Abstract
The stressors associated with poverty increase the risks for externalizing psychopathology; however, specific patterns of neurobiology and higher self‐regulation may buffer against these effects. This study leveraged a randomized control trial, aimed at increasing self‐regulation at ~11 years of age. As adults, these same individuals completed functional MRI scanning (M age = 24.88 years; intervention n = 44; control n = 49). Functional connectivity between the hippocampus and ventromedial prefrontal cortex was examined in relation to the intervention, gains in self‐regulation, and present‐day externalizing symptoms. Increased connectivity between these brain areas was noted in the intervention group compared to controls. Furthermore, individual gains in self‐regulation, instilled by the intervention, statistically explained this brain difference. These results begin to connect neurobiological and psychosocial markers of risk and resiliency.
Individuals developing in impoverished environments are at heightened risk for a host of physical and mental health difficulties across the life span (Shonkoff et al., 2012). Studies indicate that children living in poverty are at increased risk for subsequent aggression, oppositional behavior, and other forms of externalizing psychopathology (Piotrowska, Stride, Croft, & Rowe, 2015). These negative outcomes are likely due to the multiple risk factors associated with poverty, and persist into adulthood (Jensen, Berens, & Nelson, 2017). Childhood hardship is associated with a higher probability of first‐onset mood, behavioral, and substance abuse disorders at all stages of the life course (McLaughlin et al., 2011), even when controlling for income levels in adulthood (Evans & Cassells, 2014). Furthermore, quasi‐experimental work where annual income was supplemented has found moving families out of poverty specifically reduced children's symptoms of conduct and oppositional defiant disorders (Costello, Compton, Keeler, & Angold, 2003).
Even though these consistent links exist, not all children living in poverty will develop externalizing psychopathology. Indeed, research has begun to identify many potential markers of risk and resiliency. From a psychosocial perspective, differences in self‐regulation may be important for understanding the circumstances under which poverty does (and does not) become connected to externalizing outcomes (Blair & Raver, 2015; Evans & Kim, 2012). Self‐regulation is a multifaceted constellation of skills that enables the control of attention and emotion, for the purposes of setting, maintaining, and achieving goals (Bridgett, Burt, Edwards, & Deater‐Deckard, 2015; Nigg, 2016). A growing body of longitudinal studies have found that better self‐regulation in childhood is related to fewer externalizing problems, including lower rates of antisocial personality disorder, less interpersonal conflict, and reduced likelihood of being involved in criminal activity (Caspi, Moffitt, Newman, & Silva, 1996; Moffitt et al., 2011). In the aggregate, poverty is associated with lower self‐regulation abilities, but there is considerable variability in these traits within low socioeconomic status (SES) populations (Blair & Raver, 2012, 2015; Montroy, Bowles, Skibbe, McClelland, & Morrison, 2016). In addition, there is consistent evidence of protective effects, whereby individuals from low SES backgrounds with better self‐regulatory skills have lower rates of externalizing psychopathology compared with their demographically similar peers (Buckner, Mezzacappa, & Beardslee, 2003; Flouri, Midouhas, & Joshi, 2014; Lengua, Bush, Long, Kovacs, & Trancik, 2008).
Neurobiologically informed research has similarly begun to identify brain markers of risk and resiliency. Using neuroimaging, many studies have reported structural differences in the hippocampus and ventromedial portions of the prefrontal cortex (vmPFC) in relation to exposure to aspects of poverty, including lower household income and social status (Gianaros et al., 2007; Hanson, Chandra, Wolfe, & Pollak, 2011; Hanson et al., 2015; Noble et al., 2012, 2015). Past research implicates both of these brain structures as important for socioemotional functioning (Davidson & McEwen, 2012). Interestingly, structural differences in the hippocampus and vmPFC appear to mediate the relation between childhood poverty and externalizing symptoms, with smaller volumes in each region relating to greater problem behaviors (Hanson et al., 2015; Holz et al., 2014). Few studies have, however, examined resting state functional connectivity, with the preponderance of past work focused on brain structure in relation to exposure to aspects of poverty. One exception is a recent study by Sripada, Swain, Evans, Welsh, and Liberzon (2014) that found childhood poverty was related to reduced resting state functional connectivity between the hippocampus, the vmPFC, and the posterior cingulate.
The limited work examining functional brain interactions is a major limitation, as complex behavioral processes likely arise through multiple brain regions interacting and sharing information with each other (van den Heuvel & Pol, 2010). With the hippocampus and vmPFC, functional interactions between these regions may be important for using previously acquired information in goal‐directed behavior (Murty, Calabro, & Luna, 2016). For example, Gluth, Sommer, Rieskamp, and Büchel (2015) showed that decision making is limited by memory constraints, and this is associated with functional connectivity between the hippocampus and vmPFC. Studies using resting state connectivity could fill in these important gaps and provide new insights about the impact of experience on brain organization. Spontaneous brain activity (assessed at rest) is highly correlated between multiple brain regions, predicts task‐response properties of neural circuits, and can identify subjects’ aptitude for different cognitive tasks (Fox & Greicius, 2010).
Turning back to self‐regulation, although this characteristic can exert protective effects, it is not a fixed trait and this may have major implications for developmental outcomes. Psychosocial interventions have uniquely noted that improving parenting practices facilitates children's early development of self‐regulation, and this can then serve as a foundation for positive functioning in multiple domains and contexts (Brody et al., 2011; Chang, Shaw, Dishion, Gardner, & Wilson, 2014). One notable example is the Strong African American Families (SAAF) intervention—a family skills training program aimed at mitigating the negative effects of poverty and life stress on rural African American youths through a focus on youths, parents, and their family interactions (Brody, 2016). This intervention identified malleable, proximal parenting processes in a youth's immediate family context that could facilitate the development of responsive–supportive parent–child relationships; these supportive relationships could then enhance children's development of self‐regulation. In keeping with models of developmental cascades (Masten & Cicchetti, 2010), changes in parenting may cause youth: to adopt parental norms, develop the ability to govern their own behavior in the absence of external supervision, and approach stressful life events through direct action rather than through avoidance or anger. Past work (e.g., Brody, Murry, & McNair, 2005) has found support for these ideas, noting that intervention‐induced changes in parenting were linked with changes in responsive–supportive parent–child relationships and then youth self‐regulation. In addition to changes in self‐regulation, this intervention has been found to exert strong and longlasting effects on a host of other psychosocial outcomes—with major reductions in conduct problems and lessened alcohol use after participation in the program (Brody, Chen, Kogan, Murry, & Brown, 2010; Brody, Kogan, Chen, & Murry, 2008). Importantly, these effects were found long after completion of the program, with differences seen 2–5 years after intervention delivery. In addition, nearly a decade after the program, differences in inflammation and other health‐related biomarkers have been found for participants compared to controls (Brody, Yu, & Beach, 2016). Collectively, these findings underscore that fostering positive parenting can yield important self‐regulatory gains in children. Changes in self‐regulation may buffer against the psychosocial disadvantages that beset children in poverty and can foster positive mental health, as well as physical, outcomes. Indeed, these self‐regulatory gains could carry forward, facilitating exposure to more stimulating experiences across development that promote brain connectivity and other positive patterns of neurobiology.
