Abstract
Rates of heavy alcohol use in soup kitchen attendees range from 30% to 38%, but these data are based entirely on self-reported drinking. Little is known about the intensity or frequency of drinking in this population. We assessed alcohol use transdermally every 30 minutes over a 3-week period among heavy drinkers who attended local soup kitchens. In addition to transdermal alcohol monitoring, participants were randomly assigned to daily breath alcohol monitoring with or without reinforcement for alcohol-negative breath samples (BrAC). Analyses assessed feasibility of transdermal monitoring and examined alcohol use based on BrAC, transdermal, and self-report data, as well as effect sizes for these metrics based on group assignment. Nineteen participants completed the 21-day monitoring period in full; three persons removed the anklet 3–16 days early due to hospitalization, impending hospitalization, or incarceration. Participants reported minimal impacts of the monitors and severity ratings of side effects were mild. When using BrAC, transdermal, and self-report data, the percentage of non-drinking days was 93%, 58%, and 57% and the longest duration of consecutive non-drinking days averaged 10.3, 7.2, and 5.7 days, respectively. About half of drinking days involved heavy drinking (5+ drinks). Self-report and transdermal drinking days correlated significantly, p < .001, but neither index was associated with BrAC. Group comparisons indicate small-to-moderate sized effects of reinforcement compared to no reinforcement for increasing the proportion of alcohol negative breath samples and durations of consecutive non-drinking samples during the study when BrAC was the metric. Transdermal data and self-report data indicated a more complex pattern. Reinforcement participants drank more often but at lower quantities than monitoring (control) participants per both transdermal and self-report data. These data suggest that transdermal monitors are well tolerated and documented substantial heavy drinking in this population. Soup kitchens users are in need of alcohol interventions, and soup kitchens may represent a novel opportunistic setting for intervention delivery for an important and growing health disparities population.
Keywords: at-risk alcohol use, hazardous alcohol use, transdermal monitoring, brief interventions, contingency management
Soup kitchen or similar service settings are widespread, with 100,000+ emergency food programs throughout the country (Weinfield et al., 2014). The majority of users attend regularly (Feeding America, 2014), suggesting a potential for intervention delivery in community settings familiar to those who use them. These settings may also be ideal for accessing those who would not seek treatment on their own (Kayman, Gordon, Rosenblum, & Magura, 2005; Rowe, Fisk, Frey, & Davidson, 2002).
One such intervention target to focus on is alcohol misuse, which is common in soup kitchen users. Magura et al. (2000) surveyed 219 soup kitchen clients using systematic sampling methods; 43% of men and 19% of women reported past-month heavy alcohol use of 5+ drinks per day. Similarly, Nwakeze et al. (2003) randomly sampled 343 clients at two NYC soup kitchens; 38% reported drinking 5 or more drinks in a day in the past 30 days. Though drinking is prevalent among attendees, heavy drinkers in soup kitchens are less likely than drug users to recognize their use as problematic (Nwakeze, Magura, & Rosenblum, 2002), suggesting they are unlikely to seek treatment on their own and may benefit from outreach interventions targeting alcohol misuse.
Despite the high rates of heavy alcohol use, few studies have evaluated alcohol use in depth among soup kitchen users. All studies relied on self-report using global assessments of frequency of drinking (Magura et al., 2000; Nwakeze et al., 2002; Rosenblum, Magura, Kayman, & Fong, 2005). More nuanced data on alcohol consumption patterns, especially using objective indices of use, are needed. Transdermal monitors are available that provide continuous assessment of alcohol use every 30 minutes 24 hours a day, 7 days a week while the devices are worn. These monitors have several advantages over other alcohol measurement methods. They do not rely on participant recall, and they do not require participant inputs as is the case with breathalyzers (i.e., all data are collected passively); they therefore allow for measurement of alcohol use in the natural environment. Transdermal monitoring is reliable and valid, distinguishing between no, low, and high alcohol intake (Dougherty et al., 2012; Sakai, Mikulich-Gilbertson, Long, & Crowley, 2006). Overall accuracy of detected drinking events is about 57–73% (Barnett et al., 2014; Marques & Mcknight, 2009), and accuracy is dependent on quantity consumed. Detection of single drink events is low (60%), but detection rates increase to 95% to 100% for 2 and 3 standard drinks (Roache et al., 2015). Transdermal readings can also be used to estimate peak BrAC (Luczak et al., 2017; Luczak & Rosen, 2014) in both controlled (Dougherty et al., 2012) and self-paced drinking conditions (Hill-Kapturczak et al., 2014).
Transdermal alcohol assessment can be integrated into alcohol research studies to provide objective evidence for or against an intervention’s efficacy, and as noted above, low-intensity alcohol and drug abuse interventions are needed in soup kitchen settings (Kayman et al., 2005; Nwakeze et al., 2003; Wicks, Trevena, & Quine, 2006). Interventions that can be delivered by non- or para-professional staff have the greatest likelihood of adoption and robust implementation across soup kitchens. This pilot study evaluated one such intervention, known as contingency management (CM), and used transdermal monitors to assess outcomes. CM is based on behavioral principles and uses reinforcement to motivate behavior change such as reducing drinking (Petry, 2000, 2012). The intervention is brief (average of 6 min: Petry, Alessi, & Ledgerwood, 2012), and it can be readily integrated alongside other interventions or delivered on its own (Petry, 2012).
Perhaps because these monitors can detect alcohol use continuously, several investigations have designed CM protocols that base opportunities for reinforcement on data from the transdermal sensors (Barnett, Tidey, Murphy, Swift, & Colby, 2011; Barnett et al., 2017; Dougherty, Lake, et al., 2015; Dougherty, Karns, et al., 2015). While this use of transdermal monitors within CM may have applications in treatment and criminal justice settings, their likelihood of use in resource-limited settings such as soup kitchens is much lower due to high unit costs and per person daily monitoring fees. Other available assessment methods such as alcohol urine testing (EtG) also have unique obstacles that may impact adoption potential (e.g., acceptability of urine collection and testing in non-clinical settings). Thus, in this study, we examined a CM program that reinforces alcohol negative breath samples because breathalyzers are simple to operate, low cost, and more likely to be accepted by soup kitchen staff. However, in recognition of the limits of breath sample testing (i.e., it can only detect drinking that occurred over the past several hours), we also asked all participants to wear transdermal monitors for the entire intervention period. With this more complete record of alcohol use, we are able to test whether a breathalyzer-based CM intervention decreases alcohol use or merely shifts drinking to avoid detection.
