Abstract
Prospective memory (PM), “remembering to remember,” has been linked to important functional outcomes in adults. Studies of PM in children and adolescents would benefit from the development and validation of developmentally appropriate clinical measures with known psychometric properties. The Prospective Memory Assessment for Children & Youth (PROMACY), a performance-based measure of PM, was developed for the Pediatric HIV/AIDS Cohort Study Adolescent Master Protocol, Memory and Executive Functioning Substudy, and includes Summary, Time-, and Event-based scores derived from eight trials with an ongoing word search task. Fifty-four healthy perinatally HIV-exposed, uninfected children and youth, mean age 13 years, 54% female, 76% Black/non-Hispanic, and 61% impoverished were included in this psychometric analysis. PROMACY Summary Scores demonstrated low, but broadly acceptable internal consistency as measured by Cronbach’s alpha and Spearman-Brown. Better PROMACY performance was associated with older age, but no other demographic factors. Generally medium-sized correlations were observed between the PROMACY Summary Score and standard clinical measures of retrospective memory, working memory, executive functions, and IQ. Findings from this preliminary psychometric study of non-clinical children and youth provide cautious support for the internal consistency and construct validity of PROMACY’s Summary Score that awaits replication and extension in larger samples of healthy children, youth and clinical populations.
Keywords: prospective memory, children, adolescents, internal consistency, reliability
Prospective Memory (PM) describes the complex process of “remembering to remember,” which plays an integral role in the execution of many activities of daily living and health behaviors (e.g., Zogg et al., 2010), including medication adherence (i.e., Poquette et al., 2013; Woods et al., 2008a). PM is vulnerable to a wide range of conditions that affect the central nervous system, such as HIV (e.g., Carey et al., 2006; Harris et al., 2017). PM can be viewed as an umbrella construct, much like executive functions, in that its cognitive architecture is diverse and it is comprised of many different component processes that together exceed the sum of their parts. Although there was early controversy around the uniqueness of PM as a construct (e.g., Cockburn & Smith, 1991), it is now fairly well accepted that PM is separable from retrospective memory and executive functions at the levels of theory (e.g., McDaniel, Umanath, Einstein & Waldum, 2015), measurement (e.g., Gupta et al., 2010), neurobiology (e.g., Woods et al., 2006), and everyday functioning (Kamat et al., 2014) in adults. In other words, PM is reliant upon executive functions and prefrontal systems (Simons, Scholvinck, Gilbert, Frith, & Burgess, 2006; Wiley et al., 1998), as well as retrospective memory and medial temporal networks (Martin et al., 2007), but is nevertheless a unique and separable construct, consideration of which can afford incremental ecological validity (e.g., Woods et al., 2009).
According to widely-cited conceptual models of PM (e.g., Kliegel, Mackinlay, & Jager, 2008; Kliegel, Ropeter, & Mackinley, 2006; McDaniel & Einstein, 2007), “remembering to remember” requires several interrelated processes. These include encoding an intention to be performed in the future (e.g., take medication after dinner) in the presence of a given cue (or time), maintaining that cue – intention pairing in memory over time and during an ongoing activity (e.g., daily routines), monitoring the environment for the cue (or time), detecting and recognizing the cue (or time) when present (e.g., finished dinner), retrieving the intention from retrospective memory, and finally executing the intention as planned (e.g., take medication). PM cues are commonly classified as event-based (e.g., taking medication after dinner) or time-based (e.g., taking medication in an hour). Since event- and time-based PM place different demands on strategic/monitoring processes (Kerns, 2000; Mahy & Moses, 2011; McDaniel & Einstein, 2000) and may require different types of interventions, assessing both event-based and time-based PM is ideal. Yet there are very few clinical instruments available to assess PM, particularly in children and youth.
The Memory for Intentions Screening Test (MIST; Raskin, Buckheit, & Sherrod, 2010) is a standardized measure of PM in adults with strong evidence of construct validity that may provide a viable foundation to develop a validated PM measure for children and youth. The MIST involves performing eight different delayed PM tasks over a 30-minute period, while simultaneously engaged in word-search puzzles that serve as the ongoing task. PM trials are balanced in terms of delay interval (i.e., either a 2-minute or 15-minute delay), response cue (i.e., either Time-Based or Event-Based), and response mode (i.e., verbal or action). Errors are coded according to a detailed scoring system, which operationalizes common errors of omission (e.g., no response) and commission (e.g., task substitution errors). The MIST includes a multiple choice recognition post-test from which a retrieval index is calculated (see Carey et al., 2006). Finally, the MIST includes a 24-hour delayed recall task (e.g., Carey et al., 2006). The MIST has good discriminative (Carey et al., 2006; Woods, Twamley, Dawson, Narvaez, & Jeste, 2007b), convergent/divergent (Bezdicek, Raskin, Altgassen, & Ruzicka, 2014; Carey et al., 2006; Coulehan et al., 2014; Kamat et al., 2014; Raskin, 2009), and ecological validity (Woods et al., 2008b). In adults, better MIST performance is associated with younger age (e.g., Kamat et al., 2014; Raskin et al., 2010), higher educational attainment (e.g., Bezdicek et al., 2014), and gender (e.g., Bezdicek et al., 2014; Palermo et al., 2016). The MIST demonstrates strong inter-rater reliability, internal consistency, and generally satisfactory inter-relationships between the summary score, subscales, and error types (Bezdicek et al., 2014; Woods et al., 2008b). It has played a significant role in our understanding of PM in HIV (e.g., Carey et al., 2006; Poquette et al., 2013; Woods et al., 2008a; Woods et al., 2010), aging (e.g., Kamat et al., 2014), Huntington’s disease (e.g., Nicoll et al., 2014), and Parkinson’s disease (e.g., Raskin et al., 2011) in adults.
Currently, there are no clinical measures for children and adolescents that evaluate both Time- and Event-based PM and possess the strong psychometric properties of the MIST. The literature on PM in children (e.g., Kerns, 2000; Kliegel et al., 2008; Kvavilashvili, Messer, & Ebdon, 2001; Ward, Shum, McKinlay, Baker-Tweney, & Wallace, 2005; Zimmermann & Meier, 2006) supports the need for developmentally appropriate measures of PM. PM is apparent as early as preschool ages and shows developmental progression through childhood, with more advanced PM skills evident in early adolescence (Kliegel et al., 2013; Mahy, Kliegel, & Marcovitch, 2014; Voigt et al., 2014; Yang, Chan, & Shum, 2011). Additionally, an age effect in PM performance has been observed between adolescents and young adults (Zöllig et al., 2007) that may be contingent upon how related (i.e., focal) versus unrelated cues are to ongoing background activities (Wang et al., 2011). There also appear to be links between the development of PM and other executive functions such as inhibition and working memory across childhood (Kliegel et al., 2013; Kretschmer, Voigt, Friedrich, Pfeiffer, & Kliegel, 2014; Mahy et al., 2014; Mäntylä, Carelli, & Forman, 2007).
The scant literature regarding PM in pediatric conditions and diseases suggests PM performance deficits are associated with attention deficit/hyperactivity disorder (Kerns & Price, 2001; Kliegel et al., 2006), traumatic brain injury (McCauley & Levin, 2004; McCauley, McDaniel, Pedroza, Chapman, & Levin, 2009; Ward, Shum, McKinlay, Baker, & Wallace, 2007), sickle cell disease (McCauley & Pedroza, 2010), diabetes mellitus (Osipoff, Dixon, Wilson, & Preston, 2012), juvenile myoclonic epilepsy (Wandschneider et al., 2010), autism (Altgassen, Williams, Bölte, & Kliegel, 2009), and pediatric HIV disease (Harris et al., 2017). Notably, there appear to be virtually no studies on the relationship of PM to other important pediatric outcomes, such as academic performance, socioemotional functioning, or adherence to medical treatment regimens (cf., Sirois et al., 2016). Limitations of prior PM research in children (e.g., see Kerns, 2000) include but are not limited to: “Restricted range of outcome” (p. 63) due to use of single-item or small response sets; the need to balance the amount of information to be recalled (i.e., intentions), with the difficulty and/or relevance of the ongoing distractor task to be interrupted; and, lack of a validated age-appropriate measure. Kerns (2000) also indicated that the ideal PM task should engage the participant from intention formation to execution, be equally motivating to all participants, reduce the risk that the intention is remembered but not executed while simultaneously successfully distract from the to-be-remembered intention, and represent everyday intentions (Kerns, 2000, p. 68).