Interestingly, in recent neurobiological work in the SAAF cohort, the intervention was found to bolster against the adverse effects of poverty. In this work, and similar to other studies, years spent in poverty during childhood were associated with smaller adult hippocampal volumes; however, in those who completed the intervention, this relation was not seen, suggesting exposure to prevention programming in childhood could have lasting protective effects on brain development into adulthood (Brody et al., 2017). Paralleling preclinical work, smaller volumes were found in the dentate gyrus (DG) for individuals living in poverty who did not complete the intervention. Alterations in the DG are notable for a number of reasons. The DG is central to pattern separation and completion, processes that aid in adaptively guiding behavior (Nakashiba et al., 2012). More broadly, the DG is associated with affective regulation and the pathophysiology of mood disorders, contributing to stress and emotional responses, and serving as the main gateway of information for the other portions of the hippocampus (Fa et al., 2014).
Here, we return to the SAAF study to investigate links between a psychosocial intervention, neurobiology, and the behavioral gains instilled by this preventive programming. Given that this randomized control trial was designed to increase self‐regulatory abilities in low‐SES African Americans from the rural South, we first probed whether childhood self‐regulation was improved in a subsample of intervention participants who were recontacted later in adulthood (Hypothesis 1). We, next, examined resting state functional brain differences related to participation in the SAAF program. Given the recent results noting structural brain differences in the DG in SAAF participants, we set out to investigate potential alterations in the resting state connectivity of this hippocampal subregion related to SAAF. On the basis of the past research findings, we predicted that the intervention would be related to increased functional connectivity between the DG and vmPFC in the intervention group, compared to control participants (Hypothesis 2). We, next, centered in on the behavioral target of the SAAF intervention, namely developmental gains in self‐regulation. Given that SAAF improved this capacity during development, we wanted to investigate if gains in self‐regulation (instilled by the intervention) would mediate the group differences in functional connectivity (Hypothesis 3). This would be an initial step to understand the developmental factors potentially influencing neurobiological differences in adulthood. We finally, aimed to connect developmental gains, neurobiology, and present‐day behavior by examining relations between brain connectivity, self‐regulatory gains, and present‐day externalizing problems. Motivated by past research, we predicted that higher connectivity between the DG and vmPFC would be related to lessened present‐day externalizing problems (Hypothesis 4).
Method
Participants
At its inception, the original SAAF sample was 667 African American families, randomly recruited from rural communities in Georgia when the participants were approximately 11.2 years of age (SD = 0.34). At age 25, 123 individuals were recruited from this larger cohort that had participated in the SAAF randomized prevention trial. For this work, participants were recontacted at random, stratified by gender and treatment assignment, and screened for standard imaging contraindications and right handedness until a targeted sample size was reached (M age = 24.88 years; SD = 0.61; see Table 1). Additional information about the original SAAF sample and the recruitment of the neuroimaging subsample is noted in Supporting Information. The original target sample size was 100 participants with usable data on a working memory functional MRI (fMRI) task that the subjects also completed. This resulted in a slightly larger sample of participants with resting state fMRI scans (n = 123). During this visit, participants were welcomed by project staff, explained study protocols, and provided informed consent. Participants reviewed MRI clearance forms and were oriented to the MRI scanner. Participants were compensated $210, plus travel reimbursement, for this 2–3 hr laboratory visit. The University of Georgia's Institutional Review Board reviewed and approved all study procedures.
Table 1.
Characteristics of Participants in the Neuroimaging Study at Age 11 Years by Intervention Status
| Characteristics | SAAF (n = 44) | Control (n = 49) | t(91) | p | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Parent age, years | 37.34 | 9.01 | 36.96 | 6.11 | 0.24 | .81 |
| Family SES risk | 2.91 | 1.41 | 2.10 | 1.37 | 2.79 | .01 |
| Parent education | 2.25 | 0.72 | 2.39 | 0.79 | −0.88 | .38 |
| Self‐control | 29.43 | 6.82 | 29.22 | 7.37 | 0.14 | .89 |
| Delinquent behaviors | 2.39 | 1.82 | 2.00 | 2.03 | 0.95 | .34 |
| n | % | n | % | χ²(1) | p | |
|---|---|---|---|---|---|---|
| Sex, male | 20 | 45.5 | 25 | 51.0 | 0.29 | .59 |
| Family poverty status | 19 | 44.2 | 19 | 44.2 | 0 | 1.00 |
| Parent unemployment status | 9 | 20.5 | 4 | 8.2 | 2.91 | .09 |
| Single‐parent family status | 33 | 76.7 | 29 | 59.2 | 3.21 | .07 |
Parent education was coded as 1 = less than high school graduation, 2 = high school graduation or GED, 3 = some college or associate degree, 4 = bachelors degree or higher. SAAF = Strong African American Families; SES = socioeconomic status; GED = general education diploma.
Thirty participants were excluded for failure to complete MRI scanning (n = 4), excessive motion in structural images (n = 4), artifact and signal dropout in fMRI (n = 3), or excessive movement during resting state imaging (n = 19; quality control cutoffs noted below in Image Preprocessing section). This yielded 93 participants with usable MRI data. Forty‐four of these participants (46.8%) had been assigned randomly to the SAAF condition, whereas 49 participants (52.1%) were assigned randomly to the control condition as youths. As shown in Table 2, participants who did or did not take part in the imaging study at 25 years of age as a function of intervention group assignment at 11 years of age were similar for family SES disadvantage at age 11, gender, as well as on self‐control/delinquency at pretest. This suggests the neuroimaging subsample was similar to the broader SAAF (full) sample. Information related to this point is also detailed in Supporting Information.
Table 2.
Characteristics of Participants With and Without Brain Imaging Data at Age 11 Years
| Characteristics | With brain imaging data (n = 93) | Without brain imaging data (n = 574) | t(665) | p | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Parent age, years | 37.14 | 7.58 | 37.83 | 7.63 | −0.81 | .42 |
| Family SES risk | 2.48 | 1.44 | 2.28 | 1.49 | 1.25 | .21 |
| (n = 93) | (n = 571) | t(662) | p | |||
|---|---|---|---|---|---|---|
| Parent education | 2.32 | 0.75 | 2.36 | 0.91 | −0.39 | .70 |
| Self‐control | 29.32 | 7.08 | 29.37 | 8.26 | −0.05 | .96 |
| Delinquent behaviors | 2.18 | 1.93 | 2.16 | 2.23 | 0.11 | .91 |
Parent education was coded as 1 = less than high school graduation, 2 = high school graduation or GED, 3 = some college or associate degree, 4 = bachelors degree or higher. SAAF = Strong African American Families; SES = socioeconomic status; GED = general education diploma.