There were two aims of the study. The first was to assess the feasibility and acceptability of alcohol monitoring that involved daily breath samples and continuous transdermal monitoring of alcohol use to characterize alcohol use among heavy drinking soup kitchen attendees. The second aim was to examine these drinking outcomes by study conditions (monitoring vs. monitoring plus CM) using a multi-method approach that included breath samples, transdermal readings, and self-reported drinking data.
Method
Participants
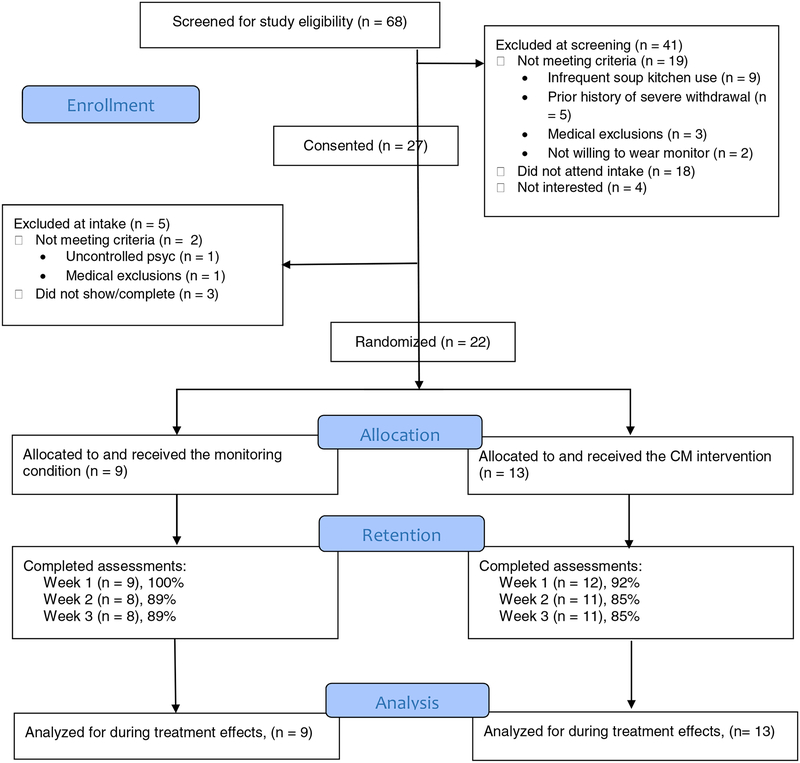
The study was conducted at a soup kitchen in the Greater Hartford, CT area. We recruited soup kitchen guests with recent heavy drinking using flyers, referrals, and word-of-mouth efforts. The Institutional Review Board approved study procedures, and participants provided written informed consent to participate. Interested individuals completed a brief screen, and those likely to be eligible were invited to schedule an intake interview. Of 69 individuals screened, 27 participants were consented and 22 were randomized to one of two study conditions (described below). See Figure 1 for the flow of participants through the study.
Figure 1.
CONSORT diagram.
Participants met the following inclusion criteria: 1) age ≥18; 2) soup kitchen use in the past month and intent to continue use for the next month; 3) past year at-risk drinking as indicated by Alcohol Use Disorders Identification Test (AUDIT; Saunders, Aasland, Babor, De La Fuenta, & Grant, 1993) score > 7 or past-month self-reported heavy drinking; 4) willing to return for study visits and to provide locators; 5) willing to wear a transdermal alcohol monitor for 3 weeks; and 6) willing to sign a property transfer form and return the transdermal monitor. The heavy drinking criteria could be met based on weekly alcohol intake or by episodic drinking that exceeds recommendations (US Department of Health and Human Services, 2005). Participants reporting total weekly consumption in excess of 14 drinks per week for men or 7 drinks per week for women for at least 1 week in the past month were considered at-risk drinkers. Those with episodes of drinking that exceeded 4 drinks per day for men or 3 drinks per day for women on 3 or more occasions in the past month were also classified as at-risk drinkers.
Exclusion criteria included: 1) uncontrolled, severe psychopathology and/or severe cognitive impairment; 2) exhibiting moderate or severe alcohol withdrawal symptoms (as assessed by the Clinical Institute Withdrawal Assessment for Alcohol Revised scale; Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989) or report a history of generalized seizures or delirium tremens; 3) non-English speaking; 4) currently wearing or planning to wear an ankle monitoring device; 5) currently living in an environment that regularly screens for alcohol use; 6) in recovery for gambling disorder; 7) has a medical condition that would interfere with transdermal alcohol readings; or 8) legal charges pending that are likely to lead to incarceration.
Procedure
For eligible individuals, research assistants fit and initialized the transdermal monitor. Participants were instructed to leave the anklet on as fitted for the duration of the monitoring period. Research staff could make adjustments for comfort as needed. Participants could shower normally but were to avoid submerging the anklet in water (i.e., avoid baths, swimming). On the same day that anklets were fitted, a computerized urn program (Charpentier, 2003) randomized participants to one of two study conditions, balancing groups by alcohol problem severity (AUDIT scores of ≤15 vs ≥ 16). The AUDIT cut-off was used to distinguish between those with medium versus higher levels of alcohol problems following the World Health Organization manual (Babor, Higgins-Biddle, Saunders, & Monteiro, 2001) that recommends more intensive intervention for those with scores of 16 or more.
Participants wore the transdermal monitor continuously over the 3-week active monitoring phase. In addition, during this phase, research staff were available before and after the lunchtime soup kitchen meal on weekdays for daily breath sample submission and to collect participants’ self-report of drinks consumed for the prior day/weekend. Once weekly, the research assistants uploaded SCRAMx data from the monitor. At the end of the SCRAMx monitoring period, participants completed the postwear survey.
All participants received $30 for completing the baseline visit, $15 for putting on the bracelet, $10 for each weekly visit, and $20 for completing the follow-up visit. In addition, participants received $15 if they wore the ankle monitor for the full 3-week period plus $40 for returning equipment in working condition at the end of the active phase. To promote regular daily visits for breath tests, participants earned $2 in small items for each daily breath alcohol sample ($30 total if all visits attended).
Measures
Self-report measures.
