Abstract
Chronic administration of nicotine or exposure to stress can produce long-lasting behavioral and physiological changes in humans and animals alike. Further, the impact of nicotine and stress exposure can be inherited by offspring to produce persistent changes in physiology and behavior. To determine if nicotine and stress interact across generations to influence offspring behavior we exposed F0 male mice to nicotine and F1 male and female mice to chronic unpredictable stress during adolescence. We then measured locomotor sensitization to repeated nicotine injections in the subsequent F2 and F3 generations. Stress exposure alone (F1) did not influence locomotor sensitization in any lineage. However, in the F1 male lineage, F0 nicotine exposure abrogated locomotor sensitization in F2 male and transiently enhanced locomotor sensitization in F2 female offspring. These effects were not passed down to the F3 generations or observed in the F1 female lineage. F1 stress exposure modulated the effects of prior F0 nicotine exposure in a sex-dependent manner. Specifically, stress blunted the nicotine-induced enhancement in locomotor sensitization observed in F2 female offspring of F1 males. The effect of F0 nicotine and F1 stress exposure in females appears to have skipped a generation and enhanced nicotine sensitization only in the F3 generation, and only in females. This novel multigenerational exposure paradigm examining the inheritance of two different environmental exposures demonstrates that nicotine responses can be modified by nicotine and stress exposure from previous generations, and these effects are strongly influenced by sex.
Keywords: transgenerational inheritance, nicotine, chronic unpredictable stress, locomotor sensitization, adolescent, mice
Lay Summary
An individual’s offspring can inherit the adverse effects of exposure to stress or nicotine, the primary addictive substance in tobacco, which may predispose them to nicotine addiction. In this paper, Yohn and colleagues investigated whether the “transgenerational” effects of nicotine in one generation are altered by chronic stress exposure that occurs in the next generation. Their results indicate that nicotine and stress appear to interact across generations to alter offspring addiction-related behaviors.
Introduction
In the United States, an estimated 40 million adult (Agaku, King, & Dube, 2014) and 3.5 million youth are smokers (Arrazola et al., 2015). In addition, the national prevalence of smokeless tobacco products continues to rise with the use of electronic cigarettes (Arrazola et al., 2015; Schoenborn & Gindi, 2015). Therefore, with approximately 4 million births occurring each year in the United States (Hamilton, Martin, Osterman, Curtin, & Matthews, 2015), a significant proportion of children will be born to nicotine (NIC) users. NIC produces its reinforcing effects in the brain to promote sustained drug use (Damsma, Day, & Fibiger, 1989; Mereu et al., 1987) concomitant with long-term behavioral and physiological changes. For example, nicotine exposure can increase or decrease anxiety- and depression-like behavior (for review see Picciotto, Brunzell, & Caldarone, 2002), enhance cognition (Heishman, Kleykamp, & Singleton, 2010), mediate changes in metabolism (Grunberg, 1990), and influence additional drug use (Levine et al., 2011).
Nicotine use and abuse is a complex behavior influenced by stress exposure. For example, stress signaling pathways in the CNS can promote NIC addiction (Koob & Volkow, 2010) and withdrawal from NIC is a stressful event that precipitates relapse and increases circulating stress hormones (i.e, cortisol) (Benwell & Balfour, 1979). Further, juvenile stress is associated with increased responses to NIC in late-adolescence and adulthood (Caruso et al., 2018; McCormick, Robarts, Gleason, & Kelsey, 2004). In addition, there is some evidence for the inheritance of stress exposure from mothers and fathers to several generations of offspring (Dietz et al., 2011; Franklin et al., 2010; Rodgers, Morgan, Bronson, Revello, & Bale, 2013; Saavedra-Rodríguez & Feig, 2013; Yehuda, Schmeidler, Wainberg, Binder-Brynes, & Duvdevani, 1998). However, no studies have examined the interaction of NIC and stress across generations and the resulting influence in future generations. Therefore, we sought to determine if two behaviors that closely interact on a physiological and molecular level within a generation, would be additive in their effects on offspring behavior across generations.
We produced four distinct lineages to examine multi- and transgenerational inheritance: F0 vehicle (VEH) and F1 no stress referred to as control (CON) exposure (F0 VEH/F1 CON), F0 VEH and F1 chronic unpredictable stress (CUS) exposure (F0 VEH/F1 CUS), F0 NIC and F1 CON exposure (F0 NIC/F1 CON), and F0 NIC and F1 CUS exposure (F0 NIC/F1 CUS). Two subsequent generations of offspring (F2, F3) from both male and female F1 CON/CUS lineages were assessed. Exposure to NIC and stress occurred during adolescence for several reasons. First, almost all adult smokers initiate NIC use during adolescence (SAMHSA, 2011). Adolescence is also a time when both gametes (Kaati, Bygren, & Edvinsson, 2002; Pembrey, 2010) and the neurobiological circuits that mediate nicotine reinforcement (Yuan, Cross, Loughlin, & Leslie, 2015) are vulnerable to environmental stimuli.
Using this novel exposure paradigm, we find that the transgenerational effects of NIC are modulated by a subsequent exposure to stress in the next generation, producing unique phenotypes in subsequent offspring. Specifically, we found NIC and stress exposure across two generations altered locomotor sensitization to NIC in subsequent offspring in a sex- and lineage-dependent manner (i.e., reduced sensitization in F2 males and females or enhanced sensitization in F3 females). This study is the first to our knowledge to explore the interaction of NIC and stress across generations and to track their influence on subsequent generations.
Methods
Animals
Male and Female C57BL/6NTac mice (6–8 weeks of age, 20–30 g) were ordered from Taconic Farms (Hudson, NY), maintained on a 12-h light/dark cycle with food and water ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee (Philadelphia, PA, USA) and National Institutes of Health care and use of laboratory animals guidelines. Mice were bred at the University of Pennsylvania for two generations to generate the F0 generation. Breeding in house decreased the impact of transportation on mice (Booker, Butt, Wehner, Heinemann, & Collins, 2007) and allowed us to isolate the effects of NIC and CUS exposure in subsequent generations of offspring. Mice remained group-housed with littermates throughout the experiments unless otherwise stated. Experimental groups were comprised of at least 1–2 mice from each litter. Efforts were made to minimize animal suffering and reduce the number of animals used for all experiments.