The paucity of research on PM in children and adolescents may be in part due to the lack of an available, psychometrically validated PM measure specifically designed for use with these age groups. Reliable and valid measurement of PM is a notoriously difficult undertaking, with strong opinions that abound on: the use of symptom-based questionnaires that can be influenced by mood and insight but capture manifest functioning; single-trial naturalistic tasks that have glaring psychometric weaknesses but have ecological relevance; mechanistic experimental measures that may be too narrowly focused to capture the nuances of PM in daily life but enhance our understanding of the construct; and, multifactorial clinical tasks that are not considered “pure” measures of PM but have shaped our understanding of PM and its daily functioning impact in neuropsychological populations (e.g., Uttl & Kibreab, 2011). According to McDaniel & Einstein (2007), the cardinal features of a PM task are that it must: 1) involve the execution of a delayed intention; 2) include an ongoing task, which is sometimes referred to as a distractor task; and, 3) the intention must be executed in a constrained window of time. Thus, it is difficult to develop a task that is brief enough to utilize in clinic, that includes an adequate number of intentions for reliable measurement, and at the same time has sufficiently long delays to dissociate the measure from attention/vigilance (see Woods et al., 2008b). It is our view that the MIST hits the mark in this regard. Unlike many of its experimental counterparts that attempted to isolate PM by including intentions (which by their very nature require encoding and retrieval from retrospective memory) by requiring simple motor (action) responses (e.g., a keyboard press), the MIST developers used clinically rooted cue-intention pairings in order to enhance the MIST’s ecological relevance (e.g., When I show you a request for records form, write your doctor’s name on it). Given the MIST’s inclusion of both Event-based and Time-based components of PM, sound psychometric properties and association with functional outcomes in adults, it is an appropriate instrument to be considered as a basis for the development of a PM measure for use with children and adolescents. Herein we describe the development and validation of the Prospective Memory Assessment for Children & Youth (PROMACY), a measure of PM informed by the MIST and designed for use with children and adolescents ages 8–21.
Two prior studies have used PROMACY in the setting of pediatric HIV disease. Harris et al. (2017) demonstrated that youth with HIV and neurocognitive disorders demonstrated significantly lower PROMACY performance across the Summary, Time-based, and Event-based scales compared to youth with HIV without neurocognitive disorders and to youth without HIV. Age at greatest HIV disease severity also was associated with PROMACY scores in this sample. In a related study of youth with and without HIV, Sirois et al. (2016) found that lower PROMACY scores (i.e., Summary, Time-based, and Event-based) were associated with poorer adaptive behavior, word reading and numerical operations, independent of age and caregiver factors (e.g., education). Thus, while PROMACY shows preliminary evidence of discriminant and ecological validity in pediatric HIV disease, little is known about its psychometric properties in healthy children and adolescents. Evaluating the internal consistency, scale interrelationships, demographic correlates, and convergent validity using data derived from healthy samples is an important next step in understanding the psychometric properties of any new neuropsychological measure intended for use in clinical samples (see Delis, Jacobson, Bondi, Hamilton, & Salmon, 2003).
Method
Participants
Participants were enrolled in the Memory and Executive Functioning substudy (herein referred to as the Memory Study) of the Pediatric HIV/AIDS Cohort Study Adolescent Master Protocol (AMP; https://phacsstudy.org). The parent AMP study is a prospective cohort-based investigation of the long-term effects of perinatal HIV infection and HIV treatments on biomedical and neurobehavioral outcomes in perinatally HIV-infected (PHIV) and perinatally HIV-exposed uninfected (PHEU) children and adolescents conducted at 15 sites in the United States and Puerto Rico. Participants were enrolled in the Memory Study at eight selected urban sites in the United States between 2010 and 2012. Eligibility criteria for the Memory Study included enrollment in the AMP parent study, age 9 to <19 years at entry, ability to participate in testing procedures, and fluency in English (given that some study measures were only available in English).
To examine the properties of PROMACY independent of the effects of HIV and to maximize generalizability of results to healthy child and adolescent populations, only data from the study’s healthy comparison sample (PHEU) were evaluated. The presence of brain injury can potentially influence the psychometrics of any given neuropsychological test (see Delis et al., 2003). As such, of the 75 PHEU participants who completed the Memory Study entry visit, three were excluded from analyses due to intellectual impairment (Full Scale Intelligence Quotient [FSIQ] <70), and 17 youth were excluded due to caregiver reported behavioral impairment on the Behavioral Assessment Systems for Children, Second Edition (Reynolds & Kamphaus, 2004, Behavioral Symptoms Index score ≥70). One additional youth met both intellectual and behavioral exclusions. Analyses reported herein include the remaining 54 healthy PHEU participants who completed PROMACY and the other Memory Study neurocognitive measures, and who represent an optimal seronegative sample within which to examine PROMACY’s psychometric properties (Delis et al., 2003).
Materials and Procedure
Institutional Review Boards at each participating Memory Study site and the Harvard T.H. Chan School of Public Health independently approved this study. Informed consent and youth assent were obtained for all participants according to local institutional guidelines. PROMACY was administered first within the larger Memory Study-specified standardized test battery of PM, retrospective memory, and executive functioning.
Measures
Prospective Memory (PM) – Primary Outcome Measure
Prospective Memory Assessment for Children & Youth (PROMACY)
PROMACY Development and Pilot Testing.
PROMACY, a performance-based test of PM that includes both Event-based and Time-based measures, was developed for the purpose of the Memory Study, adapted from the MIST (Raskin et al., 2010; described above) for use with children and adolescents ages 8–21 years, through agreement with Psychological Assessment Resources, Inc. (PAR, Inc.). To adapt the MIST for children and youth, the study team analyzed the cognitive and memory demands of each aspect of the test and made revisions in several areas. The longest delay interval was shortened from 15 to 10 minutes, thus shortening the overall length of the task from 30 to 20 minutes. Task questions were modified to be more relevant to children (i.e., “a piece of yellow paper” was substituted for “a Request for Records form”). Item vocabulary was reduced to an approximate fourth grade level, and all requests for written responses were simplified (e.g., write your name, number of pets you have). One challenge in developing PROMACY was to make it appropriate for a broad range of children and youth, who have naturally varying vocabulary/reading levels (which of course might be affected in clinical samples). In selecting a Grade 4 reading level, we rationalized that this reading level would provide an ideal balance to ensure sufficient variability in scores. Easier, more engaging word search puzzles also were created and presented in increasing difficulty as needed until the PROMACY task was completed. Finally, the 24-hour delay trial was eliminated. Initial PROMACY modifications were piloted with nine community-based healthy children. Two items were further modified and easier word search puzzles were added.