Following determination of eligibility, research assistants interviewed participants to collect demographics, homelessness history, and alcohol use characteristics. Data were entered directly into REDCap, a secure web-based data capture system (Harris et al., 2009). Information on homelessness and housing history and current housing status was assessed using the Homeless Supplement to the Diagnostic Interview Schedule (HSDIS; North et al., 2004). Alcohol-related measures included the Alcohol Use Disorder Identification Test (AUDIT; Saunders et al., 1993) and the Short Index of Problems (SIP; Miller, Tonigan, & Longabaugh, 1995). The AUDIT is a 10-item questionnaire that assesses past year frequency of alcohol use and symptoms of alcohol use disorders. Chronbach’s alpha was 0.86 in this sample. The SIP contains 15 items evaluating consequences of alcohol use, and it has good psychometric properties (Feinn, Tennen, & Kranzler, 2003; Forcehimes, Tonigan, Miller, Kenna, & Baer, 2007). Chronbach’s alpha for the SIP was 0.94.
The Timeline Follow-back (TLFB; Sobell & Sobell, 1992) retrospectively assessed alcohol use and soup kitchen use in the 30 days preceding the baseline interview and at each daily research visit. Monthly calendars were printed and used as visual cues for the participant during recall. Research assistants recorded reported alcohol use since the last visit (e.g., “…since I saw you yesterday?”). The TLFB has good reliability and validity, including in the homeless population (Sacks, Drake, Williams, Banks, & Herrell, 2003).
A satisfaction survey assessed participant experiences and satisfaction with wearing the transdermal alcohol monitors and providing daily breath samples. This measure asked about physical and social comfort on a scale of 1 to 10, with 1 indicating ‘extremely uncomfortable,’ 5 indicating ‘didn’t notice,’ and 10 indicating ‘very comfortable.’ Participants also reported interference with daily activities (e.g., clothing choices, exercise, swimming, sleep) on a scale of 1 to 10, with 1 indicating ‘not at all’ and 10 indicating ‘completely.’ Marks on skin were assessed using a scale of 0 (‘never’) to 5 (‘all the time’). Participants reported other side effects (e.g., desire to swim/bathe, clothing choices) using a scale of 1 to 10, with 1 indicating ‘not noticeable’ and 10 indicating ‘unbearable.’ We also asked about willingness to wear the anklet in the future and for longer periods of time.
Objective indices of alcohol use.
Participants submitted breath samples using Alcosensor IV monitors (Intoximeters, St. Louis, MO). The timeline between drinking episode and possible detection of alcohol use via breathalyzers is dependent on how much and how recently alcohol was consumed, and on rates of consumption, absorption and elimination. Individual characteristics such as age, gender, body weight and composition, fasting status, and liver health also affect this process. Roughly, single drink events might have a detectable window as short as 1 hour, whereas heavy drinking episodes (4–6 drinks) can be detected up to 8 hours later in healthy adults and as much as 24 hours later in those with compromised livers (Saunders & Paton, 1981).
Transdermal alcohol use data were collected using Secure Continuous Remote Alcohol Monitors (SCRAMx; Alcohol Monitoring Systems, Inc., Littleton, CO). SCRAMx is a sensitive, objective indicator of drinking that collects samples about every 30 minutes continuously and more often when drinking is detected. These readings are date- and time-stamped and stored on the anklet for up to about 4 weeks until the data are uploaded using a USB connection.
Study Conditions
Alcohol monitoring.
Breath alcohol samples were collected once daily (M-F) for 21 days. All participants wore the transdermal alcohol monitors during the active phase, but these alcohol readings intentionally were not viewed during the intervention period.
Monitoring plus Prize CM.
In addition to the SCRAMx and daily breath sample monitoring, participants in this condition earned opportunities to draw cards from a prize bowl for alcohol negative breath samples. The cards differed in prize value, ranging from $0 to $100 (below). Participants earned 1 draw for the first negative (≤ .002 g/dl) breath alcohol sample and the number of draws increased by1 draw for each consecutive negative sample up to a cap of 5 draws. Once the cap of 5 draws was achieved, participants continued to earn 5 draws for each consecutive negative sample. In addition to the daily draws earned, participants earned 10 bonus draws for each week (five consecutive days) of all negative breath samples. No draws were earned for alcohol positive samples, unexcused absences (e.g., no shows), or refused samples, and these events reset the schedule to 1 draw for the next negative sample with escalation of reinforcement resuming with consecutive negative samples. Excused absences (e.g., court appearance) were accommodated with valid proof and did not reset the reinforcement schedule.
The prize bowl contained 500 cards; 50% were winning and the remainder were non-winning (i.e., the card said “Good Job”). The majority (209/500) of winning cards were small prizes (about $1 in value); 40/500 cards were large prizes (up to $20 in value). One card (1/500) was a jumbo prize (up to $100 in value). Typical small prizes included small toiletries, food items, and bus tokens. Large prizes were small electronics, gift cards, and clothing items. Jumbo prizes included items such as small electronic tablets, TVs, or $100 in gift cards. Participants could earn 95 draws, with an expected average maximum earned per person of about $211 over 3 weeks.
Data Analysis
The main treatment outcomes for breath analysis were the percentage of BrAC-negative samples submitted and the longest duration of non-drinking days (LDND) based on negative BrAC samples. Percent negative samples was calculated with the number of negative samples as the numerator and total samples submitted in the denominator. LDND represents the longest period of consecutive days of negative BrAC samples, uninterrupted by alcohol positive or refused samples or unexcused absences.
All SCRAMx outcomes were determined by processing transdermal data through software designed for this purpose (Barnett et al., 2015). Drinking episodes were classified as: one or more TAC readings > 0.02 g/dL occurring either with an episode 1) absorption rate of < 0.05 g/dL per hour, or 2) elimination rate of < 0.025 g/dL per hr when the TAC peak is < 0.15 g/dL and < 0.035 g/dL per hr when peak is > 0.15 g/dL. Subthreshold episodes, which may represent drinking episodes of lower quantity than typically detected using the definitions above, were also estimated. These episodes required elevation in TAC that did not fully meet the above rules. Subthreshold episodes were defined as: 1) TAC ≥ 0.02 g/dL but failed both absorption and elimination rules, or 2) 0.01 ≤ TAC ≤ 0.02 g/dL and met one or both of the absorption and elimination rules (Barnett et al., 2015). Given the relaxed rules, subthreshold events are more likely to be a function of environmental noise. Thus, they are reported separately from drinking episodes and are not included in any other outcomes derived from TAC data.