F0 male nicotine exposure
Adolescent male mice were exposed to chronic NIC (18 mg/kg/day; (−) - Nicotine hydrogen tartrate salt dissolved in 0.9% saline; Sigma-Aldrich, St. Louis, MO) or saline via osmotic mini pump (model 1004; Alzet, Cupertino, CA) for 28 days from PND 28–56 (Figure 1). This dose of nicotine is in the range of doses found to produce comparable levels of NIC in the plasma of smokers following a cigarette (AlSharari et al., 2013; Benowitz & Sharp, 1989; Henningfield & Keenan, 1993; Russell, Wilson, Patel, Feyerabend, & Cole, 1975). In addition, this dose up-regulates nicotinic acetylcholine receptors in the brain of rodents (Yohn, Turner, & Blendy, 2014), a hallmark of chronic NIC use in humans (Staley, 2006). While the minipump does not recapitulate the pulsatile mode of NIC delivery experienced by human smokers, it does provide several experimental advantages. For example, minipumps allow for consistent administration of NIC doses across subjects, precise temporal control over the initiation and termination of NIC treatment, and their ease of use facilitated replication across cohorts.
Figure 1.
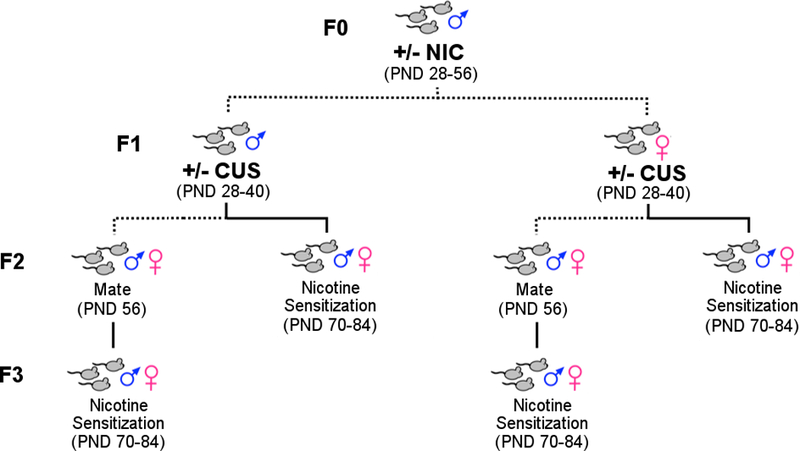
Schematic of experimental design. F0 male mice were exposed to vehicle (VEH, n=5) or nicotine (NIC, n=7) and mated (dashed line) to produce F1 offspring. Resulting F1 males and females were randomly distributed to chronic unpredictable stress (CUS) or no stress control (CON) condition and mated to produce F2 offspring. Of note, mice from a given litter were distributed evenly into CUS or CON groups, such that the resulting CUS or CON groups were comprised of mice from at least 5–7 different litters. One half of the F2 offspring were tested for locomotor sensitization to NIC (solid line) while the other half of the behaviorally-naive F2 mice were mated to produce the F3 offspring. As before, F3 mice from a single litter were evenly distributed to nicotine or saline treatments and tested for locomotor sensitization.
Mice were anesthetized with an isoflurane/oxygen mixture (1 – 3%), and osmotic minipumps were inserted subcutaneously using aseptic surgery techniques. Minipumps were placed parallel to the spine at shoulder level with the flow moderator directed away from the surgical incision. The wound was closed with 7-mm stainless steel wound clips (Reflex; Cellpoint Scientific, Gaithersburg, MD). At the end of 28 days a secondary incision site was used to remove the minipump using aseptic surgery techniques. Approximately 1 week following VEH or NIC exposure mice were housed with naive females for 7 days. This time frame allowed for matings to occur well after the elimination NIC withdrawal signs (Damaj, 2003; Turner, Castellano, & Blendy, 2011) and allowed for elimination of any sperm that may have developed prior to NIC exposure. F0 males (PND 70) were then mated with unexposed females. The presence of vaginal plugs was monitored daily, and males were removed when a plug was found. On average females were positive for vaginal plugs within 2–4 days of mating regardless of treatments. Thus, the mating period did not vary between nicotine and vehicle exposed males.
F1 adolescent chronic unpredictable stress (CUS) exposure
NIC exposure in one generation (F0 NIC) was followed with stress exposure in the subsequent generation (F1 CUS) to determine if NIC and stress interact across generations to influence offspring behavior. Both male and female offspring (F1 generation) from F0 VEH or NIC fathers were exposed to CON or CUS conditions for 12 days from PND 28–40 (Figure 1). The CUS paradigm was implemented as previously described (Yohn & Blendy, 2017). The exact stressors, duration of stressor, and sequence of exposures can be found in Supplementary Information (Supplementary Table S1) along with detailed descriptions. Briefly, mice were exposed to three stressors a day, in the morning, afternoon, and overnight, for 12 consecutive days in dedicated procedure rooms. Mice were returned to the animal colony between stressors and after the final stressor. Mice were group-housed with littermates during the CUS exposure. Following CUS exposure, male mice (PND 49) were placed with naive animals for 7 days (to allow for elimination of any sperm that may have developed prior to CUS exposure in males). F1 CON and CUS mice (PND 56) were then mated with naive animals. The presence of vaginal plugs was monitored daily, and males were removed when a plug was found. On average naïve females mated with CUS males were positive for vaginal plugs within 2–3 days, as were all Control matings. Of interest, CUS females mated with naïve males were positive for vaginal plugs within 3–5 days, perhaps reflecting some residual impact of stress exposure. Behaviorally naive F2 male and female mice were mated with naive mice to produce F3 offspring.