Procedures
SAAF Intervention
As youth, intervention participants completed the SAAF prevention program. This intervention consisted of 7 consecutive, 2‐hr weekly meetings held at community facilities, with separate skill‐building curricula for youths and for their primary caregivers, and a family curriculum. The caregiver sessions emphasized parenting skills, including the consistent provision of instrumental and emotional support, high levels of monitoring and control, adaptive racial socialization strategies, and methods for communicating about sex and alcohol use. Youth sessions focused on forming goals for the future and making plans to attain them, resistance efficacy skills, and adaptive behaviors to use when encountering racism. Details regarding the program, as well as associated outcomes for the intervention, are noted elsewhere (Brody, 2016; Brody, Yu, Chen, Beach, & Miller, 2016; Kogan et al., 2012; Miller, Brody, Yu, & Chen, 2014).
Magnetic Resonance Imaging Data Collection
Structural (high‐resolution T1‐weighted MRI; voxel size = 1 mm3) and functional resting state (two scans using single‐shot gradient echo pulse T2* sequence; voxel size = 3.5 mm3) images were acquired with a General Electric 3.0 T MRI scanner (Milwaukee, WI, USA). Additional technical information about these acquisitions are noted in Supporting Information. During the resting state scan, participants were instructed to keep their eyes open and fixed on a crosshair. After the scan, participants were asked about adherence to these instructions and all individuals reported being awake, with eyes open and fixed on the crosshairs.
Assessments and Measures
Before and after the intervention, participants completed a number of self‐report measures of sociodemographics and psychosocial functioning. To control for socioeconomic risk, six dichotomous variables formed a socioeconomic disadvantage composite at pretest. A score of 1 was assigned to each of the following: family poverty based on federal guidelines, primary caregiver unemployment, receipt of Temporary Assistance for Needy Families, primary caregiver single parenthood, primary caregiver education level less than high school graduation, and caregiver‐reported inadequacy of family income. The scores were summed to form the index. This factor and a dummy‐coded indicator for sex were used as control variables in data analyses.
Youth Measures of Self‐Regulation
To assess self‐regulation, parents completed the 12‐item Self‐Regulation Inventory (SRI; Humphrey, 1982) and the 12‐item delinquency subscale from the Child Behavior Checklist (D‐CBCL; Achenbach & Edelbrock, 1983), before (pretest) and after (posttest) the SAAF intervention. Each questionnaire involved Likert scale ratings and example items for both scales are noted in Supporting Information. We totaled parents’ ratings from the SRI and D‐CBCL and standardized scores from each questionnaire. These measures were highly correlated (rs = −.54 at pretest and −.57 at posttest, p < .001). Next, the delinquency scores were then subtracted from the self‐regulation scores to form a youth self‐regulation composite. For this measure, higher values indicated greater gains in self‐regulation. Posttest self‐regulation measures were administered on average 3 months after the end of the intervention (M = 3.32 months, SD = 1.43), and the average interval between pretest and posttest was approximately 8 months (M = 7.82 months, SD = 1.84). Using these measures (pre and postintervention), a latent difference score was calculated in Mplus (Muthen & Muthen, 2012). The change in self‐regulation between pre‐ and postintervention was modeled with the following settings: (a) the self‐regulation variable at postintervention was the single indicator of the latent difference scores (the loading was set to 1 without measurement error); (b) the self‐regulation variable at postintervention was regressed on the self‐regulation variable at preintervention and the path coefficient was set to 1; and (c) the latent difference scores were regressed on self‐regulation variable at preintervention and the path coefficient was estimated. Additional information about these procedures are noted in Supporting Information.
Present‐Day Externalizing Symptoms
To assess present day behavioral problems, we focused on total externalizing symptoms from the Adult Self‐Report (ASR; Achenbach & Rescorla, 2003), by combining responses about rule‐breaking behavior, as well as aggressive behavior (36 items total). This measure was administered near the time of the neuroimaging session (additional information regarding the ASR is noted in Supporting Information).
Measures of Brain Connectivity
All functional imaging data were preprocessed and analyzed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Preprocessing was keeping with current best practices for dealing with motion in resting state fMRI data (Hallquist, Hwang, & Luna, 2013; Power, Schlaggar, & Petersen, 2015). These steps included removing the first four functional volumes to allow for blood‐oxygen‐level dependent (BOLD) signal stabilization, “despiking” outlier volumes, slice‐time correction, intrasubject registration, motion censoring, intrasubject registration to MNI‐152 space (Montreal Neurological Institute), and partialling out nuisance regressors (eroded ventricle and white matter masks; 24 motion parameters; temporal bandpass filtering from 0.009–0.08 Hz). Participants were excluded if > 20% of frames were censored (n = 19), yielding at least 6 min of resting state data. Additional details available in Supporting Information. Next, based on past work finding alterations in the left DG, a probabilistic hippocampal atlas was used to examine subdivisions of the hippocampus (Kulaga‐Yoskovitz et al., 2015). For each participant, a regression was performed including the left DG time course, generating subject‐level maps of the correlations between this region's time course and every other voxel's time course. Given our a priori interest in DG‐vmPFC interactions, we completed voxel‐wise analyses within a vmPFC mask derived from NeuroSynth (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011). Two‐hundred fifty studies in the Neurosynth database consistently used the term “ventromedial prefrontal,” and this was used to generate a mask to limit our neuroimaging search space (total number of voxel in mask = 5925).
In our analyses, intervention status was dummy‐coded (SAAF = 1; control = 0), and subject‐level correlation maps were compared. To correct for multiple comparisons, we deployed AFNI's 3dClustSim using cluster‐size thresholding based on Monte Carlo simulation and new, mixed‐model (non‐Gaussian) auto‐correlation functions and used an initial, uncorrected statistical threshold of p < .001 (Cox, Chen, Glen, Reynolds, & Taylor, 2017). Based on this threshold, the number of comparisons in our mask, and the smoothness of our imaging data, a minimum cluster size of 18 voxels was required to have a corrected p ≤ .05. For any regions above this threshold, mean functional connectivity estimates were then extracted by averaging across every voxel in each regional cluster. Exploratory analyses were also completed using a whole‐brain approach (with an initial statistical threshold of p < .001 uncorrected and a minimum cluster size of 50 voxels was employed to balance Type I vs. II error). Further details available in Supporting Information.