Multiple drinking episodes could occur within a given day if TAC returned to 0 g/dL between episodes. Drinking episodes also could span multiple days if TAC never returned to 0 g/dL; in this case, only one episode would be recorded, but multiple days would be positive for drinking. Drinking days were defined as “social days,” defined as 24-hour periods starting at 8:00am through 7:59am the following day. This timing was intended to reduce the number of cross-over events from prior night drinking, which might artificially inflate the number of drinking days (i.e., both the start day and the next day would be coded as alcohol positive days in a cross-over event) (see Barnett et al., 2017 for further explanation). We also determined the percent of drinking days containing daytime drinking episodes, defined as TAC drinking episodes with start or peak times occurring between 8am-5pm. This variable was examined because alcohol positive breathalyzers (the basis of the reinforcement protocol) would be more likely if drinking occurred during the day, and we were specifically interested in estimating their frequency.
We used available data from the TAC monitors, and outcomes were calculated for all randomized participants, including the three individuals who removed the monitor early. From the TAC drinking episode and day data, we estimated the percentage of participants with drinking events, and we calculated outcomes parallel to the breath test outcomes, specifically percent non-drinking days and LDND. In addition, we reported the following estimates from TAC data: the number of drinking episodes and subthreshold events, episode average and peak TAC, and the percentage of daytime drinking episodes. We also derived estimates of average peak breath alcohol concentration (eBrAC) for each TAC-identified drinking episode using an algorithm (Luczak et al., 2017; Luczak & Rosen, 2014) incorporated into the Barnett et al. (2015) software.
From the self-reported alcohol data, we calculated the parallel outcomes of percent non-drinking days and LDND. In addition, we reported two additional outcomes: average drinks per drinking day and the percentage of heavy drinking days of total drinking days. Heavy drinking was defined as consuming 5 or more drinks in a day. This definition was chosen over NIH definitions that are gender-specific because it is more often reported in studies related to soup kitchen populations (Magura et al., 2000; Nwakeze et al., 2002, 2003). Pearson correlations were estimated for self-report, BrAC, and transdermal data.
For group comparisons of the alcohol outcomes, means, standard deviations, and effect size d were calculated. Significance tests were not performed due to limited power to detect differences if present. Data were analyzed using SPSS (v. 24).
Results
Baseline characteristics
No group differences were noted between study conditions on demographic characteristics, homelessness history, soup kitchen use, or alcohol-related variables. More than half were currently homeless, and emergency shelter use as the primary housing strategy was common in the past month. Past-month soup kitchen use was frequent with an average of 30 visits (SD = 21.8). Participants self-reported alcohol use on 60% of days in the past month, with a median of 6 drinks (SD = 4.7) per drinking day. See Table 1.
Table 1.
Baseline Characteristics of Heavy Drinking Soup Kitchen Attendees by Treatment Condition
| CM (n = 13) | Monitoring (n = 9) | p | |
|---|---|---|---|
| Demographics | |||
| Male, % (n) | 69 (9) | 67 (6) | .63 |
| Hispanic, % (n) | 15 (2) | 0 (0) | .34 |
| Race, % (n) | .31 | ||
| African American | 46 (6) | 44 (4) | |
| Caucasian | 31 (4) | 56 (5) | |
| Other | 23 (3) | 0 (0) | |
| Age, M (SD) | 46.8 (11.2) | 46.0 (12.6) | .90 |
| Housing/Homelessness and Soup Kitchen Use | |||
| Homelessness, % (n) | |||
| Ever homeless | 77 (10) | 100 (9) | .19 |
| Currently homeless | 46 (6) | 78 (7) | .15 |
| Lifetime homeless episodes, M (SD) | 4.6 (3.2) | 3.1 (3.3) | .16 |
| Past year shelter nights, M (SD) | 138.3 (132.2) | 115.2 (143.7) | .84 |
| Past month usual housing, % (n) | .005 | ||
| Housed (apartment or house) | 54 (7) | 0 (0) | |
| Someone else’s home | 15 (2) | 0 (0) | |
| Transitional housing | 0 (0) | 22 (2) | |
| Emergency shelter | 31 (4) | 67 (6) | |
| Literally homeless | 0 (0) | 5 (1) | |
| Soup kitchen meals in past month, M (SD) | 26.1 (21.7) | 35.8 (21.9) | .32 |
| Alcohol | |||
| Alcohol use, past 30 days, M (SD) | |||
| Drinking days | 21.0 (8.5) | 15.7 (7.3) | .13 |
| Drinks per drinking day | 8.0 (4.5) | 8.7 (5.2) | .79 |
| % non-drinking days | 30.0 (28.3) | 47.8 (24.2) | .13 |
| % 5+ drinks/drinking day | 64.8 (39.8) | 70.6 (37.4) | .70 |
| AUDIT score, M (SD) | 15.8 (10.6) | 15.9 (7.4) | .85 |
| SIP total score, M (SD) | 13.4 (13.0) | 15.9 (11.1) | .51 |
AUDIT = Alcohol Use Disorders Identification Test. SIP = Short Index of Problems (Alcohol).
Adherence
Of the 22 participants who initiated the study, 19 (86.4%) completed the 21-day monitoring period in full. Of the three participants who removed the anklet early, one had the anklet removed 3 days early due to incarceration, in one case we removed the anklet 8 days early due to a hospitalization, and the third person requested removal 16 days early because he did not want to wear the device in the days leading up to a planned hospitalization.
No data were lost to bracelet malfunctions. We adjusted anklet fit or re-initialized or replaced the anklet (e.g., due to manufacture recall) 5 times, and one participant requested a temporary removal for a medical visit (replaced same day). Tampers (n = 7) occurred for 3 participants. Five of the 7 (71%) tampers in these 3 participants coincided with SCRAMx drinking episodes. All devices were returned in working condition. Participants attended 78% (SD = 30) of daily research visits.
Acceptability
Participants responses indicate that on average they found the monitors physical and socially comfortable, and it impacted daily activities little (see Table 2). Marks on skin were relatively uncommon. Other side effects were well tolerated, with the most highly endorsed items relating to itching, trouble sleeping, and irritation. Other potential impacts of wearing the monitor (e.g., desire to swim/bathe) and completing study procedures (e.g., data uploads, daily study visits) were not generally concerning for participants. When asked if they would wear the bracelet for longer, 74% indicated that they would.
Table 2.