Behavioral testing
All behavioral testing sessions were conducted in a room that was separate from the colony room during the lights-on period between 8:00 a.m. and 5:00 p.m. On testing days, mice were transported to the behavior room at least one hour prior to testing.
Nicotine locomotor sensitization
Sensitization of the locomotor response to repeated nicotine administration was assayed in F2 (n = 5–8/group) and F3 offspring (n = 3–8/group) on PND 70–84. In order to record baseline activity on the first 2 test days, mice received intraperitoneal (i.p.) injections of 0.9% saline solution and were immediately placed in test cages that had the same dimensions as their home cage (28.9 × 17.8 × 12 cm) and contained a small layer of clean bedding. On NIC testing days 1–4, mice received 1 mg/kg (−) - Nicotine hydrogen tartrate salt (i.p.) daily and locomotor activity was recorded for 15 min. Two weeks following the last NIC injection (challenge day), mice received an additional 1mg/kg (i.p.) NIC injection and locomotor activity was recorded. Locomotor activity was detected using a photo beam frame (30 × 24 × 8 cm) with sensors arranged in an eight-beam array strip around the cage and recorded by an activity monitoring system (MED Associates, St. Albans, VT). The primary dependent variable of interest was locomotor activity (beam breaks) over 15 min because this time frame encompasses the near maximal effects of NIC injection on locomotor activity (Marks, Romm, Bealer, & Collins, 1985).
Statistical Analyses
All data are presented as the mean ± standard error of the mean (SEM). Analyses of the results for the nicotine locomotor sensitization testing was analyzed using a two-way mixed factorial analysis of variance with time (day 1, day 4, and challenge) as the within subjects repeated measure and lineage (F0 VEH/F1 CON, F0 VEH/F1 CUS, F0 NIC/F1 CON, and F0 NIC/F1 CUS) as the between subjects independent variable. Outliers were detected using Grubb’s outlier test (Grubbs, 1969) and data were excluded (locomotor sensitization: 18/534 data points excluded). Whenever significant main effects of interactions were identified post hoc analyses were performed using a Bonferroni’s multiple comparison test. An ɑ < 0.05 was considered significant for all statistical analyses including post hoc comparisons. Statistical analyses were performed using Graphpad Prism 7 (Graphpad Software, La Jolla, CA).
Results
F0 nicotine exposure blunted locomotor responses to nicotine in F2 males regardless of F1 male stress exposure
To determine if NIC and CUS interact across generations to influence offspring behavior, half of the F1 male offspring were exposed to CUS during adolescence and half were left undisturbed to serve as the no stress CON group. F2 offspring were then generated from CON- or CUS-exposed F1 (F1 CON and F1 CUS, respectively) males. In addition, to test for transgenerational inheritance of the F0 NIC and F1 CUS exposures, F2 mice were mated with naive partners to produce an F3 generation.
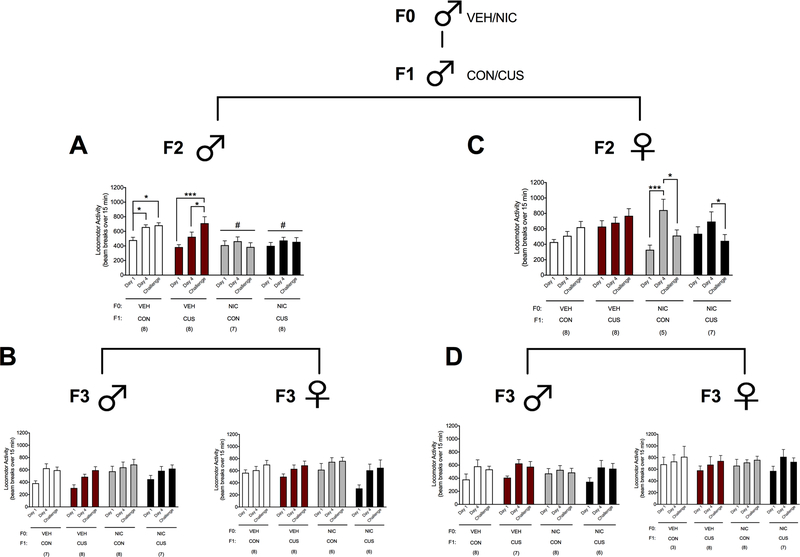
F2 males derived from the male F1 lineages displayed locomotor sensitization as indicated by greater NIC-induced (1.0 mg/kg, i.p.) locomotion on day 4 and the challenge day compared to day 1 (main effect of ‘Time’: F2,54= 8.95, p < 0.001) and F2 male offspring of the F0 VEH males derived from the male F1 CON lineage (F0 VEH/F1 CON) displayed greater NIC-induced locomotion than F2 males from the NIC-exposed F0 male lineages through the F1 CON- (F0 NIC/F1 CON) and F1 CUS-exposed (F0 NIC/F1 CUS) males (main effect of ‘Lineage’: F3,27 = 12.12; p < 0.01). A ‘Time x Lineage’ interaction (Figure 2A; F6,54 = 2.55, p < 0.05) further revealed that the F2 males from the F0 VEH/F1 CON lineage displayed greater NIC-induced locomotion on day 4 and the challenge day relative to day 1 (p’s < 0.05). F2 males from the F0 VEH- and F1 CUS-exposed (F0 VEH/F1 CUS) lineage exhibited greater NIC-induced locomotion on the challenge day relative to days 1 (p < 0.001) and 4 (p < 0.05). Alternatively, F2 males from the F0 NIC/F1 CON and F0 NIC/F1 CUS lineages displayed similar levels of NIC-induced locomotion across the test days.
Figure 2.
F0 NIC exposure prevents locomotor sensitization to NIC in male and female F2, but not F3, offspring regardless of male F1 CUS exposure. Data (mean ± SEM) represent NIC-induced (1 mg/kg, i.p.) locomotion in (A) F2 male offspring derived from the F1 male lineage, (B) F3 male and female offspring of the F2 male lineage, (C) F2 female offspring derived from the F1 male lineage, and (D) F3 male and female offspring of the F2 female lineage. Significant main effect of ‘Lineage’: # p < 0.01. Significant ‘Time x Lineage’ interaction: *p < 0.05, ***p < 0.001. Sample sizes are reported in parentheses in the figure.