With these modifications in place, prior to implementation in the Memory Study, PROMACY was further pilot tested with a separate small, diverse group of participants (n = 29), at participating Memory Study sites. Note that, the pilot study participants did not overlap with the validation study sample detailed below. Pilot participants were of mean age 12.1 years (SD = 2.7; range = 8–17 years), 62% male, 48.3% White, non-Hispanic; 17.2% Black, non-Hispanic, and 20.6% Hispanic. Average school grade level was 6.3 (SD = 2.6; range = grades 3–12). Seventeen (58.6%) pilot participants had no reported emotional, behavioral or learning diagnosis, while one or more diagnoses were reported for the remaining 12 pilot participants (41.2%), including ADHD (n = 6; 20.7%), learning disability (n = 4, 13.8%), and emotional disorder and various other conditions (n = 2, 6.9%).
The pilot sample demonstrated a range of performance with mean PROMACY Summary Score = 36.5 (SD = 8.1; range, 18–48) out of 48 possible points. The Summary Score had a modest, though nonsignificant, positive association with age (ρ = .36, p = .16). Overall, the preliminary psychometric properties obtained were encouraging given the small heterogeneous sample from which data were collected. When considering cases without a reported diagnosis (n = 17), the overall Summary Score (M = 37.0; SD = 9.1; range 18–48) demonstrated modest internal consistency (Cronbach’s α = .68) given the small sample size, and respectable split-half reliability (Spearman-Brown r = .82). With these promising preliminary results, it was expected that PROMACY would possess at least adequate basic psychometric properties to measure PM in the proposed larger study.
The Final Study Version of PROMACY.
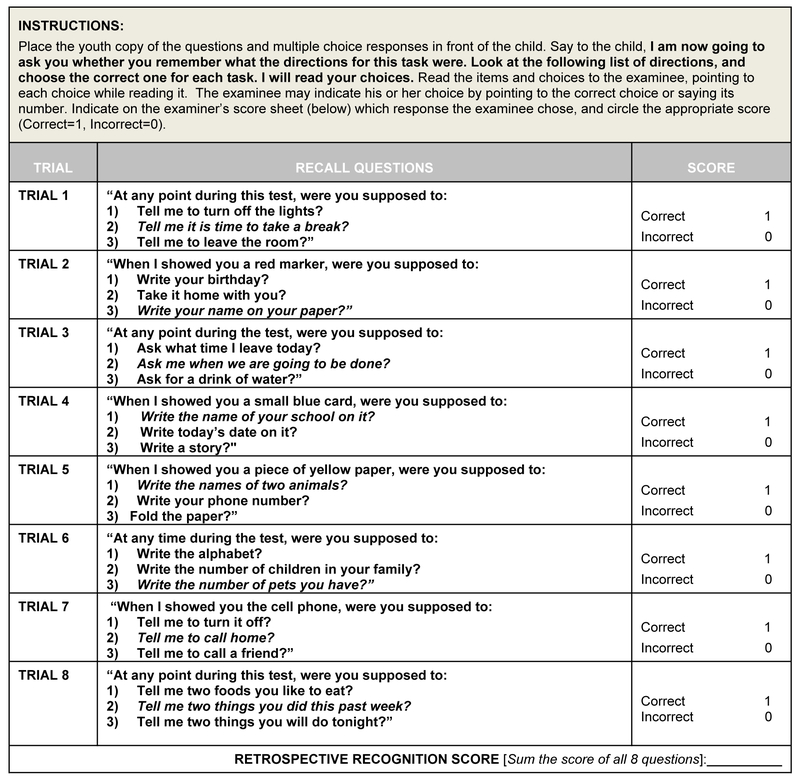
The final study version of PROMACY contains eight balanced PM items (see Table 1) that include a 2-minute or 10-minute delay, at which time a verbal response (e.g., “In 10 minutes tell me that it is time for a break”) or action (e.g., “When I show you a red pen, write your name on your paper”) is required, prompted by a Time-based cue (e.g., “In 2 minutes ask me when we’re going to be done”) or Event-based cue (e.g., “When I show you the small blue index card, write the name of your school on it”). The participant must remember to perform the specified verbal or action response while engaged in completing word search puzzles as a foreground distractor task. A digital clock was located behind the participant, but available to view at all times during the administration of this task. PROMACY is examiner-administered with items presented in time-specified intervals over a 20-minute period. During administration, the examiner codes each response for accuracy or error type(s) (see below). Immediately following PROMACY completion, an 8-item multiple choice recognition task is administered to assess retrieval (encoding or consolidation) failures, regardless of free-recall performance during PROMACY administration (also see, Woods et al., 2008b). The items on the recognition task include three response options for each trial; one option is the correct instruction given during the trial, while the other two are dummy responses.
Table 1:
Prospective Memory Assessment for Children & Youth (PROMACY): Trial Details and Descriptive Outcomes
| Order of Presentation |
Intention | Cue | Response Modality |
Time Delay (min) |
Order of Trial Execution |
Cognitive Load |
Mean Score |
SD | Cronbach α of PROMACY Summary Score with Trial Omitted |
|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | Tell me it is time to take a break. | Time | Verbal | 10 | 3 | 4 | 1.67 | 0.67 | .54 |
| Trial 2 | Write your name on your paper. | Event | Action | 10 | 4 | 3 | 1.81 | 0.48 | .63 |
| Trial 3 | Ask when we are going to be done. | Time | Verbal | 2 | 1 | 4 | 1.37 | 0.88 | .58 |
| Trial 4 | Write the names of two animals. | Event | Action | 10 | 5 | 3 | 1.72 | 0.56 | .61 |
| Trial 5 | Write the name of your school. | Event | Action | 2 | 2 | 4 | 1.63 | 0.65 | .55 |
| Trial 6 | Write the number of pets you have. | Time | Action | 10 | 8 | 1 | 1.93 | 0.38 | .59 |
| Trial 7 | Tell me to call home. | Event | Verbal | 2 | 6 | 2 | 1.74 | 0.59 | .57 |
| Trial 8 | Tell me two things you did this past week. | Time | Verbal | 2 | 7 | 2 | 1.11 | 0.86 | .52 |
Note: Min – minutes; Cognitive Load - the number of intentions to be retained until Trial execution; PROMACY – Prospective Memory Assessment for Children & Youth; SD – Standard deviation.
PROMACY’s Event-based and Time-based and two- and 10-minute trials are balanced (four of each) for which both verbal and action responses are required. Note that there is a higher number of action responses on the Time-based scale versus the Event-based scale, which differs from the MIST. Both Event-based and Time-based tasks are scored by assigning two points if the examinee performs the correct response to the correct cue or at the correct time; one point if s/he performs an incorrect response to the correct cue or at the correct time, or performs the correct response to an incorrect cue or at the incorrect time, or recognizes the cue/time but states (or otherwise indicates) s/he does not remember what to do; and, zero points if the examinee performs an incorrect response to an incorrect cue or at the incorrect time, or provides no response at all.
Error Types are coded as Omission/no response (no response provided); Task Substitution (incorrect response for an Event-based cue, or incorrect response given at the correct time for a Time-based cue); Loss of Content (recognition of the cue or recognition of the appropriate time to respond, but no recollection of the correct response itself, as indicated by the participant shaking his or her head “no,” or stating, “I forgot”); Loss of Time (correct response performed at the wrong time, with discrepancy greater than +/− 1 minute for 2-minute cues and greater than +/− 2 minutes for 10-minute cues); Place Losing Omission (only part of the task is performed because either the participant does not recall the entire task or becomes distracted prior to task completion); Random (errors that do not fit any of the other categories).
PROMACY includes six subscales based on cue type (i.e., Time- or Event-based, 2-minute or 10-minute) or response modality (i.e., action or verbal responses). Subscale scores range from 0–8 points and are derived by summing the four items that comprise each subscale (see Table 1). The PROMACY Summary Score ranges from 0–48 points and is calculated by summing the six subscale totals.
Neuropsychological Battery
The following battery of tests was administered in a specified order following completion of the PROMACY task.