Monitor Interference with Daily Activities and Side Effects (n = 19)
| Postwear survey items | M (SD) |
|---|---|
| Anklet Interference with daily activities | |
| General activity | 1.8 (1.5) |
| Exercise | 1.8 (2.1) |
| Mood | 2.0 (1.8) |
| Normal work | 1.6 (1.6) |
| Sleep | 3.6 (3.1) |
| Enjoyment of Life | 1.6 (1.5) |
| Ability to concentrate | 1.4 (1.0) |
| Social life | 1.8 (1.4) |
| Choice of clothing | 3.1 (3.3) |
| Other | 1.8 (2.6) |
| Side effects | |
| Marks on skin | 1.0 (1.2) |
| Itching | 3.3 (2.0) |
| Sweating | 2.0 (1.3) |
| Trouble sleeping | 2.9 (2.8) |
| Soreness | 2.2 (2.2) |
| Irritation | 2.8 (2.4) |
| Other | 1.3 (0.9) |
| Other potential impacts | |
| Too uncomfortable to wear for longer | 1.4 (1.5) |
| Desire to swim/bathe | 1.5 (1.8) |
| Tired of explaining the monitor to people | 0.6 (1.3) |
| Don’t want to wear due to embarrassment | 0.3 (0.8) |
| Desire to wear shorts or skirts but won’t | 0.7 (1.5) |
| Just ready to stop wearing anklet | 0.8 (1.4) |
| Dislike data uploads | 0.1 (0.2) |
| Payment would not be worth wearing longer | 0.6 (1.4) |
| Difficulty with study appointments | 0.1 (0.3) |
Interference with daily activities was assessed on a 1 to 10 scale, with 1 indicating ‘not at all’ and 10 indicating ‘completely.’ Side effects were also measured on a scale of 1 to 10, with 1 indicating ‘not noticeable’ and 10 indicating ‘unbearable,’ with the exception of marks on skin, which used a separate response scale: 0 = Never, 1 = Rarely, 2 = Sometimes, 3 = Often, and 4 = All the time. Other impacts were assessed by asking participants how true statements were for them on a scale of 0 (‘not at all true’) to 4 (‘very true’). Three participants did not complete the postwear survey.
Drinking data
Breath alcohol (BrAC).
Participants provided an average of 11.3 (SD = 4.2) breath samples of the 15 expected over three weeks, with no difference in submission rates between study conditions. Positive samples were relatively rare overall. Average percent BrAC negative samples was 93% (SD = 22), and average LDND based on BrAC samples was 10.3 days (SD = 5.7). Table 3 presents group differences. CM participants had a higher percentage of BrAC negative samples submitted (d = 0.45) and longer LDND (d = 0.39) compared to the monitoring only condition.
Table 3.
Drinking Data over 21-day Monitoring Period by Treatment Condition
| Alcohol variables | CM (n = 13) | Monitoring (n = 9) | d |
|---|---|---|---|
| Breath sample data | |||
| % negative samples | 96.2 (9.3) | 88.0 (33.1) | 0.45 |
| LDND (days) | 11.3 (5.5) | 8.8 (6.0) | 0.39 |
| TAC data | |||
| % non-drinking days | 57.2 (29.5) | 59.1 (27.1) | −0.06 |
| LDND (days) | 7.3 (5.5) | 7.0 (3.6) | 0.07 |
| Drinking episodes | 9.9 (7.8) | 6.9 (5.0) | 0.43 |
| Subthreshold episodes | 10.0 (7.2) | 4.4 (3.6) | 0.92 |
| Average TAC/episode | 0.03 (0.03) | 0.04 (0.02) | −0.23 |
| Average peak TAC/episode | 0.08 (0.09) | 0.10 (0.06) | −0.26 |
| % daytime drinking (out of drinking days) | 51.9 (25.9) | 34.8 (27.5) | 0.64 |
| Self-reported drinking | |||
| % non-drinking days | 49.2 (32.7) | 67.5 (31.0) | −0.57 |
| LDND (days) | 4.0 (3.5) | 8.1 (6.2) | −0.86 |
| Drinks per drinking day | 4.3 (3.1) | 5.9 (3.7) | −0.49 |
| % days 5+ drinks/drinking days | 38.2 (37.1) | 52.9 (34.4) | −0.41 |
LDND = longest duration of consecutive non-drinking days/samples. TAC = transdermal alcohol concentration.
Transdermal.
SCRAMx drinking occurred in 21 of 22 participants (95%). The average percentage of SCRAMx non-drinking days was 58% (SD = 28) and average LDND was 7.2 (SD = 4.7). The average number of drinking episodes was 8.6 (SD = 6.8), with an average additional 7.7 (SD =6.5) subthreshold drinking events identified per individual. The vast majority (n = 19 of 22; 86%) had positive daytime (8am-5pm) episodes, and 45% (SD = 27) of drinking positive days included daytime drinking. Average TAC during drinking episodes was 0.04 g/dL (SD = 0.03), and the average peak TAC during drinking episodes was 0.09 g/dL (SD = 0.08). This latter value corresponds to an average peak eBrAC of 0.08 (SD = 0.13) across drinking episodes.
Means and standard deviations by study condition and effect sizes are in Table 3. The monitor detected a similar percentage of drinking days in both study conditions. Focusing on variables with differences of d ≥ |0.20| between groups, CM participants had a higher number of drinking episodes and subthreshold episodes (ds = 0.43 and 0.92, respectively), and had a greater percentage of daytime drinking relative to the monitoring only condition (d = 0.64). Both average TAC and peak TAC values were lower for CM relative to monitoring participants (ds = −0.23 and −0.26, respectively).
Self-reported drinking.
The average percentage of self-reported non-drinking days was 57% (SD = 33) and average LDND was 5.7 (SD = 5.1). Average drinks consumed per drinking day was 5.0 (SD = 3.3). Participants reported consuming 5 or more drinks on average about half of all drinking days (M = 44%, SD = 36). In terms of group differences, the percentage of self-reported non-drinking days and durations of non-drinking were lower in CM relative to the monitoring group (ds = −0.57 and −0.86). CM participants, however, self-reported fewer drinks per drinking day by about one and a half standard drinks (d = −0.49).
Consistency in alcohol use among measurement methods.
