Abstract
Objective
To examine polygenic inheritance of motoric cognitive risk syndrome (MCR), a predementia syndrome characterized by the presence of subjective cognitive complaints and slow gait.
Methods
We analyzed 4,915 individuals, age 65 years and above, with European ancestry (mean age 75.0 ± 6.8 years, 56.6% women) in the Health and Retirement Study. Polygenic scores (PGS) were calculated as weighted sums of the effect of single nucleotide polymorphisms, with effect sizes derived from genome-wide association studies. The association between PGSs of 9 phenotypes (general cognition, body mass index [BMI], mean arterial pressure, education, Alzheimer disease [AD], neuroticism, well-being, waist circumference, and depressive symptoms) and MCR as well as its key components (cognitive complaints and slow gait) were examined by logistic regression, adjusting for age, sex, education, and genetic ancestry, and reported as odds ratios (ORs) with 95% confidence intervals (CIs).
Results
There were 260 prevalent MCR cases, 529 with slow gait, and 1,928 with subjective cognitive complaints. Higher PGSs for BMI (OR 1.22, 95% CI 1.07–1.39) and waist circumference (OR 1.23, 95% CI 1.07–1.40) were associated with MCR, and PGS of AD showed a suggestive association (OR 1.16, 95% CI 1.02–1.32). Higher PGS for neuroticism (OR 1.10, 95% CI 1.03–1.18) was associated with cognitive complaints, whereas higher well-being PGS (OR 0.92, 95% CI 0.87–0.98) was protective. PGS for BMI (OR 1.16, 95% CI 1.06–1.28), waist circumference (OR 1.19, 95% CI 1.08–1.31), and AD (OR 1.13, 95% CI 1.03–1.24) was associated with slow gait.
Conclusion
Obesity-related genetic traits increase risk of MCR syndrome; further investigation is required to identify potential therapeutic targets.
Motoric cognitive risk (MCR) syndrome is a predementia syndrome characterized by the presence of subjective cognitive complaints and slow gait.1,2 A pooled analysis of 26,802 older adults from 17 countries showed a prevalence of MCR of 9.7%.2 The incidence of MCR was 65.2/1,000 person-years in 4 US-based cohorts.3 Individuals with MCR are at high risk for transitioning to dementia, both Alzheimer disease (AD)2 and vascular dementia.1 MCR has incremental predictive validity for dementia compared to its individual components of cognitive complaints or slow gait.1,2
The underlying biology of MCR is not yet established. Our preliminary study showed an association of the regulatory variant in the IL10 gene with incident MCR.4 While candidate gene and genome-wide association studies (GWAS) have shown variants in multiple genetic loci to be associated with dementia,5,6 their individual effect sizes are small. Recent studies suggest that multiple regions across the genome (polygenic inheritance) may determine cognitive phenotypes.7,8 Individual level genetic predisposition is reflected by the polygenic score (PGS), constructed based on effect sizes of millions of single nucleotide polymorphisms (SNPs) derived from large GWAS conducted in the phenotype of interest.9
Obesity, stroke, Parkinson disease, physical inactivity, and depressive symptoms were reported to increase risk for MCR, whereas education was protective.2,3,10 We hypothesized that the combined genetic predisposition for these phenotypes might be associated with MCR. We conducted a preliminary investigation to examine polygenic effects on MCR and its components in the Health and Retirement Study (HRS).
Methods
Study population
The HRS is a nationally representative US-based cohort study of social, economic, and health issues in individuals age 50 years and older.11–13 The study is supported by the National Institute on Aging (NIA U01AG009740) and Social Security Administration and is conducted at the University of Michigan. The HRS conducts core interviews every 2 years using a mixed-mode design of telephone and in-person interviews. In 2006, HRS implemented “enhanced” face-to-face interviews, which included physical performance tests as well as blood and saliva samples. Half of the HRS cohort was randomly selected to receive physical performance tests (including timed gait) as part of the enhanced interview in 2006, and the other half in 2008. Additional information on the HRS design, sampling, clinical protocols, as well as genomic data are available on the HRS website (hrsonline.isr.umich.edu).
Ethical approval for the HRS was obtained from the University of Michigan institutional review board. The Einstein institutional review board approved this analysis.