To determine if F2 males derived from the F1 male lineage produced offspring with similar phenotypes we characterized behavior in their F3 male and female offspring. Overall, F3 male (Figure 2B, left; main effect of ‘Time’: F2,52 = 17.12, p < 0.001) and F3 female (Figure 2B, right; main effect of ‘Time’: F2,48 = 23.82, p < 0.001) offspring of the F2 males displayed increased NIC-induced locomotion on day 4 and the challenge day, relative to day 1. There were no significant effects of ‘Lineage’ or ‘Time x Lineage’ interactions on NIC-induced locomotion for F3 males and females derived from the F2 male lineage.
F0 nicotine exposure resulted in F2 female offspring that display transient locomotor sensitization to nicotine regardless of F1 male stress exposure.
An unusual pattern of locomotor sensitization was observed in F2 females that were derived from the NIC-exposed F0 male and F1 male lineages. F2 females exhibited greater NIC-induced locomotion on day 4 relative to day 1, but this response was significantly reduced by the challenge day, which did not differ from day 1 levels (main effect of ‘Time’: F2,48 = 6.72, p < 0.01). A ‘Time x Lineage’ interaction (Figure 2C; F6,48 = 3.99, p < 0.01) also revealed that F2 female offspring of the F0 NIC/F1 CUS male lineage exhibited increased NIC-induced locomotion on day 4 compared to day 1 (p < 0.001) and the challenge day (p < 0.05). In contrast, F2 female offspring of F0 NIC/F1 CUS male lineage displayed greater NIC-induced locomotion on day 4 compared to the challenge day (p < 0.05).
To determine if F2 females derived from the F1 male lineage produced offspring with similar phenotypes we characterized behavior in their F3 male and female offspring. Relative to day 1, F3 males derived from the F2 female lineage displayed greater NIC-induced locomotion on day 4 and the challenge day (Figure 2D, left; main effect of ‘Time’: F2,50 = 13.64, p < 0.001). Similarly, NIC-induced locomotion was greater on the challenge day relative to day 1 in F3 females of the F2 female lineage (Figure 2D, right; main effect of ‘Time’: F2,44 = 4.59, p < 0.05). There were no significant effects of ‘Lineage’ or ‘Time x Lineage’ interactions on NIC-induced locomotion for F3 males and females derived from the F2 female lineage.
F0 nicotine and F1 female stress exposures did not influence the locomotor response to nicotine in subsequent generations of male offspring.
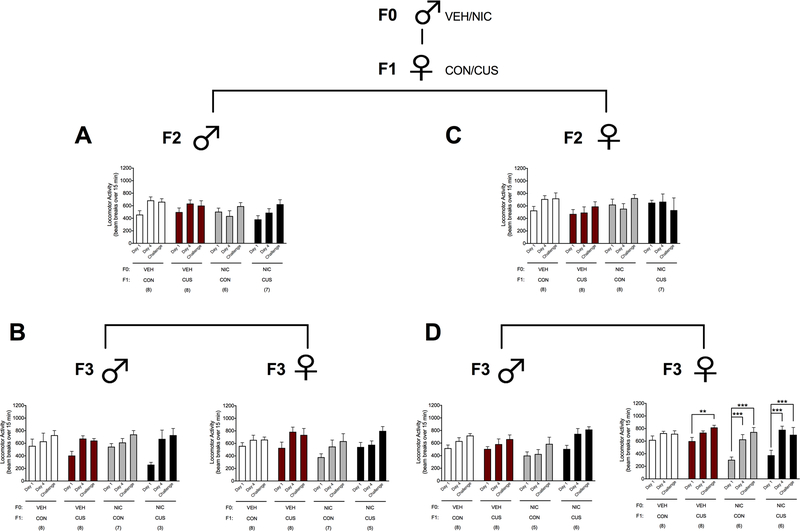
To determine if NIC and stress interact across generations in a sex specific manner, half of the F1 female offspring were exposed to CUS during adolescence and half were left undisturbed to serve as the no stress CON group. F2 offspring were then generated from CON- or CUS-exposed F1 (F1 CON and F1 CUS, respectively) females. In addition, to test for transgenerational inheritance of the F0 NIC and F1 CUS exposures, F2 mice were mated with naive partners to produce an F3 generation. Overall, F2 males derived from the female F1 lineages exhibited locomotor sensitization as indicated by greater NIC-induced (1.0 mg/kg, i.p.) locomotion on day 4 and the challenge day relative to day 1 (Figure 3A; main effect of ‘Time’: F2,50= 10.84, p < 0.001). There was no significant effect of ‘Lineage’ or ‘Time x Lineage’ interaction for F2 males. Similarly, greater NIC-induced locomotion was exhibited on day 4 and the challenge day, relative to day 1, by F3 males (Figure 3B, left; main effect of ‘Time’: F2,44= 20.50, p < 0.001) and females (Figure 3B, right; main effect of ‘Time’: F2,48= 18.68, p < 0.001). There were no significant effects of ‘Lineage’ or ‘Time x Lineage’ interaction for F3 offspring derived from the F2 male lineages.
Figure 3.
F0 NIC and female F1 CUS exposure enhances locomotor sensitization to NIC in female F3, but not F2, offspring. Data (mean ± SEM) represent NIC-induced (1 mg/kg, i.p.) locomotion in (A) F2 male offspring derived from the F1 female lineage, (B) F3 male and female offspring of the F2 male lineage, (C) F2 female offspring derived from the F1 male lineage, and (D) F3 male and female offspring of the F2 female lineage. Significant ‘Time x Lineage’ interaction: **p < 0.01, ***p < 0.001. Sample sizes are reported in parentheses in the figure.
F0 nicotine and F1 female stress exposure increased locomotor sensitization to nicotine in F3 female offspring.