Analytic Approach
To examine effects of the intervention, changes in parent reported self‐regulation behaviors from pre‐ to posttest were compared between intervention and control groups (dummy‐coded) using analysis of variance models implemented in the JMP suite of programs (SAS Institute, Cary, NC). Next, a mediation model using path analyses tested whether the SAAF intervention (X) was associated with resting state connectivity differences (Y) and whether the observed association was mediated by change in self‐regulation (M). Change in self‐regulation was operationalized as a latent difference score reflecting differences from pre‐ to posttest in self‐regulation scores. Statistical testing of mediation was done by nonparametric bootstrapping, with 95% confidence intervals for indirect mediation effects. This model included family SES disadvantage at age 11 and gender as covariates. Finally, the contemporaneous associations of resting state connectivity with externalizing behaviors were examined and the indirect pathway from SAAF intervention to externalizing behaviors through brain connectivity was tested. Of note, analyses focused on other psychosocial variables, as well as measures of self‐regulation at different developmental epochs, were also completed and are detailed in Supporting Information.
Results
Behavioral Impacts of the SAAF Intervention
As noted in past reports, participants who completed the SAAF intervention had better developmental outcomes compared to control participants. As youth, those who completed the SAAF intervention had higher gains in self‐regulation, compared to control participants at approximately 11 years of age. This was found for parental reports pre and post the randomized control trial, F(1, 87) = 5.485, p = .021, in support of Hypothesis 1. As adults, participants who completed the SAAF intervention also had lower rates of total externalizing behavior, at approximately 25 years of age, again compared to control participants (t = −2.64, p = .009). These results are shown in Figures S1 and S2.
Functional Connectivity Differences Between Intervention and Control Groups
Analyses of resting‐state fMRI data revealed that the functional coupling for the DG differed for the SAAF intervention and control groups. Specifically, connectivity between the left DG and the right vmPFC (x = +6, y = +44, z = −6, max voxel t = 4.81, p = .05 corrected) was greater for SAAF compared to control participants (related to Hypothesis 2). These differences are shown in Figure 1. Exploratory (whole‐brain) analyses revealed group‐related connectivity differences between the DG and two brain regions: (a) higher DG‐right vmPFC connectivity (in line with our a priori analyses) for SAAF compared to control participants; and (b) lower DG‐right inferior parietal lobule connectivity, again for SAAF compared to control participants. Information about these effects are noted in Table 3.
Figure 1.
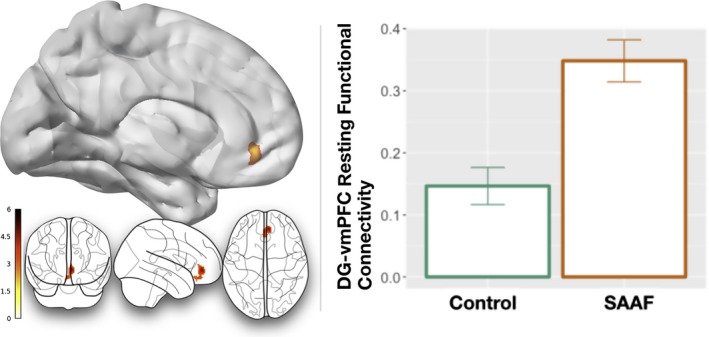
The left‐hand side of this graphic shows the spatial location of the cluster of interest that emerged. Connectivity between this portion of the ventromedial portions of the prefrontal cortex (vmPFC) and the DG emerged using a restricted (a priori) mask. Similar results were found if a larger, whole‐prefrontal cortex mask was also used. The right‐hand side of this graphic shows the functional connectivity differences between groups. Strong African American Families (SAAF) participants are shown with an orange outlined bar, and control participants are shown with a green outlined bar. The units for this graph are Fisher's Z‐transformed correlation coefficients, with higher values indicating greater coupling between brain regions.
Table 3.
Exploratory (Whole‐Brain) Analyses Examining DG Connectivity: Comparing SAAF Intervention Versus Control Participants
| Region (nearest Brodmann area or anatomical landmark) | Extent | Peak of cluster coordinates (x,y,z; in MNI coordinate space) | Direction |
|---|---|---|---|
| Right vmPFC | 59 voxels | t = +4.81 (+17.00, −3.00, −1.00) | SAAF > control |
| Right inferior parietal lobule | 50 voxels | t = −4.00 (−17.00, +31.00, +21.00) | SAAF < control |
All regions noted significant at p = .001, uncorrected with an extent of < 50 voxels (2 mm3). SAAF = Strong African American Families; vmPFC = ventromedial portions of the prefrontal cortex.
Mediation Analyses Connecting Intervention‐Related Behavioral and Brain Changes
We next tested the hypothesis that the higher vmPFC‐DG connectivity for SAAF, compared to control, participants, was attributable to intervention‐related increases in self‐regulation (Valente & MacKinnon, 2017). First, we estimated structural coefficients reflecting the association between SAAF status and increased self‐regulation (Path A) and increased self‐regulation and vmPFC‐DG connectivity (Path B). Of note, this self‐regulation was a latent difference score measuring changes from pre‐ to posttest. Second, we quantified the indirect or mediating effect of changes in self‐regulation as the product of these two coefficients (A × B). Nonparametric bootstrapping (1,000 times) was used to obtain the bias‐corrected and accelerated (BCa) confidence intervals of the indirect effect (Preacher & Hayes, 2004). Furthermore, to be considered a mediator, the strength of the direct relation between the predictor and outcome (Path C) must be diminished when the mediator is entered into the analysis (Path C′). Gender and family SES risk at pretest were controlled in the model.
The results of this analysis suggested that higher vmPFC‐DG connectivity among SAAF participants (compared to controls) were partially attributable to increased self‐regulation (Hypothesis 3). The positive coefficient for Path A indicates that participation in SAAF was associated with statistically significant increases in self‐regulation. The positive coefficient for Path B indicates that participants’ vmPFC‐DG connectivity at age 25 were positively associated with increases in self‐regulation. Multiplying these coefficients yielded an indirect mediated effect of .024 with a bootstrapped 95% confidence interval (CI) of 0.003, 0.065. Thus, the indirect pathway from SAAF to increases in self‐regulation to higher vmPFC‐DG connectivity was statistically significant. Nevertheless, SAAF remained associated with vmPFC‐DG connectivity even after accounting for changes in self‐regulation, as the significant Path C′ coefficient indicates.
We further examined contemporaneous associations between DG‐vmPFC connectivity and externalizing behaviors by including the pathway that brain connectivity predicted externalizing behaviors at age 25 (Path D). The negative coefficient for Path D indicates that higher brain connectivity (in adulthood) was associated with lower externalizing behaviors (Hypothesis 4). Furthermore, the indirect pathway from SAAF to externalizing behaviors through brain connectivity was statistically significant (indirect effect = A × B × D + C′ × D; −1.346, 95% CI [−2.604, −0.242]). Overall model fit was good (Hu & Bentler, 1999), with χ2(4) = 2.850, p = .583, comparative fit index = 1.000, and root mean square error of approximation = 0 (95% CI = 0, 0.134). Figure 2 depicts these findings.