Naturalistic Event-Based Prospective Memory Task (NEPT; McCauley et al., 2009; McCauley et al., 2011):
The NEPT is a measure of naturalistic PM previously used in studies of children with sickle cell disease (McCauley & Pedroza, 2010) and traumatic brain injury (McCauley et al., 2009; 2011). The NEPT task was embedded within a structured battery of standardized memory, executive function, attention and processing speed measures. For this task, the child is instructed to respond with, “Please give me three points,” each time the examiner gives the verbal cue, “Let’s try something different.” Three presentations of the verbal cue are provided at predetermined points during the ongoing testing tasks at approximately 15-minute intervals; the participant is to respond within five seconds of the cue presentation to be awarded points (although the participant is instructed to request three points, for scoring purposes, four points are assigned for each correct response). An Event-based score, a Retrospective Memory score and an Intention total score are obtained. The Event-based score (range 0–12 points) was the primary NEPT outcome of interest in relation to PROMACY.
Wide Range Assessment of Memory and Learning – 2nd Edition (WRAML2; Sheslow & Adams, 2003).
Two WRAML2 subtests were administered, Verbal Learning and Design Memory (including immediate recall, delayed recall, and recognition trials).
Wechsler Intelligence Scale for Children – 4th Edition (WISC-IV; Wechsler, 2003) or Wechsler Adult Intelligence Scale, 4th Edition (WAIS-IV; Wechsler, 2008).
Participants completed the age-appropriate version of the Digit Span and Coding subtests as proxies for working memory and processing speed, respectively, for the Memory Study. Within the parent study, all participants were administered subtests to obtain an FSIQ, and Working Memory Index (WMI), which were abstracted for these analyses (see Smith, et al., 2012).
Delis Kaplan Executive Function System (DKEFS; Delis, Kaplan, & Kramer, 2001).
Four DKEFS subtests were included as validation instruments with PROMACY: Verbal Fluency, Design Fluency, Color-Word Interference, and 20 Questions (ref. Strauss, Sherman, & Spreen, 2006).
Other Variables of Interest
Child and Caregiver Demographic Information.
Demographic information was collected in the parent study via interview with the primary caregiver and abstracted for these analyses, including: Child age, sex, race, ethnicity, and primary language, household income, caregiver relationship to the child, and caregiver education.
Statistical Analyses
PROMACY scores were summarized using descriptive statistics, and compared across subgroups defined by child socio-demographic characteristics, including sex (male/female), age group (9-<12, 12-<15, 15-<18, 18–19 years), race (Black/non-Black), and ethnicity (Hispanic/non-Hispanic) using Wilcoxon rank-sum tests. Frequency of error scores for various types of errors and total errors were tabulated. Internal consistency of PROMACY was evaluated using standardized measures of Spearman-Brown correlations and Cronbach’s alpha. Cronbach’s alpha coefficients were computed for the Summary Score, which included all 8 trials, and for each subscale score (Time-based, Event-based, Verbal response, Action response, 2-minute, 10-minute). Additionally, Cronbach’s alpha coefficients were computed for the Summary Score, deleting each respective individual trial from the computation demonstrating the relative contribution of each trial to the reliability of the Summary Score. Classically, internal consistency values above 0.7 are interpreted as acceptable, with values < 0.5 being unacceptable, although – as with critical alpha levels – there is much debate regarding the reasonable exceptions to these classic rules (Bhatnagar, Kim, & Many, 2014; George & Mallery, 2002). Due to skewed distribution of PROMACY scores, Spearman correlations were used to evaluate PROMACY scores against other measures of retrospective memory, executive functioning, and aspects of cognitive functioning to assess preliminary convergent construct validity. Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Included in analyses were 54 healthy youth without cognitive impairment or caregiver-reported behavioral impairment of mean age 13 years, who were 54% female, 76% Black, 24% Hispanic, with 61% living in poverty (annual household income <$20,000/year). Overall, 74% of caregivers had completed high school. Additional participant/caregiver characteristics are presented in Table 2.
Table 2:
PROMACY Child Demographic and Caregiver Characteristics
| Characteristic | Category | Mean (SD) or n (%) |
|---|---|---|
| Youth Age at PROMACY | 13.0 (2.6) | |
| Age Group | 9-<12 | 20 (37%) |
| 12-<15 | 22 (41%) | |
| 15-<18 | 10 (19%) | |
| 18-19 | 2 (4%) | |
| Gender | Male | 25 (46%) |
| Female | 29 (54%) | |
| Black Race | 41 (76%) | |
| Hispanic Ethnicity | 13 (24%) | |
| Household Income | $0- 20,000 | 33 (61%) |
| $20,001- 40,000 | 13 (24%) | |
| ≥$40,001 | 8 (15%) | |
| Caregiver is H.S. Graduate | 40 (74%) |
(n = 54). SD – Standard deviation; PROMACY – Prospective Memory Assessment for Children & Youth; H.S. – high school.
Summary statistics for PROMACY trials, the Summary Score and subscales are presented in Table 1 and 3, respectively. A series of Spearman-Brown correlations were computed to evaluate PROMACY’s internal consistency. The Spearman-Brown adjusted correlation for PROMACY (i.e., split-half reliability, even versus odd test items) was .67. Cronbach’s α coefficient for PROMACY’s Summary Score was .60. Cronbach’s α coefficients for the six individual PROMACY subscale scores tended to be lower than that of the Summary Score, ranging from α = .22-.64, with only the Time-based subscale (α = .64) rising above .6 (see Table 3). Cronbach’s α coefficients for the PROMACY Summary Score with deletion of each respective Trial ranged from α = .52 - .63 demonstrating the relative contribution of each trial to the reliability of the Summary Score as shown in Table 1. Cronbach’s α coefficients for the PROMACY Summary Score were also calculated within levels of each demographic variable and approached acceptable values for older youth (≥ 15 years, α = .58) and female gender (α = .57), and demonstrated acceptable values for Black race (α = .62), non-Hispanic ethnicity (α = .63), and male gender (α = .63). There were no significant differences in mean PROMACY Summary Score by levels of any demographic variable other than age. Older youth (≥ 15 years) scored higher on the PROMACY Summary Score than younger (< 15 years) participants (M = 44.5 vs. 37.4, respectively, p < .009).
Table 3.
Summary Statistics and Cronbach Alpha Coefficients for PROMACY Scores
| PROMACY Score | Possible Score Range |
Mean (SD) | Median | Interquartile Range (IQR) (25th, 75th percentiles) |
Standardized Cronbach Alpha |
|---|---|---|---|---|---|
| Summary | 0-48 | 38.94 (8.13) | 42.0 | (33, 45) | .60 |
| Time-Based | 0-8 | 5.89 (2.09) | 6.5 | (5, 8) | .64 |
| Event-Based | 0-8 | 7.09 (1.32) | 8.0 | (6, 8) | .47 |
| Verbal | 0-8 | 6.70 (1.63) | 7.0 | (6, 8) | .46 |
| Action | 0-8 | 6.28 (1.57) | 6.0 | (5, 8) | .42 |
| 2-minute | 0-8 | 7.15 (1.20) | 8.0 | (6, 8) | .22 |
| 10-minute | 0-8 | 5.83 (1.85) | 6.0 | (5, 7) | .45 |
| Total Errors | 0-6 | 2.06 (1.62) | 2.0 | (1, 3) | --- |
| Total Recognition | 0-8 | 7.87 (0.34) | 8.0 | (8, 8) | --- |
| Word Search | 0-105 | 39.98 (13.02) | 37.0 | (33, 48) | --- |
PROMACY – Prospective Memory Assessment for Children &Youth; SD – Standard deviation.