MCR syndrome
MCR syndrome was diagnosed in HRS participants based on established criteria.1–3 In brief, MCR adapts definitions of mild cognitive impairment (MCI),14 substituting the objective cognitive impairment criterion based on cognitive tests used in MCI with slow gait speed. MCR is defined as presence of subjective cognitive complaints and slow gait in older individuals without dementia or mobility disability. Subjective cognitive complaints were classified based on a positive response by the participant to either of the following 2 questions on the core HRS interview: (1) “How would you rate your memory at the present time? Would you say it is excellent, very good, good, fair, or poor?” “Fair” and “poor” responses were coded as positive. (2) “Compared with the previous interview, would you say your memory is better now, about the same, or worse now than it was then?” A response of “worse” was coded as positive. Participants aged 65 years and older were timed while walking at normal pace over a 98.5-inch (8 feet) course with start and stop points marked on the floor. The mean of 2 trials was calculated. Walking times were converted to gait speed (m/s). Slow gait was defined as gait speed 1 SD or more below age- and sex-specific means.15 Age- and sex-adjusted slow gait cut scores (m/s) were as follows: men <75 years = 0.61, men ≥75 years = 0.48, women <75 years = 0.54, and women ≥75 years = 0.42. We have reported risk factors for incident slow gait in HRS using the same definition of slow gait.15 We also defined MCR in HRS using the same operational crtieria.16,17
Genetic data and PGS construction
Genomic data and quality control
DNA samples for the 2006 wave were collected from buccal swabs, and extracted using the Qiagen (Venlo, the Netherlands) Autopure method. In 2008, saliva samples were collected and DNA extracted with Oragene. Around 12,500 individuals in these 2 phases were genotyped for ∼2.4 million SNPs using the Illumina (San Diego, CA) HumanOmni2.5-4v1 array at the Center for Inherited Disease Research. Standard quality control procedures led to removal of individuals with missing call rates (>2%), chromosomal anomalies, and first-degree relatives. SNPs with call rates <98% and HWE p value <0.0001 were also removed. For our study, only participants with European ancestry were included to minimize population stratification issues. European ancestry (European American) was defined as individuals who reported non-Hispanic white/Caucasian ethnicity and fell within 1 SD for eigenvectors 1 and 2 in the principal components analysis (PCA) of all unrelated study participants. PCA have been further defined in the quality control report available on the HRS website (hrs.isr.umich.edu/data-products/genetic-data).
Polygenic scores
We examined the association of 9 PGS with MCR and its components, subjective cognitive complaints, and slow gait. The PGS were those associated with the following 9 phenotypes: general cognition, body mass index (BMI), mean arterial pressure, education, AD, neuroticism, subjective well-being, waist circumference, and depressive symptoms. These phenotypes were selected based on their associations with MCR or dementia in epidemiologic studies.2,3,10
Polygenic scores construction
PGSs are the weighted sum of SNPs across the genome associated with the phenotype for an individual, and are calculated based on large GWAS meta-analysis studies. PGS data for various phenotypes were released by HRS in July 2017, and made accessible online (hrs.isr.umich.edu/news/hrs-polygenic-scores-2006-2010-genetic-data). All available SNPs, independent of any linkage disequilibrium (LD) or p value threshold, were used to construct PGS. MHC region in chromosome 6 (26–33 Mb) was omitted due to long range LD. PGS were calculated as weighted sums, with odds ratio (OR) or beta estimates from the GWAS meta-analysis file for the phenotypes of the interest used to define weights. GWAS meta-analyses that did not include HRS data in discovery analysis were selected or when analysis was repeated after removing HRS participants. All the GWAS meta-analyses SNP files were converted to National Center for Biotechnology Information build 37 annotations for compatibility with HRS SNP data. In case of negative beta values (or OR < 1), the beta/OR measures are converted to positive values by flipping reference allele to contribute to phenotype-increasing PGSs. The following formula is used to derive PGSs:
 |
where i is individual i (i = 1 to N), j is SNP j (j = 1 to J), W is the meta-analysis effect size for SNP j, and G is the genotype, or the number of reference alleles (0, 1, or 2), for individual i at SNP j. All the PGSs were standardized within ethnicity to a standard normal curve with mean = 0 and SD = 1. Further details on PGS construction can be accessed from the HRS website (hrs.isr.umich.edu/news/hrs-polygenic-scores-2006-2010-genetic-data).