As with the males, F2 females displayed greater NIC-induced locomotion on the challenge day, relative to day 1, indicating locomotor sensitization (Figure 3C; main effect of ‘Time’: F2,40= 4.87, p < 0.05), but there was no significant effect of ‘Lineage’ or ‘Time x Lineage’ interaction. F3 male offspring derived from the F2 females also displayed increased NIC-induced locomotion on day 4 as compared to day 1 and the challenge day relative to days 1 and 4 (Figure 3D, left; main effect of ‘Time’: F2,46= 18.60, p < 0.001) with no significant effect of ‘Lineage’ or ‘Time x Lineage’ interaction. Finally, F3 female offspring derived from the F2 female lineages displayed greater NIC-induced locomotion on day 4 and the challenge day relative to day 1 (main effect of ‘Time’: F2,48= 37.46, p < 0.05). This change in NIC-induced locomotion was dependent on lineage (Figure 3D, right; ‘Time x Lineage’ interaction: F6,48= 3.93, p < 0.05). Specifically, NIC-induced locomotion did not differ across the three test days for F3 female offspring of the F0 VEH/F1 CON female lineage. Alternatively, F3 female offspring derived from the F0 VEH/F1 CUS female lineage displayed greater NIC-induced locomotion on the challenge day relative to day 1 (p < 0.01). F3 females derived from the F0 NIC-exposed female lineages (i.e., F0 NIC/F1 CON and F0 NIC/F1 CUS) displayed greater NIC-induced locomotion on day 4 and the challenge day compared to day 1 (p’s < 0.001).
Discussion
Transgenerational inheritance of environmental exposures from parent to offspring suggests that the quality of the offspring’s life can be affected by the actions and experiences of the parents (Skinner et al., 2014). The use of rodent models of environmental exposures has allowed for substantial progress to be made studying the genetic and epigenetic inheritance of parental exposures to exogenous stimuli. Parental exposure to changes in diet (Ng et al., 2010), environmental toxins (Skinner et al., 2014), and stress (Dietz et al., 2011; Franklin et al., 2010; Rodgers et al., 2013; Saavedra-Rodríguez & Feig, 2013; Yehuda et al., 1998) promote altered behavior, physiology, and disease predisposition in the offspring of future generations. These findings have been extended to drugs of abuse. The effects of parental exposure to cocaine (Vassoler, White, Schmidt, Sadri-Vakili, & Pierce, 2013), morphine (Byrnes, Johnson, Carini, & Byrnes, 2013), cannabinoids (Szutorisz et al., 2014), and alcohol (Finegersh & Homanics, 2014) have been studied and each demonstrates some impact on offspring behavior. NIC exposure may also impact multi- and transgenerational inheritance. For example, mice exposed to NIC in utero produce two generations of offspring with hyperactivity (Zhu, Lee, Spencer, Biederman, & Bhide, 2014), altered metabolism (Holloway, Cuu, Morrison, Gerstein, & Tarnopolsky, 2007), and a predisposition to respiratory disease (Rehan et al., 2012).
We utilized a novel multigenerational exposure paradigm to identify the effects of two environmental exposures during a vulnerable developmental window on behavior in future generations of offspring. F0 NIC and F1 CUS exposure produced striking changes in the F2 and F3 offspring’s NIC responses. By including both males and females in our analysis, we considered sex as a relevant biological variable that could affect exposure inheritance across generations (Clayton, 2016). As a result, our work is the first to assess transgenerational the effects of NIC and stress exposure across three generations in both male and female mice.
Despite epidemiological evidence supporting the inheritance of NIC exposure in offspring (Hillemacher et al., 2008; Mill & Petronis, 2008), only a few studies have utilized rodent models to examine the multi- and transgenerational effects of NIC exposure (Holloway et al., 2007; Rehan et al., 2012; Zhu et al., 2014). For the most part these previous studies used in utero NIC administration paradigms and produced mice that were directly exposed to drug. NIC is known to cross the placental barrier and thus can directly affect the developing fetus (Jordanov, 1990). In addition, in utero exposure paradigms include maternal responses and distress (Lambers & Clark, 1996) that could influence fetal development and have a major impact on behavior. To eliminate these factors and evaluate the inheritance of postnatal NIC exposure, we exposed F0 male mice to NIC via osmotic minipumps during adolescence, removed the pumps following 1-month of exposure, and mated them to produce a generation of F1 offspring.
We focused exclusively on paternal NIC exposure in the F0 generation. When drug exposure occurs in F0 males, the germ cells that produce the F1 generation are also “exposed”. Therefore, phenotypes found in F0 and F1 animals are multigenerational. In contrast, phenotypes in F2 animals and all subsequent generations are transgenerational; F2 animals are the first generation whose cells have not been exposed to drug (for review see Yohn, Bartolomei, & Blendy, 2015). Further, males were no longer administered NIC at mating and were removed from the mating cage prior to the birth of pups. By ensuring no interaction with NIC-exposed fathers and a short mating window, we attempted to minimize the impact of paternal NIC exposure on maternal care (Curley, Mashoodh, & Champagne, 2011).
The experience of stress is a particularly salient environmental exposure in both humans and animals. Chronic stress promotes maladaptive responses and disease states in the individual exposed to stress (McEwen & Stellar, 1993) as well as altered physiology and behavior in several generations of offspring in both humans and animals (Dietz et al., 2011; Franklin et al., 2010; Rodgers et al., 2013; Saavedra-Rodríguez & Feig, 2013; Yehuda et al., 1998). However, we wanted to know if stress exposure across generations would interact with NIC to influence subsequent behavior. Previous work by Zhu and colleagues (2014) identified a hyperactive phenotype in the male and female offspring of mice that were exposed to NIC in utero. Thus, we decided to focus on NIC-induced locomotor activity.