In evaluating PROMACY error scores, the most common error type was Omission (M = 0.96, n = 27) followed by Task Substitution (M = 0.61, n = 24) and Loss of Content errors (M = 0.39, n = 17). Loss of Time (n = 3) and Place Losing (n = 1) were much less frequently observed and Random errors did not occur in this healthy sample. The mean number of errors was 2. One-fifth of participants did not commit any errors, 24% committed only one error, 17% two errors, 15% three errors, and 24% ≥ four errors. The mean Recognition post-task score was 7.87 (out of 8), demonstrating that most participants recognized the required PM tasks after the fact, regardless of task performance.
Correlations of PROMACY with other Neuropsychological Measures:
Correlations of neurocognitive measures with the PROMACY Summary Score are reported in Table 4. The following tests/subtests demonstrated significant correlations with the PROMACY Summary Score: WRAML2 Verbal Learning Immediate and Delayed Recall, WISC-IV/WAIS-IV Working Memory Index and FSIQ, and the Letter Fluency and Category Fluency, Color-Word Interference Word Reading, and 20 Questions Initial Abstraction Score from the DKEFS. For these significant results the Spearman correlations ranged from ρ = .30 to .53 (M = .41). In a linear regression model considering all of these correlated assessments, only the WISC-IV/WAIS-IV Working Memory Index and the DKEFS Category Fluency score were significantly associated with the PROMACY Summary Score after adjustment for age. There was no evidence of collinearity in regression models, with no indication of instability of parameter estimates and variance inflation factors close to 1 for all predictors. PROMACY was not associated with the NEPT (p > .10). Correlations between the standard clinical measures and the Time-based scales of PROMACY are provided in Table 4 for descriptive purposes. Correlations with Event-based scales are not reported due to low reliability.
Table 4:
Spearman Correlation Coefficients of PROMACY Scores with Other Neuropsychological Measures
| Construct (Test/Subtest) | Subscale Mean(SD) |
PROMACY Summary Score Correlation |
PROMACY TB Score Correlation |
|
|---|---|---|---|---|
| Prospective Memory (NEPT) | ||||
| Event-based score | 6.74 (4.96) | −.04 | −.10 | |
| Retrospective Memory (WRAML2) | ||||
| Verbal Learning Total | 9.26 (2.46) | .39** | .30 | |
| Verbal Learning Recall | 9.68 (2.93) | .38** | .38 | |
| Design Memory Total | 8.64 (2.96) | .27 | .28 | |
| Design Memory Recognition | 8.94 (3.38) | .17 | .24 | |
| Working Memory (WISC-IV/WAIS-IV) | ||||
| Working Memory Index (n=50) | 94.16 (11.83) | .53** | .44 | |
| Processing Speed (WISC-IV/WAIS-IV) | ||||
| Coding | 7.78 (2.96) | .19 | .22 | |
| Executive Function (DKEFS) | ||||
| Verbal Fluency | ||||
| Letter Fluency | 8.72 (2.57) | .46** | .48 | |
| Category Fluency | 10.06 (2.99) | .42** | .36 | |
| Category Switching | 9.11 (2.75) | .20 | .24 | |
| Design Fluency | ||||
| Filled Dots | 8.37 (2.11) | .18 | .14 | |
| Empty Dots | 8.67 (2.22) | .20 | .22 | |
| Switching | 9.11 (2.55) | −.08 | −.10 | |
|
Inhibition/Interference
(DKEFS Color-Word Interference) | ||||
| Color Naming | 8.13 (3.57) | .20 | .10 | |
| Word Reading | 9.54 (3.44) | .37** | .29 | |
| Inhibition | 8.64 (2.89) | .14 | .14 | |
| Inhibition/Switching | 8.44 (3.25) | .15 | .09 | |
|
Problem-Solving/Concept Formation (DKEFS 20 Questions) | ||||
| Abstraction | 8.38 (2.76) | .30** | .27 | |
| FSIQ | ||||
| WISC-IV/WAIS-IV FSIQ (n=50) | 92.24 (11.22) | .45** | .42 | |
p < .05;
p < .01
(n = 54).PROMACY – Prospective Memory Assessment for Children & Youth; SD – Standard Deviation; EB – Event-based; TB – Time-based; NEPT – Naturalistic Event-Based Prospective Memory Task; WRAML2 – Wide Range Assessment of Memory & Learning, 2nd Edition; DKEFS – Delis-Kaplan Executive Function System; WISC-IV – Wechsler Intelligence Scale for Children, 4th Edition; WAIS-IV – Wechsler Adult Intelligence Scale, 4th Edition; FSIQ – Full Scale Intelligence Quotient. Note: Significance testing was not performed on the Time--based correlations, which are provided only for descriptive purposes. Event-based correlations are not reported due to low reliability.
Discussion
PM is a unique construct that has tremendous ecological relevance; however, there is currently a lack of validated, psychometrically sound PM measures for use with children and adolescents. Here we describe the development and initial psychometric properties of PROMACY, which is a new PM measure that was adapted from a well-validated adult task (i.e., MIST; Raskin et al., 2010). Recent data from our colleagues provided preliminary support for the discriminant (Harris et al., 2017) and ecological (Sirois et al., 2016) validity of PROMACY in the context of pediatric HIV disease. In the current study of 54 healthy children and adolescents without HIV infection or cognitive or behavioral impairment, PROMACY demonstrates both strengths and limitations as a measure of PM. At face value, examiner observation revealed that the level of engagement, motivation and effort put forth by participants demonstrated PROMACY is acceptable for children, adolescents, and youth regardless of age. That is, youth appropriately engaged in completing the distractor task word search puzzles, with older youth completing more word searches than younger participants.
In terms of reliability, the PROMACY Summary Score had low but acceptable internal consistency. By traditional standards, Summary Score reliability values < .7 would be considered questionable. However, one must keep in mind the necessary brevity of any clinical PM task, which demands sufficiently long intervals between targets to distinguish the construct from other aspects of cognition (e.g., vigilance), and thereby reduces the number of available items. For cognitive tasks with fewer items, the observed reliability values would be considered “acceptable” (e.g., Bhatnagar, Kim, & Many, 2014; George & Mallery, 2002) and are consistent with adult studies on the MIST (e.g., Bezdicek et al., 2014; Woods et al., 2008). The MIST, for example, has a Spearman-Brown coefficient of .7 in a small sample of healthy adults. The PROMACY Summary Score approached acceptable internal consistency reliability for older youth (≥ 15 years) and female gender, and demonstrated acceptable internal consistency reliability for Black race, non-Hispanic ethnicity and male gender. Aside from the Time-based subscale score, no other PROMACY subscale demonstrated acceptable reliability, even according to standards for shorter measures. It is possible that such low reliability coefficients reflect the instability of the task or the restricted range of scores that are sometimes derived from healthy samples, as has been shown in the adult MIST literature (e.g., Raskin et al., 2011). When individual trials were removed from the Cronbach’s α calculation for the Summary Score, no Cronbach’s α coefficient was below .52, suggesting each trial meaningfully contributes to the internal consistency of the Summary Score, despite relatively restricted range of scores.
PROMACY’s Event-based scale showed very limited reliability and notable ceiling effects that clearly limit its usefulness. One possible contributor to these psychometric problems is the lower item grade level, which may have been too easy for the older youth in this study (as evidenced in the strong ceiling effects in that subgroup). Furthermore, it is possible that the unequal distribution of the action and verbal responses on the Event- versus Time-based scales may have contributed to the differences in reliabilities and ceiling effects on these scales. However, the Event-based scale had more verbal responses, which might actually be expected to be associated with lower performance (e.g., Cohen, 1989). Thus it will be important for future studies to follow the recommendations of Delis et al. (2003) to examine the internal consistency and test-retest reliability of PROMACY in a wide range of clinical samples, which may provide a larger range of scores, particularly on Event-based trials. Future studies may also wish to increase the difficulty level of PROMACY’s Event-based trials, for example by reducing their semantic relatedness (e.g., Woods et al., 2010).