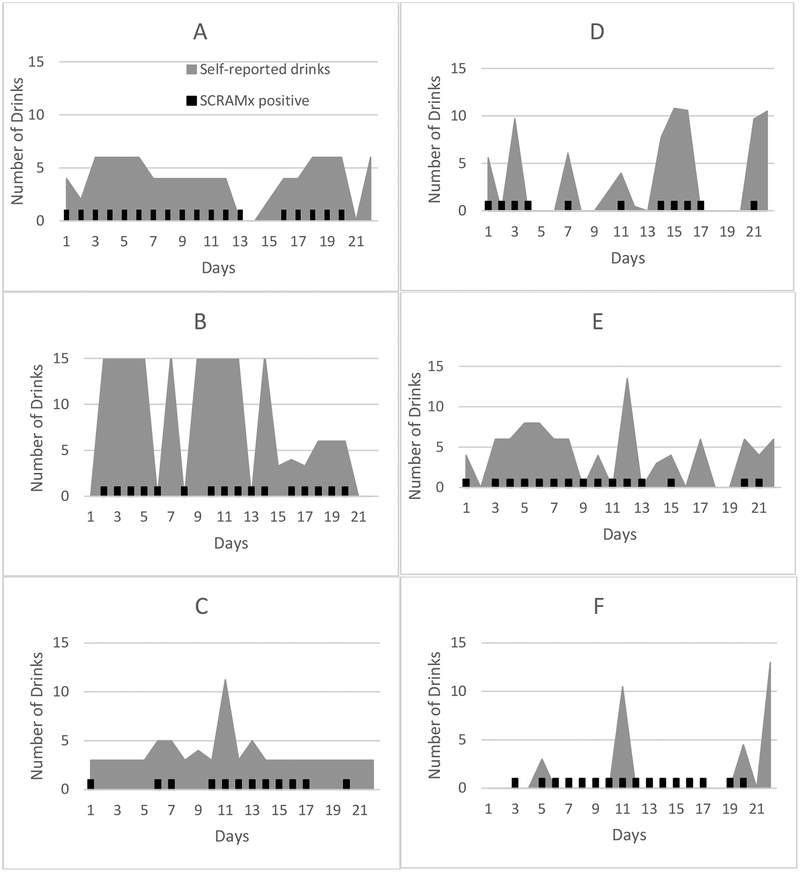
We examined consistency in alcohol use as measured by BrAC, SCRAMx, and self-report. The percent of non-drinking days per self-report and as detected by SCRAMx was significantly correlated, r (n = 22) = 0.68, p < .001, but neither of these metrics correlated significantly with BrAC percent negative. Figure 2 presents graphic displays of the relation between self-reported number of alcoholic drinks (grey) overlaid with SCRAM positive days (black indicators) for 6 participants. The top four panels show SCRAMx and self-report data that are largely consistent; the bottom two panels display two different patterns with less consistency in these measures. A supplemental figure presents concordance among the three indices by day for each participant.
Figure 2.
Self-reported number of standardized alcoholic drinks per day and SCRAMx-detected drinking days. Self-reported number of standardized drinks is shown in grey; black hash marks indicate days that were SCRAMx-detected positive drinking days. The left-side panels (A, B, C) portray frequent drinking with occasional heavy episodes. The first two participants (D, E) in the right-side panel display a more episodic pattern, largely consistent with SCRAMx-detected drinking days. The last participant (F) reported infrequent drinking that was not consistent with SCRAMx reports. Participant C also displays some inconsistency, with more self-reported drinking than detected by the monitor.
We also examined these same relations within each condition. For those in the monitoring group, the above described pattern was observed, with a significant relation between SCRAMx and self-reported percent non-drinking days, r (n = 9) = 0.73, p = .03, and no significant correlations of either variable with BrAC. In the CM group, SCRAMx and self-report were again significantly correlated, r (n = 13) = 0.69, p = .01. No correlation between self-report and BrAC was present for the CM group. However, SCRAMx percent non-drinking days correlated significantly with the same metric derived from BrAC data, r (n = 13) = 0.58, p = .04 for CM participants. When examined LDND, no significant correlations were present across the three metrics either for the full sample or within condition.
Discussion
This study examined the feasibility of multi-method alcohol assessment and a daily alcohol use intervention in soup kitchen attendees. Results suggest that daily breath and continuous transdermal alcohol monitoring are well tolerated, and that these individuals are willing to attend daily brief study visits nearly 80% of the time over a 3-week period. Drinking measurement in this sample of heavy drinkers confirmed that alcohol use is frequent, as is heavy alcohol use, with participants consuming alcohol at a hazardous (i.e., heavy drinking) level on 44% of drinking days. Breathalyzer assessment was rarely positive despite ongoing frequent (including daytime) drinking detected by the transdermal monitors. Parallel outcomes derived from independent breathalyzer and transdermal data indicate more drinking based on transdermal data, as expected given its ability to monitor alcohol use at regular intervals in the natural environment.
Soup kitchen attendees appear willing to wear transdermal monitors when compensated for doing so. In prior work (Alessi et al., 2017), we assessed acceptability of these monitors among patients enrolled in outpatient alcohol treatment. In that study, participants wore the anklets for 12 weeks (with similar compensation), and they still reported minimal impacts on daily living or from side effects. Notably, the SCRAMx monitor is large, and in the Alessi et al. (2017) study, the most common suggestion related to reduction of the size or weight of the unit. The National Institutes of Health sponsored a competition to spur development of wearable alcohol biosensors; the development of a transdermal monitor similar in size to fitness tracker bracelets that maintains the properties of these anklets would greatly enhance its acceptability and may eliminate the need to compensate participants simply for wearing the device. As this technology continues to improve, access and affordability will improve, potentially increasing utility by researchers and treatment providers.
Although the pilot nature of this study did not provide sufficient sample size to detect significant group differences, we reported effect sizes for drinking outcomes. Group comparisons suggested that CM improved the percent negative drinking days and longest duration of non-drinking per breathalyzer data; however, transdermal data indicated these outcomes were similar between groups. Review of other frequency and quantity indicators suggested that contrary to expectations, CM participants increased frequency but decreased quantity of alcohol use relative to the monitoring condition. These patterns were evident in both the self-report and SCRAMx data. In terms of change from baseline behavior, the only pre-post metric of change available in this study was self-reported alcohol use. The percentage of heavy drinking days decreased from 67% at baseline to 38% during the active phase for CM participants and drinks per drinking day decreased from 8.3 to 4.3 drinks. These results may suggest that once-daily breathalyzer-based reinforcement interventions may be useful in decreasing heavy drinking episodes, but its utility may be limited if the intervention goal is decreased drinking days or abstinence. This finding may have important implications for harm reduction. Episodic heavy drinking events comprise the majority of costs related to alcohol use (CDC, 2015). Interventions that reduce heavy drinking have high value even if they do not reduce overall frequency of use, as such reductions decrease the likelihood of negative consequences of binge drinking, such as driving under the influence, violence, unintentional injuries, etc. However, given the small sample size, we recommend caution in interpreting between-groups differences.