Figure 2.
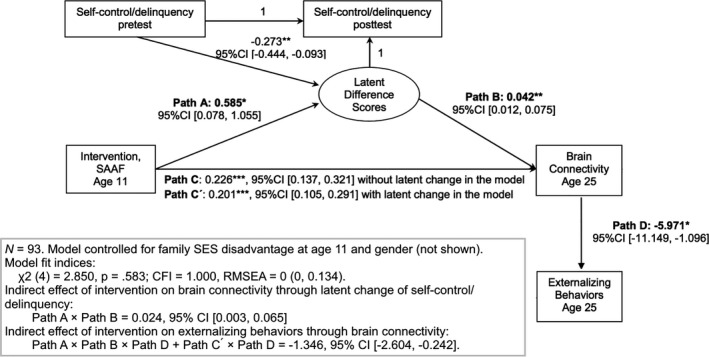
The figure shows our structural equation model with intervention status, gain in self‐regulation, brain connectivity, and present‐day externalizing symptoms. To unpack these effects, higher brain connectivity between the hippocampus and ventromedial portions of the prefrontal cortex (vmPFC) was found for the Strong African American Families (SAAF) intervention participants (Path C). Participation in SAAF was associated with statistically significant increases in self‐regulation (Path A). Path B indicated that higher brain connectivity at age 25 was positively associated with increase in self‐regulation (a latent difference score). Path C′ indicates that increases in self‐regulation partially explained the relation between intervention status and higher brain connectivity at age 25. DG‐vmPFC connectivity was associated with externalizing behaviors at age 25 (higher connectivity relating to lower symptoms; Path D). Finally, the indirect pathway from SAAF to externalizing behaviors through brain connectivity was statistically significant (Path A × Path B × Path D + Path C′ × Path D). *p < .05. **p < .01. ***p < .001.
To probe the specificity of these effects, we first completed similar mediation analyses for parietal lobe‐DG connectivity (using SAAF intervention status, latent difference scores of self‐regulation gains, and present‐day externalizing symptoms). No relations were found for this regional connectivity and change in self‐regulation and/or present‐day externalizing symptoms (all p's > .12, additional details in Supporting Information). We also completed supplemental analyses to examine if other psychosocial variables (e.g., family chaos; measures of the parent–child relationship) might explain variability in vmPFC‐DG connectivity. Results indicated no significant association between any of the psychosocial variables and measures of adult brain connectivity (additional details in Supporting Information). Finally, analyses using measures of self‐regulation from other developmental epochs are also noted in Supporting Information.
Discussion
Here, we report on a unique study that combined longitudinal assessments, a randomized control trial intervention (SAAF), and rich measures of neurobiology. These sources of data were leveraged to determine how this prevention program impacted behavior during childhood and then brain functioning in adulthood. In line with past results (Brody et al., 2008), we first find that individuals who participated in the SAAF intervention had improvements in self‐regulation shortly after the intervention. Drilling into neurobiology, we found that individuals who participated in SAAF had higher coupling between the DG and vmPFC, two brain regions involved with socioemotional functioning and self‐regulation, compared to control participants. Interestingly, gains in self‐regulation partially (statistically) explained the group differences noted in DG‐vmPFC connectivity. Finally, we also found that individual differences in this functional coupling related to present‐day externalizing symptomatology. All of these results were in keeping with our study predictions.
These findings converge with earlier reports describing structural alterations in the hippocampus and vmPFC in individuals exposed to childhood poverty, or who suffered other forms of early adversity (Hanson et al., 2010; Hanson et al., 2015; Holz et al., 2014). Similarly, longitudinal research suggests that parenting impacts hippocampal development, with greater parental warmth and support predicting larger hippocampal volumes later in development (Luby, Belden, Harms, Tillman, & Barch, 2016; Luby et al., 2012). Functionally, childhood poverty has been associated with reduced hippocampal activation in adulthood, as well as decreased functional connectivity between left amygdala and portions of the prefrontal cortex (Javanbakht et al., 2015; Liberzon et al., 2015). In addition, work by Sripada et al. (2014) found childhood poverty was related to reduced functional connectivity between the hippocampus, the vmPFC, and the posterior cingulate in adulthood. Building upon this earlier research, we capitalized here on data from a randomized trial, whose design provides additional leverage for making causal inferences about self‐regulation, brain function, and psychosocial risk and resiliency.
By combining rich assessments of neurobiology and psychosocial processes, our results suggests malleable neural systems involved with self‐regulation, a foundational skill for behavioral development (Baumeister & Vohs, 2004). Contextualizing this project with data from developmental science, we feel our results fit with ideas of “developmental cascades.” The SAAF intervention impacted parenting practices and these changes then precipitated changes in self‐regulation. At the start of this preventative program, youth were still developing their self‐regulatory capabilities and this time period is also a critical time of brain development (Belsky & De Haan, 2011). Changes in self‐regulation may then facilitate exposure to more stimulating experiences, strengthening positive patterns of neurobiology. Indeed, our data suggest that these changes in self‐regulation may then go on to impact neurobiology. Connecting our results with insights from basic cognitive neuroscience, emerging theories of vmPFC‐hippocampal functioning suggest these brain regions play central roles in flexible cognition, learning, memory‐guided decision making, and adapting to different environmental contexts (Preston & Eichenbaum, 2013; Schlichting & Preston, 2016). A large body of work has found the vmPFC is related to the processing of reward and value‐based decision making (Rangel, Camerer, & Montague, 2008). Research indicates that the vmPFC tracks subjective value across multiple types of stimuli, and activity in this region predicts choice behavior (Berkman, 2018). The hippocampus, specifically the DG, plays an important role in contextual processing and memory encoding (Kesner, 2013). The DG may influence value‐based decisions through these processes. During decision making, increased interactions between the hippocampus and vmPFC has been linked to making more decisions that integrate memories and past experiences (Gershman, Blei, & Niv, 2010; Gluth et al., 2015). As such, the heightened connectivity between these regions in the intervention group (compared to controls) may represent a greater reliance on past experiences and a greater influence of current context when making decisions. Though speculative, the SAAF intervention may impact self‐regulation through these more proximal processes. In future work with this cohort, we hope to explicitly examine decision‐making patterns and memory processes through novel experimental paradigms and computational modeling (similar to Hanson et al., 2017).