Statistical analysis
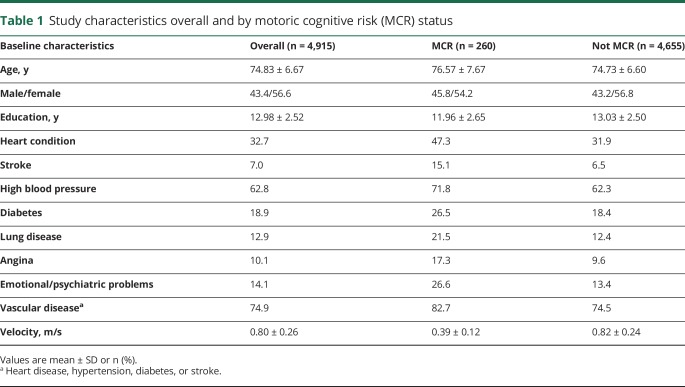
Baseline characteristics of participants with and without MCR were compared by t test for continuous variables and χ2 test for categorical variables (table 1). MCR was diagnosed based on data from either the 2006 or 2008 wave depending on which wave the participant completed the physical function assessment. Association of PGSs with MCR and individual components was conducted using logistic regression, and reported as ORs with 95% confidence intervals (CIs). All analyses were adjusted for age, sex, education, and 5 ancestry-specific principal components (to correct for potential bias due to population stratification). The phenotypes and trait analyzed in this study and the meta-analysis used for the construction of PGSs are described in table 2. Statistical analyses were conducted using SPSS software (version 24; IBM Corporation, Armonk, NY).
Table 1.
Study characteristics overall and by motoric cognitive risk (MCR) status
Table 2.
Genome-wide association studies (GWAS) meta-analysis used by the Health and Retirement Study (HRS) to derive polygenic scores for different phenotypes
Sensitivity analysis
We did not statistically correct our primary analyses as they were empirically based with phenotypes selected based on previous studies.2,3,10 As a secondary approach, the results were adjusted for multiple testing using the Benjamini and Hochberg18 false discovery rate (FDR) procedure. FDR p values less than 0.05 were considered significant. Due to the preliminary nature of this study and to avoid missing important insights, in analyses where the FDR p value was more than 0.05, a p value less than 0.05 was described as being of suggestive significance.
To account for the presence of individuals with individual clinical MCR components (cognitive complaints or slow gait) in the non-MCR control group, we conducted sensitivity analysis excluding these individuals from the control group.
Pairwise interaction analysis was carried out between PGSs on MCR. This analysis was exploratory in nature, as such analyses require a larger sample size to have adequate statistical power. The logistic regression model contained both the individual PGSs as well as the interaction variable. All interaction analyses were adjusted for age, sex, education, and genetic ancestry.
Data availability statement
The datasets used for the primary and supplementary analyses are available from the HRS website (hrs.isr.umich.edu/data-products/access-to-public-data).
Results
Study population
Of the 9,991 HRS participants with European ancestry with PGS available, 8,958 participated in the 2006 or 2008 enhanced HRS interview, and 5,671 were age 65 and older. We excluded 756 older individuals from this analysis for the following reasons: self-reported or proxy-reported dementia diagnosis at baseline (n = 155), participants with total scores that were 2 SDs or below the mean on the modified Telephone Interview for Cognitive Status used to assess general mental status in HRS (n = 164),19 incomplete timed walk assessments due to safety concerns (n = 170), refusal (n = 39), inability to walk due to a health condition (n = 70), space constraints or equipment malfunction (n = 149), or inaccurate or missing walk time (n = 9). Hence, 4,915 HRS participants, age 65 years and older, formed the eligible sample.
Of the 4,915 eligible participants, 260 individuals had MCR diagnosed at either the 2006 or 2008 wave, 1,928 subjective cognitive complaints, 529 slow gait, and 2,718 were free from both slow gait and cognitive complaints. The study characteristics overall and by MCR status are presented in table 1. Overall, the mean age of participants was 75.0 ± 6.8 years (range 65–101 years). The mean years of education were 12.9 ± 2.6, and 56.6% of participants were women. There was higher prevalence of heart-related disorders, stroke, high blood pressure, diabetes, and lung disease as well as emotional/psychiatric problems in participants with MCR compared to those without MCR (table 1).
PGS analysis
MCR syndrome
The association of PGS of multiple traits with MCR adjusted for age, sex, education, and genetic ancestry is shown in table 3. Increased PGS for BMI (OR 1.22, p = 0.004, pFDR = 0.027) and waist circumference (OR 1.23, p = 0.003, pFDR = 0.027) were associated with MCR syndrome. Increased PGS for AD (OR 1.16, p = 0.021, pFDR = 0.071) showed a trend towards increased risk of MCR. The other 6 PGS were not associated with MCR (table 3).