Of course, it is possible for a shorter neuropsychological test such as PROMACY (i.e., with only 8 items) to demonstrate modest internal consistency, but still be clinically useful in discriminating clinical populations, detecting everyday functioning difficulties, and relating to other neurocognitive constructs (see Loevinger, 1954). In this regard, prior studies show that PROMACY differentiates youth with HIV and neurocognitive disorders from youth with HIV without cognitive disorders and from youth without HIV, independent of relevant clinicodemographic co-factors (Harris et al., 2017). Moreover, PROMACY is an independent predictor of relevant functional outcomes, including adaptive behavior and academic performance (Sirois et al., 2016). The current study also showed that the PROMACY Summary Score demonstrated moderate associations (i.e., convergent validity) with well-validated neurocognitive tests, including measures of verbal learning and recall, working memory, Full Scale IQ, letter and category fluency, word reading, and problem solving (see Table 4). In general, verbally-based neurocognitive tests demonstrated larger associations with PROMACY Summary Scores than non-verbal tests. Specifically, working memory and category fluency emerged as the strongest predictors of the PROMACY Summary Score while Full Scale IQ was the weakest predictor. The direction and magnitude of these associations were highly consistent with findings from psychometric studies of the MIST in middle-aged (e.g., Carey et al., 2006) and older (e.g., Bezdicek et al., 2014; Kamat et al., 2014) healthy adults, as well as in some clinical samples (e.g., Carey et al., 2006; Coulehan et al., 2014). Such data fit with current conceptual models regarding the cognitive demands of PM, particularly in regard to retrospective memory and various executive functions (see McDaniel & Einstein, 2007). Studies of divergent validity in larger healthy and clinical populations are needed in order to establish the specificity of PROMACY (and MIST).
Contrary to our expectation, PROMACY did not correlate significantly with the NEPT and that null association was accompanied by a small effect size. This finding was surprising as both tasks demonstrate some evidence of discriminant validity in clinical populations (Harris et al., 2017) and ecological relevance (Sirois et al., 2016). From our vantage point, there are several possible reasons why these two PM measures did not correlate with one another in this study. First, it is possible that the current sample of non-clinical youth produced a very restricted range of scores that resulted in a ceiling effect and risk of Type II error. This was especially evident on the PROMACY Event-based trials for which low reliability coefficients were observed. Second, it is possible that these two tasks measure two different aspects of PM. The NEPT is a very naturalistic, habitual task that only includes event-based trials and has minimal retrospective memory demands. By way of comparison, PROMACY includes both time- and event-based trials that are interspersed with one another among eight different cue-intention pairings, which place considerable demands on strategic monitoring and retrospective memory (as shown with the correlational analyses, Table 4). In order to resolve this issue, future studies might: 1) examine the associations between these two clinical PM tasks with traditional experimental tests of PM, such as the classic McDaniel and Einstein paradigms (e.g., 2007), which would allow for a theory-driven analysis of component processes; and 2) measure the association between the NEPT and PROMACY in clinical samples with greater variability in PM scores.
As anticipated, a relationship with age was observed whereby older youth (≥ 15 years) scored higher on the PROMACY Summary Score than younger participants (< 15 years). This was a robust effect that is consistent with prior reports of improved PM performance associated with developmental progression in adolescents over children (Kliegel et al., 2013; Mahy et al., 2014; Voigt et al., 2014; Ward et al., 2005; Yang et al., 2011; Zimmerman & Meier, 2006). Furthermore, the positive age association observed here is consistent with findings from the MIST in adults (see Kamat et al., 2014; Raskin et al., 2010). Similarly, the absence of an association between PROMACY and gender and race/ethnicity aligns with adult MIST studies (e.g., Woods et al., 2008; Raskin et al., 2010; cf. Palermo et al., 2016). Of course, such findings do not preclude the possibility that gender and race/ethnicity may interact with other demographic or disease-related factors in influencing PROMACY performance.
With regard to component analyses, the most common error type observed in this small sample of healthy children and youth was omissions, followed by task substitution and loss of content. The majority of participants committed at least one error, with one-fourth of the sample committing four or more errors, suggesting a sizable subgroup had difficulty maintaining the intention, or alternately with execution. Nonetheless, participants performed well on post-task recognition of PROMACY items independent of PROMACY task performance. These distributions and error patterns align nicely with studies of the MIST conducted in healthy adults across the lifespan (e.g., Bezdicek et al., 2014; Kamat et al., 2014; Raskin et al., 2010; Woods et al., 2008).
This study is not without limitations. First, the study sample represents a cohort of healthy children and adolescents who were exposed to HIV in-utero, most of whom were also likely exposed to anti-HIV prophylactic medications postnatally. Thus, results may not generalize to other healthy children and adolescents without such exposures. Additionally, the sample was relatively homogeneous, being primarily Black, non-Hispanic, and impoverished, which may further limit generalizability to children and adolescents of other demographic backgrounds. The study sample did not include participants younger than 8 years of age; thus, future studies may wish to examine the potential usefulness of PROMACY in younger children. The sample also was relatively small and thus not of appropriate size for some psychometric analyses that will be important in future work (e.g., norms). Furthermore, scores obtained on neurocognitive measures for this cohort were generally within the Low Average to Average range of functioning, thus supporting their inclusion as the PROMACY validation sample. However, obtained PROMACY scores were relatively restricted in range, which may have limited statistical analyses. Range restrictions (and ceiling effects) are common among event-based PM tasks, especially those with low strategic demands. As noted above, it remains a challenge to develop a reliable PM measure with a sufficient number of intentions separated by a reasonable delay interval that is also brief enough to be implemented in a clinical setting with children and adolescents. Although our sample may represent children at risk for chronic health conditions well, replicating validation of PROMACY with a more representative demographic sample, that includes clinical samples, is needed. Including children and adolescents with cognitive, behavioral, and/or health conditions would likely address the observed score range restriction resulting in strengthened psychometric properties.
The ability to assess PM in children and adolescents is an important factor in fully understanding their developmental neurocognitive progression and daily functioning, but available clinical measures are non-existent. In this study, we present preliminary evidence of the internal consistency, convergent validity, and demographic correlates of PROMACY. When paired with two prior studies showing the discriminant and ecological validity of PROMACY (Harris et al., 2017; Sirois et al., 2016), the current findings are cautiously encouraging, particularly for the Summary Score. PROMACY is the first of its kind, easy to administer, comprehensive measure, that includes both Event-based and Time-based tasks of PM evaluated for use with children and adolescents. However, PROMACY needs to be further validated with a larger, more demographically diverse healthy sample and various clinical populations before being considered for clinical use. Future analyses will allow for validation of an alternate form and test-retest reliability, which will help inform longitudinal comparisons.
Figure 1:
Post-PROMACY Retrospective Recognition Task
*Note: PAR, Inc. holds the copyright to Prospective Memory Assessment for Children & Youth (PROMACY) and should not be used or reproduced without the written permission from PAR, Inc.