This study has implications for both research and practice. Conceptually, building upon the observational studies described previously, suggest buffering influences of enhanced parenting on brain functioning through self‐regulatory changes. In that regard, our results converge with the “parental effects” commonly observed in animal models, wherein parental caregiving tendencies exert lasting influences on offspring behavior and physiology, especially in the brain (Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015). Furthermore, our results suggest a neural circuit related to self‐regulation, namely the vmPFC‐hippocampus. It will be important to examine functional interactions of these brain regions in relation to normative development in self‐regulatory processes, especially using multimethod approaches. Clinically, the findings suggest that SAAF and perhaps other interventions focused on strengthening parenting and families could bolster core psychological capacities through alterations in brain circuitry and brain development. This should serve as an additional motivator for practitioners and policy makers, as neurobiological effects of the intervention were seen in adulthood and related to important variations in externalizing behaviors.
Several limitations of this study must be noted. First, the SAAF trial was not designed with brain functioning as an endpoint. As a result, we did not collect pretrial neuroimaging scans that could be used to determine whether the intervention and control groups’ neural profiles changed differentially over time. At study entry, the SAAF and control groups were similar in terms of SES, parenting quality, mental health, and other factors. This would suggest that the groups began the trial with similar patterns of brain connectivity. Nevertheless, until pre–post data are available, conclusions about the true capacity of SAAF to bring about changes in neural circuitry must be viewed as tentative. Second, we obtained brain imaging from only a subset of participants. Statistical models would suggest that before SAAF, all participants who completed neuroimaging were similar to the broader trial population, again in terms of SES, parenting quality, mental health, and other factors. With that said, these families may have been more actively engaged in the intervention and perhaps as a consequence showed greater improvements in parenting, self‐regulation, and patterns of neurobiology. Third, our measures of self‐regulation were parental self‐reports. There may be potential biases present in these measures, as families were completing an intervention focused on changing related constructs. Moving forward, we hope to integrate behavioral and self‐report data of self‐regulation to understand intervention effects at multiple levels of analysis (similar to work on risk taking by Harden et al., 2017). Fourth, although we believe changes in DG‐vmPFC connectivity may underlie externalizing symptoms in adulthood, these two variables were measured at the same time. A causal relation from brain connectivity to psychopathology, therefore, cannot be assumed. There may be significant bidirectional interactions between these important variables, and our measures of interest in our study. Further follow‐up of this neuroimaging subsample could more definitively speak to whether DG‐vmPFC connectivity is indeed mediating connections between the SAAF intervention, gains in self‐regulation, and externalizing issues later in development. Finally, we examined changes in childhood self‐regulation as a behavioral mediator for the neural differences seen in our adult participants. The SAAF intervention may have impacted numerous behavioral processes, all of which could have contributed to neural differences.
Limitations notwithstanding, this study provides initial evidence that a family oriented intervention can influence brain connectivity, in part by improving self‐regulatory abilities. Additional research is needed to confirm and clarify the relations between self‐regulation, neurobiology, and prevention programming, but our data provide suggestions regarding neurobiological mechanisms. This information could provide an additional vantage point for understanding risk and resiliency, as well as how environmental experience (both positive and negative) becomes biologically embedded. Furthermore, these results should motivate policy makers to invest in evidence‐based prevention programs in adolescence, as well as childhood. Such practices are in increasing need, as the rates of childhood poverty have risen steadily in recent years, and this trend has been especially pronounced in rural, African American communities.
Supporting information
Appendix S1. Participant Information and Study Procedure and Measure Details. This document contains additional information about participants, as well as greater details about study procedures and measures. Additional analyses using other brain connectivity measures, other psychosocial variables, and self‐regulation at different developmental time‐points are also included here
We thank all the staff at the Center for Translational & Prevention Science and The Center for Family Research, both at The University of Georgia, Athens, for their assistance with data collection and study management. This work was supported by National Institute on Drug Abuse Grants P30‐DA027827 (to Gene H. Brody).
References
- Achenbach, T. M. , & Edelbrock, C. S. (1983). Manual for the child behavior checklist: And revised child behavior profile. Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- Achenbach, T. M. , & Rescorla, L. A. (2003). Manual for the ASEBA adult forms & profiles. Burlington, VT: University of Vermont. [Google Scholar]
- Baumeister R. F., & Vohs K. D. (Eds.). (2004). Handbook of self‐regulation Research, theory, and applications. New York, NY: Guilford. [Google Scholar]
- Belsky, J. , & De Haan, M. (2011). Annual research review: Parenting and children's brain development: The end of the beginning. Journal of Child Psychology and Psychiatry, 52, 409–428. 10.1111/j.1469-7610.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Berkman, E. T. (2018). Value‐based choice: An integrative, neuroscience‐informed model of health goals. Psychology & health, 33(1), 40–57. 10.1080/08870446.2017.1316847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, C. , & Raver, C. C. (2012). Individual development and evolution: Experiential canalization of self‐regulation. Developmental Psychology, 48, 647–657. 10.1037/a0026472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, C. , & Raver, C. C. (2015). School readiness and self‐regulation: A developmental psychobiological approach. Annual Review of Psychology, 66, 711–731. 10.1146/annurev-psych-010814-015221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett, D. J. , Burt, N. M. , Edwards, E. S. , & Deater‐Deckard, K. (2015). Intergenerational transmission of self‐regulation: A multidisciplinary review and integrative conceptual framework. Psychological Bulletin, 141, 602–654. 10.1037/a0038662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, G. H. (2016). The Strong African American Families Program (SAAF), the Strong African American Families‐Teen Program (SAAF‐T), and the Adults in the Making Program (AIM) In Van Ryzin M. J., Kumpfer K. L., Fosco G. M., & Greenberg M. T. (Eds.), Family‐based prevention programs for children and adolescents: Theory, research, and large‐scale dissemination (pp. 282–307). New York, NY: Psychology Press. [Google Scholar]