Table 3.
Logistic regression analysis of polygenic score (PGS) of multiple phenotypes with motoric cognitive risk syndrome adjusted for age, sex, education, and genetic ancestry
Sensitivity analysis excluding individuals with isolated cognitive complaints or slow gait confirmed the association of BMI (OR 1.23, 95% CI 1.07–1.41, p = 0.003) and waist circumference (OR 1.24, 95% CI 1.08–1.43, p = 0.002) PGS with MCR. Further, the association of PGS associated with AD (OR 1.17, 95% CI 1.02–1.33, p = 0.021) were strengthened in the sensitivity analysis (table 4).
Table 4.
Logistic regression analysis of polygenic score (PGS) of multiple phenotypes with motoric cognitive risk adjusted for age, sex, education, and genetic ancestry (sensitivity analysis)
We conducted a post hoc analysis, including the trait along with its related PGS in the logistic regression analysis for those showing significant results. Increased PGS for BMI (OR 1.16, 95% CI 1.01–1.33, p = 0.027) and waist circumference (OR 1.17, 95% CI 1.02–1.34, p = 0.021) were still associated with MCR in these models, though the level of significance was reduced.
Subjective cognitive complaints
Increased neuroticism PGS was associated with subjective cognitive complaints (OR 1.10, p = 0.007, pFDR 0.036) (table 5). The PGS for subjective wellbeing was associated with reduced risk of cognitive complaints (OR 0.92, p = 0.012, pFDR 0.046). There was no association between the other PGS and cognitive complaints (table 5).
Table 5.
Logistic regression analysis of polygenic score (PGS) of multiple phenotypes with cognitive complaints adjusted for age, sex, education, and genetic ancestry
Slow gait
Similar to MCR, PGS for BMI (OR 1.16, p = 0.002, pFDR 0.027) and waist circumference (OR 1.19, p = 0.001, pFDR 0.027) were associated with the slow gait component of MCR (table 6). AD PGS was associated with slow gait (OR 1.13, p = 0.008, pFDR 0.036). No other PGSs were associated with slow gait (table 6).
Table 6.
Logistic regression analysis of polygenic score (PGS) of multiple phenotypes with slow gait adjusted for age, sex, education, and genetic ancestry
Of all pairwise interactions between the 9 PGSs, only 2 were nominally significant. AD–neuroticism (OR 1.153, 95% CI 1.027–1.294, p = 0.016) as well as mean arterial pressure–educational attainment (OR 0.880, 95% CI 0.785–0.986, p = 0.027) PGS interactions were associated with MCR. However, neither association survived FDR correction.
Discussion
Our study based in the nationally representative HRS cohort provides insights into the genetic predisposition of MCR syndrome. We found associations between (1) obesity-related PGS and MCR, (2) neuroticism, depressive symptoms, and subjective well-being PGS with cognitive complaints, and (3) obesity-related and AD PGS with slow gait speed. Although the study was carried out in relatively small sample size and not all associations survived corrections for multiple testing, all effects were in the expected directions, and the inferences were consistent with biological pathways (e.g., AD PGS was associated with MCR, brain-related PGS were more strongly associated with cognitive complaints, whereas anthropometric PGS were more strongly associated with gait). Furthermore, the MCR-associated genetic traits were consistent with the clinical risk factors identified for MCR in multiple aging cohorts.2,3,10 The findings from our preliminary investigation will help decipher the genetic risk shared with other complex phenotypes as well as in understanding specific biological pathways underlying MCR. To our knowledge, this is the first study that has examined individual-level genetic burden in relation to a predementia syndrome.
Higher BMI and waist circumference PGS were associated with increased risk for MCR in HRS. Support for our findings comes from both epidemiologic and genetic studies. Obesity in midlife is a strong risk factor for AD as well as vascular dementia.20–22 Higher midlife BMI predicted early onset of AD as well as vascular dementia.23–25 High BMI is also associated with reduced default mode connectivity26,27 as well as global and regional gray matter volume loss.28 Higher BMI was associated with incident MCR in a multicenter study involving 3,128 older adults in United States3 as well as with prevalent MCR in a cross-sectional Japanese study.10 Waist circumference has been suggested as a better indicator of obesity-related health risk than BMI.29 It was shown to be associated with dementia in persons 65–75 years old.30 An age-related paradox in the association of obesity with dementia has been reported, with obesity being a risk factor for dementia in midlife and protective of cognition in advanced ages. However, this has less influence on our genetic results. The obesity-related genetic predisposition to MCR may be due to pleiotropic effects of SNPs related with obesity and shared pathways with dementia rather than a direct effect. At the genetic level, variations in the fat and obesity-associated FTO gene have been associated with dementia, mainly AD.31,32 FTO obesity-associated risk allele carriers also showed lower brain volumes compared to noncarriers.33 Inclusion of associated traits (BMI and waist circumference) reduces but does not take away the effect of the related PGS with MCR, suggesting that other pathways might also be involved in this association. Biological studies are needed to further explore these associations.