The influence of F0 NIC exposure on subsequent generations was dependent on both the sex of the F1 parents and their offspring. When derived from the F1 male CON/CUS lineage, the F2 females displayed increased sensitivity the locomotor effects of NIC, as indicated by a robust increase in NIC-induced locomotion on day 4. These results reflect an alteration in the induction of sensitization. However, the expression of sensitization was not affected by F0 NIC exposure, as the F2 females’ NIC-induced locomotor responses on the challenge day were similar to that of day 1. Notably, there are unique NIC-induced neurobiological adaptations that contribute to the induction, but not the expression, of sensitization. Furthermore, these adaptations are required, but they are not sufficient for the expression of sensitization (DiFranza & Wellman, 2007). As such, it is possible that the decreased response to nicotine on the challenge day results from a lack of maintenance of the sensitized response to repeated NIC exposures and may reflect the development of tolerance (Tapper et al., 2004). The influence on the induction of sensitization was transient and no changes were observed in the F3 offspring.
Surprisingly, when offspring were derived from the F1 female CON/CUS lineage the effects of F0 NIC exposure manifested as an enhancement in the induction and expression of sensitization one generation removed in the F3 offspring, and then only in females. Sex differences in the regulation of central dopaminergic neurotransmission may be a contributing factor in modulating nicotine locomotor responses. For example, the increase in the extracellular dopamine concentration in the nucleus accumbens has been reported to be higher in female rats than in male rats following systemic nicotine administration (Pogun, 2001). The long-lasting facilitation of locomotor sensitization in these F3 females suggests that NIC exposure might target unique neurobiological processes when transmitted through the female lineage. The two main phases of locomotor sensitization are the induction and expression (Robinson, Browman, Crombag, & Badiani, 1998; Todtenkopf, Mihalakopoulos, & Stellar, 2002). The induction phase relies on behavioral and physiological events elicited by the repeated psychostimulant administration, resulting in enduring neurochemical, molecular and, depending on drug class, morphological alterations in mesolimbic-cortical pathways. During expression, it is thought that the long-term changes developed during induction are now consolidated giving rise to the subsequent sensitization responses observed weeks to months after the last drug exposure (Robinson & Berridge, 1993). Thus, prior exposure to nicotine and stress, may differentially impact these mechanisms in males and females.
Future studies are required to address mechanisms underlying lineage-dependent effects of F0 NIC exposure. Epigenetic modifications could underlie the longevity of psychiatric conditions such as drug abuse both within, as well as across generations (Nielsen, Utrankar, Reyes, Simons, & Kosten, 2012). Studies show that non-imprinted genes and repetitive genomic elements can escape loss of methylation patterning that typically occurs following reprogramming events (Lane et al., 2003; Orozco et al., 2014). Thus, the retention of genomic methylation patterns in sperm of exposed parents and brains of offspring may occur following generational stress (Franklin et al., 2010) or drug-exposure (Govorko, Bekdash, Zhang, & Sarkar, 2012). Interestingly, Dai et al. (2017) found that paternal nicotine exposure downregulates miR-15b expression, due to DNA hypermethylation, in both F0 sperm and the thalamus of F1 offspring. Further, virally-mediated overexpression of thalamic miR-15b also prevented the reduction in anxiety- and depression-like behavior that was displayed by F1 males following F0 NIC exposure. As such, our findings may reflect the contribution of similar epigenetic modifications that arise from the female germline. To date, few studies have examined the transgenerational effects of F0 exposure in females, possibly due to the confound of maternal care, even if offspring are cross-fostered, and the effect on F1 generations.
Overall, our findings raise important questions about the impact of parental exposure of drugs on their offspring’s susceptibility to responses to similar agents. However, caution must be exercised when interpreting these results. Given that sensitization is associated with neurobiological adaptations in the same brain reward circuits that are implicated in addiction (DiFranza & Wellman, 2007; Robinson & Berridge, 1993), it is tempting to speculate that enhanced NIC sensitization translates to increased risk for NIC addiction in F2/F3 offspring. However, it is unknown whether sensitization occurs in human smokers. Theories postulate NIC sensitization enhances the salience of reward-related cues associated with NIC. These sensitized cues may drive compulsive craving and NIC-seeking behavior (Robinson & Berridge, 1993). Alternatively, sensitization may enhance NIC’s ability to suppress the generation of cravings. Repeat NIC exposure would therefore result in a homeostatic imbalance that, in the absence of NIC, enhances craving and drives NIC-seeking behavior (DiFranza & Wellman, 2007). Few, if any, studies have tested these theories experimentally. In lieu of empirical evidence supporting a role for sensitization in NIC addiction, any such interpretation remains speculative.
Conclusion
In this study we exposed F0 males to nicotine and F1 males to stress and determined the transgenerational interaction of nicotine and stress on offspring behavior. Remarkably, we found that environmental exposures are subject to cross-generational inheritance and produce unique phenotypes in offspring. In addition, we identified novel phenotypes in several generations of offspring derived from paternal nicotine exposure. When considering these results, we might infer that an epigenetic mechanism is in play as our behaviors occur over multiple generations. Importantly, the occurrence of a behavioral phenotype may occur proximal to the exposure (e.g., from F1 male CON/CUS to the next F2 generation) or it may skip generations (e.g., from F1 female CON/CUS to the F3 generation). Future work to mechanistically identify and probe cellular changes that mediate the phenotypes characterized for functional significance will greatly add to our knowledge of transgenerational interactions and reprogramming of the offspring brain.
Supplementary Material
Acknowledgement
This work was supported by the National Institutes of Health grants from the National Institute of Drug Abuse DA033646 (J.A.B.) and T32DA28874 (N.L.Y). We thank Kenneth Bisson and Gavin Huang for experimental assistance.