- Brody, G. H. , Chen, Y. F. , Kogan, S. M. , Murry, V. M. , & Brown, A. C. (2010). Long‐term effects of the strong african american families program on youths’ alcohol use. Journal of consulting and clinical psychology, 78(2), 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, G. H. , Chen, Y. F. , Kogan, S. M. , Yu, T. , Molgaard, V. K. , DiClemente, R. J. , & Wingood, G. M. (2011). Family‐centered program deters substance use, conduct problems, and depressive symptoms in black adolescents. Pediatrics, 129, 108–115. 10.1542/peds.2011-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, G. H. , Gray, J. C. , Yu, T. , Barton, A. W. , Beach, S. R. H. , Galván, A. , … Sweet, L. H. (2017). Protective prevention effects on the association of poverty with brain development. JAMA Pediatrics, 171, 46–52. 10.1001/jamapediatrics.2016.2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, G. H. , Kogan, S. M. , Chen, Y.‐F. , & Murry, V. M. (2008). Long‐term effects of the Strong African American Families program on youths’ conduct problems. Journal of Adolescent Health, 43, 474–481. 10.1016/j.jadohealth.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, G. H. , Murry, V. M. , & McNair, L. (2005). Linking changes in parenting to parent–child relationship quality and youth self‐control: The Strong African American Families program. Journal of Research on Adolescence, 15, 47–69. 10.1111/j.1532-7795.2005.00086.x [DOI] [Google Scholar]
- Brody, G. H. , Yu, T. , & Beach, S. R. (2016). Resilience to adversity and the early origins of disease. Development and Psychopathology, 28, 1347–1365. 10.1017/S0954579416000894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, G. H. , Yu, T. , Chen, E. , Beach, S. R. H. , & Miller, G. E. (2016). Family‐centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. Journal of Child Psychology and Psychiatry, 57, 566–574. 10.1111/jcpp.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, J. C. , Mezzacappa, E. , & Beardslee, W. R. (2003). Characteristics of resilient youths living in poverty: The role of self‐regulatory processes. Development and Psychopathology, 15, 139–162. 10.1017/S0954579403000087 [DOI] [PubMed] [Google Scholar]
- Caspi, A. , Moffitt, T. E. , Newman, D. L. , & Silva, P. A. (1996). Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry, 53, 1033–1039. 10.1192/pb.bp.108.021196 [DOI] [PubMed] [Google Scholar]
- Chang, H. , Shaw, D. S. , Dishion, T. J. , Gardner, F. , & Wilson, M. N. (2014). Direct and indirect effects of the family check‐up on self‐regulation from toddlerhood to early school‐age. Journal of Abnormal Child Psychology, 42, 1117–1128. 10.1007/s10802-014-9859-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello, E. J. , Compton, S. N. , Keeler, G. , & Angold, A. (2003). Relationships between poverty and psychopathology: A natural experiment. Journal of the American Medical Association, 290, 2023–2029. 10.1001/jama.290.15.2023 [DOI] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox, R. W. , Chen, G. , Glen, D. R. , Reynolds, R. C. , & Taylor, P. A. (2017). FMRI clustering in AFNI: False‐positive rates redux. Brain Connectivity, 7, 152–171. 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. J. , & McEwen, B. S. (2012). Social influences on neuroplasticity: Stress and interventions to promote well‐being. Nature Neuroscience, 15, 689–695. 10.1038/nn.3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, G. W. , & Cassells, R. C. (2014). Childhood poverty, cumulative risk exposure, and mental health in emerging adults. Clinical Psychological Science, 2, 287–296. 10.1177/2167702613501496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, G. W. , & Kim, P. (2012). Childhood poverty, chronic stress, self‐regulation, and coping. Child Development Perspectives, 7, 43–48. 10.1111/cdep.12013 [DOI] [Google Scholar]
- Fa, M. , Xia, L. , Anunu, R. , Kehat, O. , Kriebel, M. , Volkmer, H. , & Richter‐Levin, G. (2014). Stress modulation of hippocampal activity–spotlight on the dentate gyrus. Neurobiology of Learning and Memory, 112, 53–60. 10.1016/j.nlm.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Flouri, E. , Midouhas, E. , & Joshi, H. (2014). Family poverty and trajectories of children's emotional and behavioural problems: The moderating roles of self‐regulation and verbal cognitive ability. Journal of Abnormal Child Psychology, 42, 1043–1056. 10.1007/s10802-013-9848-3 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , & Greicius, M. (2010). Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience, 4, 19 10.3389/fnsys.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman, S. J. , Blei, D. M. , & Niv, Y. (2010). Context, learning, and extinction. Psychological Review, 117, 197–209. 10.1037/a0017808 [DOI] [PubMed] [Google Scholar]
- Gianaros, P. J. , Horenstein, J. A. , Cohen, S. , Matthews, K. A. , Brown, S. M. , Flory, J. D. , … & Hariri, A. R. (2007). Perigenual anterior cingulate morphology covaries with perceived social standing. Social cognitive and affective neuroscience, 2(3), 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluth, S. , Sommer, T. , Rieskamp, J. , & Büchel, C. (2015). Effective connectivity between hippocampus and ventromedial prefrontal cortex controls preferential choices from memory. Neuron, 86, 1078–1090. 10.1016/j.neuron.2015.04.023 [DOI] [PubMed] [Google Scholar]
- Gunnar, M. R. , Hostinar, C. E. , Sanchez, M. M. , Tottenham, N. , & Sullivan, R. M. (2015). Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social Neuroscience, 10, 474–478. 10.1080/17470919.2015.1070198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist, M. N. , Hwang, K. , & Luna, B. (2013). The nuisance of nuisance regression: Spectral misspecification in a common approach to resting‐state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage, 82, 208–225. 10.1016/j.neuroimage.2013.05.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Chandra, A. , Wolfe, B. L. , & Pollak, S. D. (2011). Association between income and the hippocampus. PLoS ONE, 6, e18712 10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Chung, M. K. , Avants, B. B. , Shirtcliff, E. A. , Gee, J. C. , Davidson, R. J. , & Pollak, S. D. (2010). Early stress is associated with alterations in the orbitofrontal cortex: a tensor‐based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30(22), 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Nacewicz, B. M. , Sutterer, M. J. , Cayo, A. A. , Schaefer, S. M. , Rudolph, K. D. , … & Davidson, R. J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77, 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , van den Bos, W. , Roeber, B. J. , Rudolph, K. D. , Davidson, R. J. , & Pollak, S. D. (2017). Early adversity and learning: Implications for typical and atypical behavioral development. Journal of Child Psychology and Psychiatry, 456, 245–249. 10.1111/jcpp.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden, K. P. , Kretsch, N. , Mann, F. D. , Herzhoff, K. , Tackett, J. L. , Steinberg, L. , & Tucker‐Drob, E. M. (2017). Beyond dual systems: A genetically‐informed, latent factor model of behavioral and self‐report measures related to adolescent risk‐taking. Developmental Cognitive Neuroscience, 25, 221–234. 10.1016/j.dcn.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz, N. E. , Boecker, R. , Hohm, E. , Zohsel, K. , Buchmann, A. F. , Blomeyer, D. , … & Plichta, M. M. (2014). The long‐term impact of early life poverty on orbitofrontal cortex volume in adulthood: Results from a prospective study over 25 years. Neuropsychopharmacology, 40, 996–1004. 10.1038/npp.2014.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, L. T. , & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Humphrey, L. L. (1982). Children's and teachers’ perspectives on children's self‐control: The development of two rating scales. Journal of Consulting and Clinical Psychology, 50, 624–633. 10.1037/0022-006X.50.5.624 [DOI] [PubMed] [Google Scholar]