Acknowledgments
The following institutions (in alphabetical order), clinical site investigators and staff participated in conducting PHACS AMP; sites participating in the Memory and Executive Functioning Study and the site PI for the substudy are marked with an asterisk: Ann & Robert H. Lurie Children’s Hospital of Chicago*: Ram Yogev, Margaret Ann Sanders, Kathleen Malee*, Scott Hunter; Baylor College of Medicine*: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris*; Boston Children’s Hospital*: Sandra Burchett, Nancy Karthas, Betsy Kammerer*; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children’s Diagnostic & Treatment Center*: Ana Puga, Sandra Navarro, Patricia Garvie*, James Blood; Jacobi Medical Center*: Andrew Wiznia, Marlene Burey, Molly Nozyce*; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Susan Adubato; St. Christopher’s Hospital for Children: Janet Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St. Jude Children’s Research Hospital*: Katherine Knapp, Kim Allison, Megan Wilkins*; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center*: Margarita Silio, Medea Jones, Patricia Sirois*; University of California, San Diego*: Stephen Spector, Kim Norris, Sharon Nichols*; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Alisa Katai, Jennifer Dunn, Suzanne Paul; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
Funding: The Pediatric HIV/AIDS Cohort Study (PHACS) Memory and Executive Functioning Substudy was supported by the National Institute of Mental Health [MH084794; PI: Sharon Nichols]. Data analysis services were provided by the Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health (PI: Paige Williams); data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski); and regulatory services and logistical support were provided by Westat, Inc. (PI: Julie Davidson). PHACS was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T. H. Chan School of Public Health [HD052102] (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis).
The authors extend their appreciation to Sarah A. Raskin, Ph.D., Psychological Assessment Resources, Inc. (PAR, Inc.), holder of the copyright for the Memory for Intentions Screening Test (MIST; Raskin, Buckheit, & Sherrod, 2010), for permitting access to the MIST as a foundation for development of the Prospective Memory Assessment for Children & Youth (PROMACY). Reproduced by special permission of the Publisher, Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida, 33549, from the Memory for Intentions Screening Test (MIST) by Sarah Raskin, Ph.D. and Carol Buckheit, Copyright 1998, 2009 by PAR, Inc. Further reproduction is prohibited without permission from PAR, Inc.
The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Footnotes
Disclosure Statement: All authors have declared that they have no conflict of interest to report related to the work presented herein.
Contributor Information
Patricia A. Garvie, Research Department, Children’s Diagnostic & Treatment Center, 1401 South Federal Highway, Fort Lauderdale, FL 33316, pgarvie@browardhealth.org.
Sharon L. Nichols, Department of Neurosciences, University of California, San Diego, 9500 Gilman Drive #0935, La Jolla, CA 92093, slnichols@ucsd.edu.
Paige L. Williams, Department of Biostatistics, Harvard T. H. Chan School of Public Health, 665 Huntington Avenue, Boston, MA 02115, paige@sdac.harvard.edu.
Lynnette L. Harris, Department of Pediatrics, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030, llharri1@texaschildrens.org.
Betsy Kammerer, Department of Psychiatry, Boston Children’s Hospital, Fegan 8, 300 Longwood Avenue, Boston MA 02115, betsy.kammerer@childrens.harvard.edu.
Miriam C. Chernoff, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, 651 Huntington Avenue, Boston, MA 02115, mchernoff@sdac.harvard.edu.
Veronica Figueroa, Department of Pediatrics, Mother-Child-Adolescent HIV Program, University of California San Diego, 4076 Third Ave, Suite 301, San Diego, CA 92103, vfigueroa@ucsd.edu.
Steven Paul Woods, Department of Psychiatry, University of California, San Diego, La Jolla, CA and Department of Psychology, University of Houston, 126 Heyne Building, Houston, TX 77004, spwoods@uh.edu.
References
- Altgassen M, Williams TI, Bölte S, & Kliegel M (2009). Time-based prospective memory in children with autism spectrum disorder. Brain Impairment, 10(1), 52–58. [Google Scholar]
- Bezdicek O, Raskin SA, Altgassen M, Ruzicka E (2014). Assessment of prospective memory: A validity study of Memory for Intentions Screening Test. Czech and Slovak Neurology and Neurosurgery, 77/110(4), 435–443. [Google Scholar]
- Bhatnagar R Kim J, & Many JE (2014). Candidate surveys on program evaluation: Examining instrument reliability, validity and program effectiveness. American Journal of Educational Research, 2(8): 683–690. [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I, & the HNRC Group. (2006). Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology, 28, 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn J, & Smith PT (1991). The relative influence of intelligence and age on everyday memory. Journal of Gerontology, 46(1), 31–36. [DOI] [PubMed] [Google Scholar]
- Cohen RL (1989). Memory for action events: The power of enactment. Educational Psychology Review, 1(1), 57–80. [Google Scholar]
- Coulehan K, Byrd D, Arentoft A, Monzones J, Fuentes A, Fraser F, …& Mindt MR (2014). The role of decision-making ability in HIV/AIDS: Impact on prospective memory. Journal of Clinical and Experimental Neuropsychology, 36(7), 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Jacobson M, Bondi MW, Hamilton JM, & Salmon DP (2003). The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society, 9, 936–946. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- George D, & Mallery P (2002). SPSS for Windows Step by Step: A Simple Guide and Reference, 11.0 update, 4th ed Boston: Allyn & Bacon. [Google Scholar]
- Gupta S, Woods SP, Weber E, Dawson MS, Grant I, & the HNRC Group. (2010). Is prospective memory a dissociable cognitive function in HIV infection? Journal of Clinical and Experimental Neuropsychology, 32, 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LL, Chernoff MC, Nichols SL, Williams PL, Garvie PA, Yildrim C, … Woods SP (2017). Prospective memory in youth with perinatally-acquired HIV infection. Child Neuropsychology. DOI: 10.1080/09297049.2017.1360854. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Weinborn M, Kellogg E, Bucks R Velnoweth A, & Woods SP (2014). Construct validity of the Memory for Intentions Screening Test in older adults. Assessment, 21, 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA (2000). The CyberCruiser: An investigation of development of prospective memory in children. Journal of International Neuropsychological Society, 6, 62–70. [DOI] [PubMed] [Google Scholar]
- Kerns KA, & Price KJ (2001). An investigation of prospective memory in children with ADHD. Child Neuropsychology, 7, 162–171. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Mackinlay R, & Jager T (2008). Complex prospective memory: Development across the lifespan and the role of task interruption. Developmental Psychology, 44, 612–617. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Mahy CEV, Voigt B, Henry JD, Rendell PG, & Aberle I (2013). The development of prospective memory in young schoolchildren: The impact of ongoing task absorption, cue salience, and cue centrality. Journal of Experimental Child Psychology, 116, 792–810. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Ropeter A, & Mackinley R (2006). Complex prospective memory in children with ADHD. Child Neuropsychology, 12, 407–419. [DOI] [PubMed] [Google Scholar]
- Kretschmer A, Voigt B, Friedrich S, Pfeiffer K, & Kliegel M (2014). Time-based prospective memory in young children: Exploring executive functions as a developmental mechanism. Child Neuropsychology, 20(6), 662–676. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L, Messer DJ, & Ebdon P (2001). Prospective memory in children: The effects of age and task interruption. Developmental Psychology, 37, 428–430. [DOI] [PubMed] [Google Scholar]
- Loevinger J (1954). The attenuation paradox in test theory. Psychological Bulletin, 51(5), 493–504. [DOI] [PubMed] [Google Scholar]
- Mackinlay RJ, Kliegel M, & Mäntylä T (2009). Predictors of time based prospective memory in children. Journal of Experimental Child Psychology, 102, 251–264. [DOI] [PubMed] [Google Scholar]
- Mahy CEV, Kliegel M, Marcovitch S (2014). Emerging themes in the development of prospective memory during childhood. Journal of Experimental Child Psychology, 127, 1–7. [DOI] [PubMed] [Google Scholar]
- Mahy CEV, & Moses LJ (2011). Executive functioning and prospective memory in young children. Cognitive Development, 26, 269–281. [Google Scholar]
