Pediatr, 171(6), 927–33. [DOI] [PubMed] [Google Scholar]
- 10.Zhu C, Xu Y, Duan Y et al. (2017). Exogenous melatonin in the treatment of pain: A systematic review and meta-analysis. Oncotarget, 8(59), 100582–100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marseglia L, D’angelo G, Manti S et al. (2016). Potential utility of melatonin in preeclampsia, intrauterine fetal growth retardation, and perinatal asphyxia. Reprod Sci, 23(8), 970–7. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Korkmaz A et al. (2014). Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update, 20(2), 293–307. [DOI] [PubMed] [Google Scholar]
- 13.Weaver DR, Lockley SW Melatonin regulation of circadian rhythmicity in vertebrates. In: Encyclopedia of neuroscience, 2009; pp. 721–732. [Google Scholar]
- 14.West KE, Jablonski MR, Warfield B et al. (2011). Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol, 110(3), 619–626. [DOI] [PubMed] [Google Scholar]
- 15.Gooley JJ, Rajaratnam SMW, Brainard GC et al. (2010). Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med, 2(31), 31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rüger M, St Hilaire MA, Brainard GC et al. (2013). Human phase response curve to a single 6.5h pulse of short-wavelength light. J Physiol, 591(Pt 1), 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brainard GC, Sliney D, Hanifin JP et al. (2008). Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms, 23(5), 379–386. [DOI] [PubMed] [Google Scholar]
- 18.Roenneberg T, Daan S, Merrow M (2003). The art of entrainment. J Biol Rhythms, 18(3), 183–194. [DOI] [PubMed] [Google Scholar]
- 19.Lucas RJ, Douglas RH, Foster RG (2001). Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci, 4(6), 621–626. [DOI] [PubMed] [Google Scholar]
- 20.Hattar S, Liao H-W, Takao M et al. (2002). Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science, 295, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karsch FJ, Bittman EL, Foster DL et al. Neuroendocrine basis of seasonal reproduction. In: Proceedings of the 1983 laurentian hormone conference Greep RO ed., Academic Press, Orlando, Florida, 1984; pp. 185–232. [DOI] [PubMed] [Google Scholar]
- 22.Bittman EL, Kaynard AH, Olster DH et al. (1985). Pineal melatonin mediates photoperiodic control of pulsatile luteinizing hormone secretion in the ewe. Neuroendocrinology, 40(5), 409–418. [DOI] [PubMed] [Google Scholar]
- 23.Bartness TJ, Powers JB, Hastings MH et al. (1993). The timed infusion paradigm for melatonin delivery; what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res, 15(4), 161–190. [DOI] [PubMed] [Google Scholar]
- 24.Cohen M, Roselle D, Chabner B et al. (1978). Evidence for a cytoplasmic melatonin receptor. Nature, 274(5674), 894–5. [DOI] [PubMed] [Google Scholar]
- 25.Olcese J Melatonin receptors in the human reproductive tract In: Endocrinology ii: Lectures on the temporal structure of endocrine systems. Peschke E ed., Saxon Academy of Science, Leipzig, 2005; pp. 47–56. [Google Scholar]
- 26.Schlabritz-Loutsevitch N, Hellner N, Middendorf R et al. (2003). The human myometrium as a target for melatonin. J Clin Endocrinol Metab, 88(2), 908–13. [DOI] [PubMed] [Google Scholar]
- 27.Sharkey JT, Puttaramu R, Word RA et al. (2009). Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J Clin Endocrinol Metab, 94(2), 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharkey JT, Cable C, Olcese J (2010). Melatonin sensitizes human myometrial cells to oxytocin in a protein kinase c alpha/extracellular-signal regulated kinase-dependent manner. J Clin Endocrinol Metab, 95(6), 2902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ralph CL, Mull D, Lynch HJ et al. (1971). A melatonin rhythm persists in rat pineals in darkness. Endocrinology, 89, 1361–1366. [DOI] [PubMed] [Google Scholar]
- 30.Dijk DJ, Cajochen C (1997). Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep eeg. J Biol Rhythms, 12(6), 627–635. [DOI] [PubMed] [Google Scholar]
- 31.Hertz-Eshel M, Rahamimoff R (1965). Effect of melatonin on uterine contractility. Life Sci, 4(14), 1367–72. [DOI] [PubMed] [Google Scholar]
- 32.Burns JK (1972). Effects of melatonin on some blood constituents and on uterine contractility in the rat. J Physiol, 226(2), 106P–107P. [PubMed] [Google Scholar]
- 33.Gimeno MF, Landa A, Sterin-Speziale N et al. (1980). Melatonin blocks in vitro generation of prostaglandin by the uterus and hypothalamus. Eur J Pharmacol, 62(4), 309–17. [DOI] [PubMed] [Google Scholar]
- 34.Abd-Allah AR, El-Sayed El SM, Abdel-Wahab MH et al. (2003). Effect of melatonin on estrogen and progesterone receptors in relation to uterine contraction in rats. Pharmacol Res, 47(4), 349–54. [DOI] [PubMed] [Google Scholar]
- 35.Takayama H, Nakamura Y, Tamura H et al. (2003). Pineal gland (melatonin) affects the parturition time, but not luteal function and fetal growth, in pregnant rats. Endocr J, 50(1), 37–43. [DOI] [PubMed] [Google Scholar]
- 36.Smith R (2007). Parturition. N Engl J Med, 356(3), 271–83. [DOI] [PubMed] [Google Scholar]
- 37.In: Preterm birth: Causes, consequences, and prevention. Behrman RE, Butler AS eds., Washington (DC), 2007. [PubMed] [Google Scholar]
- 38.Laughon SK, Branch DW, Beaver J et al. (2012). Changes in labor patterns over 50 years. Am J Obstet Gynecol, 206(5), 419 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czeisler CA (2013). Perspective: Casting light on sleep deficiency. Nature, 497(7450), S13. [DOI] [PubMed] [Google Scholar]