- Javanbakht, A. , King, A. P. , Evans, G. W. , Swain, J. E. , Angstadt, M. , Phan, K. L. , & Liberzon, I. (2015). Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Frontiers in Behavioral Neuroscience, 9, 154 10.3389/fnbeh.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, S. K. G. , Berens, A. E. , & Nelson, 3rd, C. A. (2017). Effects of poverty on interacting biological systems underlying child development. Lancet Child & Adolescent Health, 1, 225–239. 10.1016/S2352-4642(17)30024-X [DOI] [PubMed] [Google Scholar]
- Kesner, R. P. (2013). An analysis of the dentate gyrus function. Behavioural Brain Research, 254, 1–7. 10.1016/j.bbr.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Kogan, S. M. , Brody, G. H. , Molgaard, V. K. , Grange, C. M. , Oliver, D. A. H. , Anderson, T. N. , … Sperr, M. C. (2012). The Strong African American Families‐teen trial: Rationale, design, engagement processes, and family‐specific effects. Prevention Science, 13, 206–217. 10.1007/s11121-011-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaga‐Yoskovitz, J. , Bernhardt, B. C. , Hong, S.‐J. , Mansi, T. , Liang, K. E. , van der Kouwe, A. J. W. , … Bernasconi, N. (2015). Multi‐contrast submillimetric 3 Tesla hippocampal subfield segmentation protocol and dataset. Scientific Data, 2, 150059 10.1038/sdata.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua, L. J. , Bush, N. R. , Long, A. C. , Kovacs, E. A. , & Trancik, A. M. (2008). Effortful control as a moderator of the relation between contextual risk factors and growth in adjustment problems. Development and psychopathology, 20(2), 509–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon, I. , Ma, S. T. , Okada, G. , Ho, S. S. , Swain, J. E. , & Evans, G. W. (2015). Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Social Cognitive and Affective Neuroscience, 10, 1596–1606. 10.1093/scan/nsv045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby, J. L. , Barch, D. M. , Belden, A. , Gaffrey, M. S. , Tillman, R. , Babb, C. , … Botteron, K. N. (2012). Maternal support in early childhood predicts larger hippocampal volumes at school age. Proceedings of the National Academy of Sciences of the United States of America, 109, 2854–2859. 10.1073/pnas.1118003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby, J. L. , Belden, A. , Harms, M. P. , Tillman, R. , & Barch, D. M. (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proceedings of the National Academy of Sciences of the United States of America, 113, 5742–5747. 10.1073/pnas.1601443113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten, A. S. , & Cicchetti, D. (2010). Developmental cascades. Development and Psychopathology, 22, 491–495. 10.1017/S0954579410000222 [DOI] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Breslau, J. , Green, J. G. , Lakoma, M. D. , Sampson, N. A. , Zaslavsky, A. M. , & Kessler, R. C. (2011). Childhood socio‐economic status and the onset, persistence, and severity of DSM–IV mental disorders in a US national sample. Social Science & Medicine, 73, 1088–1096. 10.1016/j.socscimed.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. E. , Brody, G. H. , Yu, T. , & Chen, E. (2014). A family‐oriented psychosocial intervention reduces inflammation in low‐SES African American youth. Proceedings of the National Academy of Sciences of the United States of America, 111, 11287–11292. 10.1073/pnas.1406578111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt, T. E. , Arseneault, L. , Belsky, D. , Dickson, N. , Hancox, R. J. , Harrington, H. , … & Sears, M. R. (2011). A gradient of childhood self‐control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America, 108, 2693–2698. 10.1073/pnas.1010076108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montroy, J. J. , Bowles, R. P. , Skibbe, L. E. , McClelland, M. M. , & Morrison, F. J. (2016). The development of self‐regulation across early childhood. Developmental Psychology, 52, 1744–1762. 10.1037/dev0000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty, V. P. , Calabro, F. , & Luna, B. (2016). The role of experience in adolescent cognitive development: Integration of executive, memory, and mesolimbic systems. Neuroscience and Biobehavioral Reviews, 70, 46–58. 10.1016/j.neubiorev.2016.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen L. K., & Muthen B. O. (Eds.). (2012). Mplus user's guide (7th ed.). Los Angeles, CA: Author. [Google Scholar]
- Nakashiba, T. , Cushman, J. D. , Pelkey, K. A. , Renaudineau, S. , Buhl, D. L. , McHugh, T. J. , … Fanselow, M. S. (2012). Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell, 149, 188–201. 10.1016/j.cell.2012.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, J. T. (2016). Annual research review: On the relations among self‐regulation, self‐control, executive functioning, effortful control, cognitive control, impulsivity, risk‐taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58, 361–383. 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Kan, E. , & Sowell, E. R. (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental science, 15(4), 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Brito, N. H. , Bartsch, H. , Kan, E. , Kuperman, J. M. , … & Schork, N. J. (2015). Family income, parental education and brain structure in children and adolescents. Nature neuroscience, 18(5), 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska, P. J. , Stride, C. B. , Croft, S. E. , & Rowe, R. (2015). Socioeconomic status and antisocial behaviour among children and adolescents: A systematic review and meta‐analysis. Clinical Psychology Review, 35, 47–55. 10.1016/j.cpr.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Schlaggar, B. L. , & Petersen, S. E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher, K. J. , & Hayes, A. F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36, 717–731. 10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Preston, A. R. , & Eichenbaum, H. (2013). Interplay of hippocampus and prefrontal review cortex in memory. Current Biology, 23, R764–R773. 10.1016/j.cub.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel, A. , Camerer, C. , & Montague, P. R. (2008). A framework for studying the neurobiology of value‐based decision making. Nature Reviews Neuroscience, 9, 545–556. 10.1038/nrn2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting, M. L. , & Preston, A. R. (2016). Hippocampal‐medial prefrontal circuit supports memory updating during learning and post‐encoding rest. Neurobiology of Learning and Memory, 134, 91–106. 10.1016/j.nlm.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff, J. P. , Garner, A. S. , Siegel, B. S. , Dobbins, M. I. , Earls, M. F. , McGuinn, L. , … & Wegner, L. M. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129, e232–e246. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Sripada, R. K. , Swain, J. E. , Evans, G. W. , Welsh, R. C. , & Liberzon, I. (2014). Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology, 39, 2244–2251. 10.1038/npp.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente, M. J. , & MacKinnon, D. P. (2017). Comparing models of change to estimate the mediated effect in the pretest‐posttest control group design. Structural Equation Modeling, 24, 428–450. 10.1080/10705511.2016.1274657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , & Pol, H. E. H. (2010). Exploring the brain network: a review on resting‐state fMRI functional connectivity. European neuropsychopharmacology, 20(8), 519–534. [DOI] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Nichols, T. E. , Van Essen, D. C. , & Wager, T. D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–670. 10.1038/nmeth.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Participant Information and Study Procedure and Measure Details. This document contains additional information about participants, as well as greater details about study procedures and measures. Additional analyses using other brain connectivity measures, other psychosocial variables, and self‐regulation at different developmental time‐points are also included here