As in prior studies (Barnett et al., 2017, 2011; Dougherty et al., 2014; Simons, Wills, Emery, & Marks, 2015), we found overall good agreement between self-report and transdermal data in this non-treatment seeking heavy drinking sample, using both global indices and via day-to-day graphic overlay of these two data sources. The correlation between these indices, but not between these indices and BrAC, might be due to participant’s shifting their drinking patterns to avoid detection by the breathalyzer. In this study, CM participants knew when they were attending the research visit and could have delayed drinking onset until after the appointment, but may have still accurately self-reported alcohol use because these self-reports did not affect reinforcement. BrACs may be more amenable to homeless facilities, where staff could increase testing frequency to mornings and evenings as part of the daily routine. Future studies might also explore the use of random breath tests or use transdermal or urine assessment methodologies in determining CM reinforcement, both of which would be less influenced by these types of temporal shifts in drinking. Nonetheless, even with this limitation of BrACs, participants exposed to CM appeared to reduce heavy drinking behavior according to both transdermal and self-report data.
Consistent with an emphasis on detecting higher quantity alcohol use, we used established rules for detecting alcohol use episodes of TAC greater than 0.02 g/dL with specific absorption and elimination criteria (Barnett et al., 2011). However, this threshold may miss lower level drinking. For example, in Barnett et al. (2014), only 73% of self-reported drinking episodes were detected by the monitors, and the average number of drinks per episode of 2.6 for missed events was lower relative to the average of 7.7 drinks for detected drinking events. Exploration of lower thresholds for detection (Dougherty et al., 2012; Roache et al., 2015) may be important for this population, particularly if used in treatment studies, as our data suggested many subthreshold episodes and more frequent lower level drinking in the intervention condition.
The threshold used to detect drinking also has the potential to affect day-to-day concordance between alcohol use metrics as displayed in Figure 2 and the Supplemental Figure. For example, a participant may have self-reported alcohol use, but the quantity was not sufficient to be coded as an alcohol positive day via the TAC criteria. This pattern would result in fewer SCRAMx positive days compared to self-reported alcohol days. Other potential influences on concordance include the inherent delay in transdermal alcohol detection relative to consumption and cross-over events, in which alcohol consumption started on one day is still detectable in TAC the following day. We used a ‘social day’ (8:00am through 7:59am) to determine drinking days rather than calendar days (i.e., with a cut-off of midnight) to reduce such events, but depending on when alcohol consumption occurred, these influences may be present and contribute to lack of consistency across measures.
Limitations.
Future studies will need to assess rates of alcohol and heavy alcohol use more broadly in the soup kitchen population for updated prevalence estimates, as our study specifically targeted recruitment of heavy drinkers. Larger samples and recruitment from multiple soup kitchens will also be important next steps for studies seeking to characterize alcohol use in this population. An additional limitation was the inability to collect postwear surveys from the three participants who removed the anklets early. This study also did not assess alcohol use beyond the intervention phase, and thus we do not know if the observed heavy drinking reductions were sustained post-intervention. Post-intervention effects on drinking will be critical to establishing the efficacy of brief interventions that can be delivered in soup kitchen settings by non-professionals.
In sum, this study highlights regular ongoing heavy drinking and the need for alcohol interventions in this population. Soup kitchens represent an ideal location for implementing brief interventions, as patients are readily accessible and frequently attend. Further, participants in this study were willing to engage in regular study visits and may attend brief multi-visit formatted interventions if scheduled around the unique needs of this population (i.e., arranged before or after but not conflicting with soup kitchen meal times). These findings are encouraging and should spur more research with this population that has been largely overlooked by the research and treatment communities.
Supplementary Material
Highlights.
Three methods of assessing alcohol use are compared in soup kitchen attendees.
Transdermal monitoring of alcohol use is feasible and acceptable in this setting.
Heavy drinking soup kitchen attendees are in need of alcohol interventions.
Acknowledgments
Financial support: This work was supported by the National Institutes of Health [grant numbers R21-DA031897, P50-DA009241, P60-AA03510, R01-HD075630, R01-AA021446, R01-AA023502, and R01-DA013444]; and the Connecticut Institute for Clinical and Translational Science (CICATS) at the University of Connecticut. The content is solely the responsibility of the authors and does not necessarily represent the official views of CICATS and NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi SM, Barnett NP, & Petry NM (2017). Experiences with SCRAMx alcohol monitoring technology in 100 alcohol treatment outpatients. Drug and Alcohol Dependence, 178(May), 417–424. 10.1016/j.drugalcdep.2017.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, & Monteiro MG (2001). AUDIT: The Alcohol Use Disorders Indetification Test. Geneva, Switzerland: World Health Organization, Department of Mental Health and Substance Dependence. [Google Scholar]
- Barnett NP, Celio MA, Tidey JW, Murphy JG, Colby SM, & Swift RM (2017). A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction, 112(6), 1025–1035. 10.1111/add.13767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Meade EB, & Glynn TR (2014). Predictors of detection of alcohol use episodes using a transdermal alcohol sensor. Experimental and Clinical Psychopharmacology, 22(1), 86–96. 10.1037/a0034821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Souza T, Rosen IG, Luczak SE, Glynn TR, & Swift R (2015). Transdermal alcohol sensor data macro (Version 1.4). Brown University. [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, & Colby SM (2011). Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug and Alcohol Dependence, 118(2–3), 391–399. 10.1016/j.drugalcdep.2011.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control (2015). Excessive alcohol use continues to drain the American economy CDC Newsroom Releases. Washington, DC: US Department of Health and Human Services. [Google Scholar]
- Charpentier PA (2003). gRand urn randomization program, version 1.10. New Haven: Yale Univeristy. [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, John S, Furr RM, & Hill-Kapturczak N (2012). Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Experimental and Clinical Psychopharmacology, 20(5), 373–81. 10.1037/a0029021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL, … Roache JD (2014). Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug and Alcohol Dependence, 142, 301–306. 10.1016/j.drugalcdep.2014.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Karns TE, Mullen J, Liang Y, Lake SL, Roache JD, & Hill-Kapturczak N (2015). Transdermal alcohol concentration data collected during a contingency management program to reduce at-risk drinking. Drug and Alcohol Dependence, 148, 77–84. 10.1016/j.drugalcdep.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Hill-Kapturczak N, Liang Y, Karns TE, Mullen J, & Roache JD (2015). Using contingency management procedures to reduce at-risk drinking in heavy drinkers. Alcoholism: Clinical and Experimental Research, 39(4), 743–751. 10.1111/acer.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeding America. (2014). Hunger in America 2014 Feeding America, 1–28. Retrieved from http://www.feedingamerica.org/hunger-in-america/our-research/hunger-in-america/ [Google Scholar]