Footnotes
Conflicts of Interest
The authors declare no conflict of interests
References
- Agaku IT, King BA, & Dube SR (2014). Current cigarette smoking among adults - United States, 2005–2012. MMWR. Morbidity and Mortality Weekly Report, 63(2), 29–34. 10.1001/jama.2012.114523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari SD, Akbarali HI, Abdullah RA, Shahab O, Auttachoat W, Ferreira GA, … Damaj MI (2013). Novel Insights on the Effect of Nicotine in a Murine Colitis Model. Journal of Pharmacology and Experimental Therapeutics, 344(1), 207–217. 10.1124/jpet.112.198796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, … Diehl K (2015). Tobacco use among middle and high school students - United States, 2011–2014. MMWR. Morbidity and Mortality Weekly Report, 64(14), 381–385. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25879896 [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, & Sharp DS (1989). Inverse relation between serum cotinine concentration and blood pressure in cigarette smokers. Circulation, 80(5), 1309–1312. 10.1161/01.CIR.80.5.1309 [DOI] [PubMed] [Google Scholar]
- Benwell MEM, & Balfour DJK (1979). Effects of nicotine administration and its withdrawal on plasma corticosterone and brain 5-hydroxyindoles. Psychopharmacology, 63(1), 7–11. 10.1007/BF00426913 [DOI] [PubMed] [Google Scholar]
- Booker TK, Butt CM, Wehner JM, Heinemann SF, & Collins AC (2007). Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacology Biochemistry and Behavior, 87(1), 146–157. 10.1016/j.pbb.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Carini LM, & Byrnes EM (2013). Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology, 227(2), 263–272. 10.1007/s00213-012-2960-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso MJ, Reiss DE, Caulfield JI, Thomas JL, Baker AN, Cavigelli SA, & Kamens HM (2018). Adolescent chronic variable social stress influences exploratory behavior and nicotine responses in male, but not female, BALB/cJ mice. Brain Research Bulletin, 138(July 2017), 37–49. 10.1016/j.brainresbull.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA (2016). Studying both sexes: A guiding principle for biomedicine. FASEB Journal, 30(2), 519–524. 10.1096/fj.15-279554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R, & Champagne FA (2011). Epigenetics and the origins of paternal effects. Hormones and Behavior 10.1016/j.yhbeh.2010.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Wang Z, Xu W, Zhang M, Zhu Z, Zhao X, … Qiao Z (2017). Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-miR-15b. Scientific Reports, 7(1), 1–15. 10.1038/s41598-017-07920-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI (2003). Characterization of Spontaneous and Precipitated Nicotine Withdrawal in the Mouse. Journal of Pharmacology and Experimental Therapeutics, 307(2), 526–534. 10.1124/jpet.103.054908 [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, & Fibiger HC (1989). Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. European Journal of Pharmacology, 168(3), 363–368. 10.1016/0014-2999(89)90798-X [DOI] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, … Nestler EJ (2011). Paternal Transmission of Stress-Induced Pathologies. Biological Psychiatry, 70(5), 408–414. 10.1016/j.biopsych.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, & Wellman RJ (2007). Sensitization to nicotine: How the animal literature might inform future human research. Nicotine and Tobacco Research, 9(1), 9–20. 10.1080/14622200601078277 [DOI] [PubMed] [Google Scholar]
- Finegersh A, & Homanics GE (2014). Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS ONE, 9(6). 10.1371/journal.pone.0099078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Grff J, Linder N, Michalon A, … Mansuy IM (2010). Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry, 68(5), 408–415. 10.1016/j.biopsych.2010.05.036 [DOI] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, & Sarkar DK (2012). Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biological Psychiatry, 72(5), 378–388. 10.1016/j.biopsych.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE (1990). The inverse relationship between tobacco use and body weight. In Kozlowski LT, Annis HM, Cappell HD, Glaser FB, Goodstadt MS, Israel Y, … Vingilis ER (Eds.), Research Advances in Alcohol and Drug Problems (pp. 273–315). Boston, MA: Springer US; [Google Scholar]
- Hamilton BE, Martin JA, Osterman MJK, Curtin SC, & Matthews TJ (2015). Births: Final Data for 2014. National Vital Statistics Reports : From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 64(12), 1–64. https://doi.org/May 8, 2013 [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, & Singleton EG (2010). Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology 10.1007/s00213-010-1848-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, & Keenan RM (1993). Nicotine delivery kinetics and abuse liability. Journal of Consulting and Clinical Psychology, 61(5), 743–750. 10.1037/0022-006X.61.5.743 [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Moskau S, Muschler MAN, Semmler A, Kornhuber J, … Linnebank M (2008). Global DNA methylation is influenced by smoking behaviour. European Neuropsychopharmacology, 18(4), 295–298. 10.1016/j.euroneuro.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Holloway AC, Cuu DQ, Morrison KM, Gerstein HC, & Tarnopolsky MA (2007). Transgenerational effects of fetal and neonatal exposure to nicotine. Endocrine, 31(3), 254–259. 10.1007/s12020-007-0043-6 [DOI] [PubMed] [Google Scholar]
- Jordanov JS (1990). Cotinine concentrations in amniotic fluid and urine of smoking, passive smoking and non-smoking pregnant women at term and in the urine of their neonates on 1st day of life. European Journal of Pediatrics, 149(10), 734–737. 10.1007/BF01959534 [DOI] [PubMed] [Google Scholar]
- Kaati G, Bygren LO, & Edvinsson S (2002). Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. European Journal of Human Genetics, 10(11), 682–688. 10.1038/sj.ejhg.5200859 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers DS, & Clark KE (1996). The maternal and fetal physiologic effects of nicotine. Seminars in Perinatology, 20(2), 115–126. 10.1016/S0146-0005(96)80079-6 [DOI] [PubMed] [Google Scholar]
- Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, & Reik W (2003). Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis, 35(2), 88–93. 10.1002/gene.10168 [DOI] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Pollak DD, Xu S, … Kandel ER (2011). Molecular Mechanism for a Gateway Drug: Epigenetic Changes Initiated by Nicotine Prime Gene Expression by Cocaine. Science Translational Medicine, 3(107), 107ra109–107ra109. 10.1126/scitranslmed.3003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Romm E, Bealer SM, & Collins AC (1985). A test battery for measuring nicotine effects in mice. Pharmacology, Biochemistry and Behavior, 23(2), 325–330. 10.1016/0091-3057(85)90577-5 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, & Kelsey JE (2004). Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Hormones and Behavior, 46(4), 458–466. 10.1016/j.yhbeh.2004.05.004 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the Individual: Mechanisms Leading to Disease. Archives of Internal Medicine, 153(18), 2093–2101. 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- Mereu G, Yoon KWP, Boi V, Gessa GL, Naes L, & Westfall TC (1987). Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. European Journal of Pharmacology, 141(3), 395–399. 10.1016/0014-2999(87)90556-5 [DOI] [PubMed] [Google Scholar]