- Mäntylä T, Carelli MG, & Forman H (2007). Time monitoring and executive functioning in children and adults. Journal of Experimental Child Psychology, 96, 1–19. [DOI] [PubMed] [Google Scholar]
- Martin T, McDaniel MA, Guynn MJ, Houck JM, Woodruff CC, Bish JP, … Tesche CD (2007). Brain regions and their dynamics in prospective memory retrieval: A MEG study. International Journal of Psychophysiology, 64(3), 247–58. [DOI] [PubMed] [Google Scholar]
- McCauley SR, & Levin HS (2004). Prospective memory in pediatric traumatic brain injury: A preliminary study. Developmental Neuropsychology, 25(1&2), 5–20. [DOI] [PubMed] [Google Scholar]
- McCauley SR, McDaniel MA, Pedroza C, Chapman SB, & Levin HS (2009). Incentive effects on event-based prospective memory performance in children and adolescents with traumatic brain injury. Neuropsychology, 23(2), 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SR, Pedroza C (2010). Event-based prospective memory in children with sickle cell disease: Effect of cue distinctiveness. Child Neuropsychology, 16, 293–312. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Pedroza C, Chapman SB, Cook LG, Vasquez AC, & Levin HS (2011). Monetary incentive effects on event-based prospective memory three months after traumatic brain injury in children. Journal of Clinical and Experimental Neuropsychology, 33(6), 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, & Einstein GO (2000). Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology, 14, S127–S144. [Google Scholar]
- McDaniel MA & Einstein GO (2007). Prospective memory: An overview and synthesis of an emerging field. Los Angeles, CA: Sage Publications. [Google Scholar]
- McDaniel MA, Umanath S, Einstein GO, Waldum ER (2015). Dual pathways to prospective remembering. Frontiers in Human Neuroscience, 14(9), 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll DR, Pirogovsky E, Woods SP, Filoteo JV, Goldstein J, Corey-Bloom J, & Gilbert PE (2014). “Forgetting to remember” in Huntington’s Disease: A study of laboratory, semi-naturalistic, and self-perceptions of prospective memory. Journal of the International Neuropsychological Society, 20, 192–199. [DOI] [PubMed] [Google Scholar]
- Osipoff JN, Dixon D, Wilson TA, & Preston T (2012). Prospective memory and glycemic control in children with type 1 diabetes mellitus: A cross-sectional study. International Journal of Pediatric Endocrinology, 2012(29), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo L, Cinelli MC, Piccardi L, Ciurli C, Incoccia C, Zompanti L, & Guariglia C (2016). Women outperform men in remembering to remember. Quarterly Journal of Experimental Psychology, 69(1), 65–74. [DOI] [PubMed] [Google Scholar]
- Poquette A, Moore DJ, Gouaux B, Morgan EE, Grant I, Woods SP, & the HNRC Group. (2013). Prospective memory and antiretroviral medication non-adherence in HIV: An analysis of ongoing task delay length using the Memory for Intentions Screening Test. Journal of the International Neuropsychological Society, 19, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin SA (2009). Memory for intentions screening test: Psychometric properties and clinical evidence. Brain Impairment, 10(1), 23–33. [Google Scholar]
- Raskin S, Buckheit C, & Sherrod C (2010). Memory for Intentions Test. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Raskin S, Woods SP, Poquette A, McTaggart AB, Sethna J, Williams RC, & Tröster AI (2011). A differential deficit in time- versus event-based prospective memory in Parkinson’s disease. Neuropsychology, 25, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW (2004). BASC-2: Behavior Assessment System for Children, Second Edition Circle Pines, MN: American Guidance Service. [Google Scholar]
- Sheslow D, & Adams W (2003). Wide Range Assessment of Memory and Learning, Second Edition Wilmington, DE: Wide Range, Inc. [Google Scholar]
- Simons JS, Schölvinck ML, Gilbert SJ, Frith CD, & Burgess PW (2006). Differential components of prospective memory?: Evidence from fMRI. Neuropsychologia, 44, 1388–1397. [DOI] [PubMed] [Google Scholar]
- Sirois PA, Chernoff MC, Kammerer BL, Garvie PA, Harris LL, Woods SP, …for the Pediatric HIV/AIDS Cohort Study. (2016). Associations of memory and executive functioning with academic and adaptive functioning among youth with perinatal HIV exposure and/or infection. Journal of the Pediatric Infectious Diseases Society, 5(Supp 1), S24–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, … for the Pediatric HIV/AIDS Cohort Study (PHACS). (2012). Impact of Human Immunodeficiency Virus severity on cognitive and adaptive functioning during childhood and adolescence. Pediatric Infectious Diseases Journal, 31(6), 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, & Spreen O (2006). A compendium of neuropsychological tests: Administration, norms and commentary (3rd ed). New York, NY: Oxford University Press. [Google Scholar]
- Uttl B, & Kibreab M (2011). Self-Report Measures of Prospective Memory Are Reliable but Not Valid. Canadian Journal of Experimental Psychology, 65(1), 57–68. [DOI] [PubMed] [Google Scholar]
- Voigt B, Mahy CEV, Ellis J, Schnitzspahn K, Krause I, Altgassen M, Kliegel M (2014). The development of time-based prospective memory in childhood: The role of working memory updating. Developmental Psychology, 50(10), 2393–2404. [DOI] [PubMed] [Google Scholar]
- Wandschneider B, Kopp UA, Kliegel M, Stephani U, Kurlemann G, Janz D, & Schmitz B (2010). Prospective memory in patients with juvenile myoclonic epilepsy and their healthy siblings. Neurology, 75, 2161–2167. [DOI] [PubMed] [Google Scholar]
- Wang L, Altgassen M, Liu W, Xiong W, Akgun C, & Kliegel M (2011). Brief Report: Prospective memory across adolescence: The effects of age and cue focality. Developmental Psychology, 47(1), 226–232. [DOI] [PubMed] [Google Scholar]
- Ward H, Shum D, McKinlay L, Baker-Tweney S, & Wallace G (2005). Development of prospective memory: Tasks based on the prefrontal-lobe model. Child Neuropsychology, 11, 527–549. [DOI] [PubMed] [Google Scholar]
- Ward H, Shum D, McKinlay L, Baker S, & Wallace G (2007). Prospective memory and pediatric traumatic brain injury: Effects of cognitive demand. Child Neuropsychology, 13, 219–239. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003). Manual for the Wechsler Intelligence Scale for Children, Fourth Edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (2008). Manual for the Wechsler Adult Intelligence Scale, Fourth Edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, … Achim CL (1998). Distribution of brain HIV load in AIDS. Brain Pathology, 8, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I & the HNRC Group. (2007a). Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology, 22, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH & the HNRC Group. (2009). Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society, 15(1), 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson M, Weber E, Grant I, & the HNRC Group. (2010). The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology, 32, 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, … the HNRC Group. (2008a). Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology, 23(3), 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I, & the HNRC Group. (2008b). Psychometric characteristics of the Memory for Intentions Screening Test. The Clinical Neuropsychologist, 22, 864–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre SL, & the HNRC Group. (2006). Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cognitive Behavioral Neurology, 19(4), 217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Twamley EW, Dawson MS, Narvaez JM, Jeste DV (2007b). Deficits in cue detection and intention retrieval underlie prospective memory impairment in schizophrenia. Schizophrenia Research, 90, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TX, Chan RC, & Shum D (2011). The development of prospective memory in typically developing children. Neuropsychology, 25, 342–352. [DOI] [PubMed] [Google Scholar]
- Zimmermann TD, & Meier B (2006). The rise and decline of prospective memory performance across the lifespan. The Quarterly Journal of Experimental Psychology, 59, 2040–2046. [DOI] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Weber E, Iudicello JE, Dawson MS, Grant I, & the HRNC Group. (2010). HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance. The Clinical Neuropsychologist, 24(6), 945–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllig J, West R, Martin M, Altgassen M, Lemke U, & Kliegel M (2007). Neural correlates of prospective memory across the lifespan. Neuropsychologia, 45, 3299–3314. [DOI] [PubMed] [Google Scholar]