- Feinn R, Tennen H, & Kranzler HR (2003). Psychometric properties of the Short Index of Problems as a measure of recent alcohol-related problems. Alcoholism: Clinical & Experimental Research, 27(9), 1436–1441. 10.1097/01.ALC.0000087582.44674.AF [DOI] [PubMed] [Google Scholar]
- Forcehimes AA, Tonigan JS, Miller WR, Kenna GA, & Baer JS (2007). Psychometrics of the Drinker Inventory of Consequences (DrInC). Addictive Behaviors, 32(8), 1699–1704. 10.1016/j.addbeh.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Lake SL, Roache JD, Cates SE, Liang Y, & Dougherty DM (2014). Do variable rates of alcohol drinking alter the ability to use transdermal alcohol monitors to estimate peak breath alcohol and total number of drinks? Alcoholism: Clinical and Experimental Research, 38(10), 2517–2522. 10.1111/acer.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayman DJ, Gordon C, Rosenblum A, & Magura S (2005). A port in a storm: Client perceptions of substance abuse treatment outreach in a soup kitchen. Journal of Social Work Practice, 5(4), 3–25. 10.1300/J160v05n04 [DOI] [Google Scholar]
- Luczak SE, Hawkins AL, Dai Z, Wichmann R, Wang C, & Rosen IG (2017). Obtaining continuous BrAC/BAC estimates in the field: A hybrid system integrating transdermal alcohol biosensor, Intellidrink smartphone app, and BrAC Estimator software tools. Addictive Behaviors, (November), 1–8. 10.1016/j.addbeh.2017.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, & Rosen IG (2014). Estimating BrAC from transdermal alcohol concentration data using the BrAC Estimator Software Program. Alcoholism: Clinical & Experimental Research, 38(8), 2243–2252. 10.1111/acer.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, Rosenblum A, & Joseph H (2000). Substance misuse and related infectious diseases in a soup kitchen population. Substance Use & Misuse, 35(4), 551–583. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10741541 [DOI] [PubMed] [Google Scholar]
- Marques PR, & Mcknight AS (2009). Field and laboratory alcohol detection with 2 types of transdermal devices. Alcoholism: Clinical & Experimental Research, 33(4), 703–711. 10.1111/j.1530-0277.2008.00887.x [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, & Longabaugh R (1995). The Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse (Test Manual) In NIAAA Project MATCH Monograph Series, Vol. 4 NIH Publication No. 95–3911. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- North CS, Eyrich KM, Pollio DE, Foster DA, Cottler LB, & Spitznagel EL (2004). The Homeless Supplement to the Diagnostic Interview Schedule: Test-retest analyses. International Journal of Methods in Psychiatric Research, 13(3), 184–191. 10.1002/mpr.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwakeze PC, Magura S, & Rosenblum A (2002). Drug problem recognition, desire for help, and treatment readiness in a soup kitchen population. Substance Use and Misuse, 37(3), 291–312. 10.1081/JA-120002480 [DOI] [PubMed] [Google Scholar]
- Nwakeze PC, Magura S, Rosenblum A, & Joseph H (2003). Homelessness, substance misuse, and access to public entitlements in a soup kitchen population. Substance Use & Misuse, 38(3–6), 645–668. [DOI] [PubMed] [Google Scholar]
- Petry NM (2000). A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence, 58(1–2), 9–25. 10.1016/S0376-8716(99)00071-X [DOI] [PubMed] [Google Scholar]
- Petry NM (2012). Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York: Routledge. [Google Scholar]
- Petry NM, Alessi SM, & Ledgerwood DM (2012). A randomized trial of contingency management delivered by community therapists. Journal of Consulting and Clinical Psychology, 80(2), 286–298. 10.1037/a0026826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roache JD, Karns TE, Hill-Kapturczak N, Mullen J, Liang Y, Lamb RJ, & Dougherty DM (2015). Using transdermal alcohol monitoring to detect low-level drinking. Alcoholism: Clinical and Experimental Research, 39(7), 1120–1127. 10.1111/acer.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Magura S, Kayman DJ, & Fong C (2005). Motivationally enhanced group counseling for substance users in a soup kitchen: A randomized clinical trial. Drug and Alcohol Dependence, 80(1), 91–103. http://doi.org/10.1016Zj.drugalcdep.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Rowe M, Fisk D, Frey J, & Davidson L (2002). Engaging persons with substance use disorders: Lessons from homeless outreach. Administration and Policy in Mental Health, 29(3), 263–273. 10.1023/A:1015147710813 [DOI] [PubMed] [Google Scholar]
- Sacks JAY, Drake RE, Williams VF, Banks SM, & Herrell JM (2003). Utility of the Time-Line Follow-Back to assess substance use among homeless adults. The Journal of Nervous and Mental Disease, 191(3), 145–153. 10.1097/01.NMD.0000054930.03048.64 [DOI] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Long RJ, & Crowley TJ (2006). Validity of transdermal alcohol monitoring: Fixed and self-regulated dosing. Alcoholism: Clinical & Experimental Research, 30(1), 26–33. 10.1111/j.1530.0277.2006.00004.x [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuenta JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction, 88(6), 791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Saunders J, & Paton A (1981). ABC of alcohol: Alcohol in the body. British Medical Journal, 283(6303), 1380–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Wills TA, Emery NN, & Marks RM (2015). Quantifying alcohol consumption: Self-report, transdermal assessment, and prediction of dependence symptoms. Addictive Behaviors, 50, 205–212. 10.1016/j.addbeh.2015.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline Follow-back: A technique for assessing self-reported alcohol consumption In Litten R & Allen R (Eds.), Measuring Alcohol Consumption (pp. 41–71). Totowa, NJ: Humana Press. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo C. a, & Sellers EM (1989). Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWAAr). British Journal of Addiction, 84(11), 1353–1357. 10.1111/j.1360-0443.1989.tb00737.x [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2005). Helping patients who drink too much: A clinician’s guide. Washington, DC: Retrieved from http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf [Google Scholar]
- Weinfield NS, Mills G, Borger C, Gearing M, Macaluso T, Montaquila J, & Zedlewski S (2014). Hunger in America 2014: National Report, (August), 176.
- Babor TF, Higgins-Biddle JC, Saunders JB, & Monteiro MG (2001). AUDIT: The Alcohol Use Disorders Indetification Test. Geneva, Switzerland: World Health Organization, Department of Mental Health and Substance Dependence. [Google Scholar]
- Wicks R, Trevena LJ, & Quine S (2006). Experiences of food insecurity among urban soup kitchen consumers: Insights for improving nutrition and well-being. Journal of the American Dietetic Association, 106(6), 921–924. http://doi.org/10.1016Zj.jada.2006.03.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.