Increased genetic predisposition for neuroticism as well as lower subjective well-being PGS were risk factors for subjective cognitive complaints in HRS. Neuroticism is one of the Big Five higher-order personality traits, and higher levels of neuroticism were associated with dementia and MCI.34 Neuroticism PGS was suggestively associated with MCR in our sensitivity analysis. Midlife neuroticism was found to be associated with increased risk of AD dementia over a 38-year follow-up period in women in a population-based study.35 Neuroticism was correlated with subjective cognitive complaints in the Sydney Memory and Ageing Study.36 Interaction analysis showed an association of neuroticism–AD PGS pair with MCR. But this interaction needs to be validated in a larger sample. Lower PGSs for subjective well-being were associated with increased risk for MCR in our analysis.
Our analysis revealed shared genetic predisposition for AD and obesity-associated traits with slow gait. Growing evidence supports slow gait as an early predictor of cognitive decline and dementia.37 Slowing of gait has been reported to precede mild cognitive impairment syndrome as well as dementia.38,39 MCR syndrome was shown to predict AD as well as vascular dementia.1,2 In the Einstein aging study, we found that APOE ɛ4 allele was associated with faster gait speed decline in older men.40 PGS of obesity-related phenotypes (BMI and waist circumference) were associated with slow gait, similar to MCR. Elevated BMI is a well-established risk factor for slow gait.41,42 Thus, our observation in regard to higher PGSs for BMI and waist circumference being associated with slow gait aligns with earlier studies linking obesity and gait performance. PGS for educational attainment had a suggestive protective effect on slow gait. Education, a marker of cognitive reserve, is protective for AD.43
The strengths of this study include the well-defined PGS based on comparative studies carried out in HRS44 and the use of best available estimates of genetic effects from large meta-analyses. We were consistent in defining MCR as in our previous reports.1–3,10 Strengths of the MCR construct are that slow gait is defined objectively, independent of clinical gait evaluations that may be prone to variable sensitivity and specificity as well as being examiner-dependent. Though slow gait is multifactorial in nature with both neurologic and non-neurologic etiologies,15 multiple studies have shown that slow gait predicts cognitive decline irrespective of the underlying etiology.39 Our analytical approach was empiric; we identified phenotypes of interest from previous MCR studies.2,3,10 Finally, the large, well-characterized HRS sample and the systematic gait assessments are also strengths. There are limitations that need to be noted. We may have missed other unstudied phenotypes that might also influence MCR and dementia pathogenesis. The analysis was restricted to European ancestry; our findings need to be verified in other ethnicities. The analysis was cross-sectional in nature.
We found an association of obesity-related genetic traits with MCR syndrome. These observations have to be studied further to identify underlying biological mechanisms and potential therapeutic targets in dementia pathogenesis.