- Mill J, & Petronis A (2008). Pre- and peri-natal environmental risks for attention-deficit hyperactivity disorder (ADHD): The potential role of epigenetic processes in mediating susceptibility. Journal of Child Psychology and Psychiatry and Allied Disciplines 10.1111/j.1469-7610.2008.01909.x [DOI] [PubMed] [Google Scholar]
- Ng S-F, Lin RCY, Laybutt DR, Barres R, Owens JA, & Morris MJ (2010). Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature, 467(7318), 963–966. 10.1038/nature09491 [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Utrankar A, Reyes JA, Simons DD, & Kosten TR (2012). Epigenetics of drug abuse: Predisposition or response. Pharmacogenomics 10.2217/pgs.12.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco LD, Rubbi L, Martin LJ, Fang F, Hormozdiari F, Che N, … Pellegrini M (2014). Intergenerational genomic DNA methylation patterns in mouse hybrid strains. Genome Biology, 15(5), R68 10.1186/gb-2014-15-5-r68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME (2010). Male-line transgenerational responses in humans. Human Fertility 10.3109/14647273.2010.524721 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, & Caldarone BJ (2002). Effect of nicotine and nicotinic receptors on anxiety and depression. NeuroReport 10.1097/00001756-200207020-00006 [DOI] [PubMed] [Google Scholar]
- Pogun S (2001). Sex differences in brain and behavior: Emphasis on nicotine, nitric oxide and place learning. In International Journal of Psychophysiology (Vol. 42, pp. 195–208). 10.1016/S0167-8760(01)00168-4 [DOI] [PubMed] [Google Scholar]
- Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, … Torday JS (2012). Perinatal nicotine exposure induces asthma in second generation offspring. BMC Medicine, 10 10.1186/1741-7015-10-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews, 18(3), 247–291. 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, & Badiani A (1998). Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neuroscience and Biobehavioral Reviews 10.1016/S0149-7634(97)00020-1 [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, & Bale TL (2013). Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. Journal of Neuroscience, 33(21), 9003–9012. 10.1523/JNEUROSCI.0914-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MAH, Wilson C, Patel UA, Feyerabend C, & Cole PV (1975). Plasma Nicotine Levels after Smoking Cigarettes with High, Medium, and Low Nicotine Yields. British Medical Journal, 2(5968), 414–416. 10.1136/bmj.2.5968.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodríguez L, & Feig LA (2013). Chronic social instability induces anxiety and defective social interactions across generations. Biological Psychiatry, 73(1), 44–53. 10.1016/j.biopsych.2012.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn C, & Gindi R (2015). Electronic cigarette use among adults: United States, 2014. National Center for Health Statistics, (217), 1–7. Retrieved from http://www.cdc.gov/nchs/data/databriefs/db217.pdf [PubMed] [Google Scholar]
- Skinner MK, Yao Y, Robinson A, Zucchi F, Robbins J, Babenko O, … Elbert T (2014). Environmental stress and epigenetic transgenerational inheritance. BMC Medicine, 12(1), 153 10.1186/s12916-014-0153-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK (2006). Human Tobacco Smokers in Early Abstinence Have Higher Levels of beta2* Nicotinic Acetylcholine Receptors than Nonsmokers. Journal of Neuroscience, 26(34), 8707–8714. 10.1523/JNEUROSCI.0546-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2011). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse, (HHS Publication No. (SMA) 11–4658), 1–143. https://doi.org/NSDUH Series H-41, HHS Publication No. (SMA) 11–4658 [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, … Hurd YL (2014). Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology, 39(6), 1315–1323. 10.1038/npp.2013.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, … Lester HA (2004). Nicotine Activation of ɑ4* Receptors: Sufficient for Reward, Tolerance, and Sensitization. Science, 306(5698), 1029–1032. 10.1126/science.1099420 [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Mihalakopoulos A, & Stellar JR (2002). Withdrawal duration differentially affects c-fos expression in the medial prefrontal cortex and discrete subregions of the nucleus accumbens in cocaine-sensitized rats. Neuroscience, 114(4), 1061–1069. 10.1016/S0306-4522(02)00272-5 [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, & Blendy JA (2011). Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine and Tobacco Research, 13(1), 41–46. 10.1093/ntr/ntq206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, & Pierce RC (2013). Epigenetic inheritance of a cocaine-resistance phenotype. Nature Neuroscience, 16(1), 42–47. 10.1038/nn.3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Schmeidler J, Wainberg M, Binder-Brynes K, & Duvdevani T (1998). Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. American Journal of Psychiatry, 155(0002–953X (Print)), 1163–1171. 10.1176/ajp.155.9.1163 [DOI] [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, & Blendy JA (2015). Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, andnicotine. Progress in Biophysics and Molecular Biology 10.1016/j.pbiomolbio.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, & Blendy JA (2017). Adolescent Chronic Unpredictable Stress Exposure Is a Sensitive Window for Long-Term Changes in Adult Behavior in Mice. Neuropsychopharmacology, 42(8), 1670–1678. 10.1038/npp.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, Turner JR, & Blendy JA (2014). Activation of α4β2*/α6β2* nicotinic receptors alleviates anxiety during nicotine withdrawal without upregulating nicotinic receptors. The Journal of Pharmacology and Experimental Therapeutics, 349(2), 348–354. 10.1124/jpet.113.211706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, & Leslie FM (2015). N icotine and the adolescent brain. The Journal of Physiology, 593(16), 3397–3412. 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lee KP, Spencer TJ, Biederman J, & Bhide PG (2014). Transgenerational Transmission of Hyperactivity in a Mouse Model of ADHD. Journal of Neuroscience, 34(8), 2768–2773. 10.1523/JNEUROSCI.4402-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.