Glossary
- AD
Alzheimer disease
- BMI
body mass index
- CI
confidence interval
- FDR
false discovery rate
- GWAS
genome-wide association studies
- HRS
Health and Retirement Study
- LD
linkage disequilibrium
- MCI
mild cognitive impairment
- MCR
motoric cognitive risk
- OR
odds ratio
- PCA
principal components analysis
- PGS
polygenic score
- SNP
single nucleotide polymorphism
Appendix. Authors

Study funding
This study was supported by a National Institute on Aging grant (1R56AG057548-01). The sponsors had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 2013;68:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 2014;83:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verghese J, Ayers E, Barzilai N, et al. Motoric cognitive risk syndrome: multicenter incidence study. Neurology 2014;83:2278–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sathyan S, Barzilai N, Atzmon G, Milman S, Ayers E, Verghese J. Association of anti-inflammatory cytokine IL10 polymorphisms with motoric cognitive risk syndrome in an Ashkenazi Jewish population. Neurobiol Aging 2017;58:238.e231–238.e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 2013;45:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bettens K, Sleegers K, Van Broeckhoven C. Genetic insights in Alzheimer's disease. Lancet Neurol 2013;12:92–104. [DOI] [PubMed] [Google Scholar]
- 7.Davies G, Armstrong N, Bis JC, et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N=53949). Mol Psychiatry 2015;20:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies G, Marioni RE, Liewald DC, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112 151). Mol Psychiatry 2016;21:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet 2013;9:e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi T, Verghese J, Shimada H, et al. Motoric cognitive risk syndrome: prevalence and risk factors in Japanese seniors. J Am Med Directors Assoc 2015;16:1103.e21–1103.e25. [DOI] [PubMed] [Google Scholar]
- 11.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour 1995:S7–S56. [Google Scholar]
- 12.Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the Health and Retirement Study. J Am Geriatr Soc 2009;57:511–516. [DOI] [PubMed] [Google Scholar]
- 13.Zivin K, Llewellyn DJ, Lang IA, et al. Depression among older adults in the United States and England. Am J Geriatr Psychiatry 2010;18:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC. Clinical practice: mild cognitive impairment. N Engl J Med 2011;364:2227–2234. [DOI] [PubMed] [Google Scholar]
- 15.Verghese J, Wang C, Allali G, Holtzer R, Ayers E. Modifiable risk factors for new-onset slow gait in older adults. J Am Med Dir Assoc 2016;17:421–425. [DOI] [PubMed] [Google Scholar]
- 16.Ayers E, Verghese J. Motoric cognitive risk syndrome and risk of mortality in older adults. Alzheimers Dement 2016;12:556–564. [DOI] [PubMed] [Google Scholar]
- 17.Callisaya ML, Ayers E, Barzilai N, et al. Motoric cognitive risk syndrome and falls risk: a multi-center study. J Alzheimers Dis 2016;53:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 19.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 2011;66(suppl 1):i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razay G, Raza G, Vreugdenhil A. Obesity in middle age and future risk of dementia: midlife obesity increases risk of future dementia. BMJ 2005;331:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med 2003;163:1524–1528. [DOI] [PubMed] [Google Scholar]
- 23.Chuang YF, An Y, Bilgel M, et al. Midlife adiposity predicts earlier onset of Alzheimer's dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry 2016;21:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitmer RA, Gunderson EP, Quesenberry CP, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103–109. [DOI] [PubMed] [Google Scholar]
- 25.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age and ageing 2006;36:23–29. [DOI] [PubMed] [Google Scholar]
- 26.Beyer F, Kharabian Masouleh S, Huntenburg JM, et al. Higher body mass index is associated with reduced posterior default mode connectivity in older adults. Hum Brain Mapp 2017;38:3502–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorg C, Riedl V, Mühlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA 2007;104:18760–18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity 2008;16:119–124. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004;79:379–384. [DOI] [PubMed] [Google Scholar]
- 30.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol 2007;64:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitz C, Tosto G, Mayeux R, Luchsinger JA. Genetic variants in the fat and obesity-associated (FTO) gene and risk of Alzheimer's disease. PLoS One 2012;7:e50354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer's disease risk: a prospective cohort study. J Alzheimers Dis 2011;23:461–469. [DOI] [PubMed] [Google Scholar]
- 33.Ho AJ, Stein JL, Hua X, et al. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci USA 2010;107:8404–8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low L-F, Harrison F, Lackersteen SM. Does personality affect risk for dementia? A systematic review and meta-analysis. Am J Geriatr Psychiatry 2013;21:713–728. [DOI] [PubMed] [Google Scholar]
- 35.Johansson L, Guo X, Duberstein PR, et al. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology 2014;83:1538–1544. [DOI] [PubMed] [Google Scholar]
- 36.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 2010;18:701–710. [DOI] [PubMed] [Google Scholar]
- 37.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol 2012;68:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 2010;67:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 2007;78:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verghese J, Holtzer R, Wang C, Katz MJ, Barzilai N, Lipton RB. Role of APOE genotype in gait decline and disability in aging. J Gerontol A Biol Sci Med Sci 2013;68:1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev 2010;11:568–579. [DOI] [PubMed] [Google Scholar]
- 42.Beavers KM, Beavers DP, Houston DK, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition Study. Am J Clin Nutr 2013;97:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shpanskaya KS, Choudhury KR, Hostage C, et al. Educational attainment and hippocampal atrophy in the Alzheimer's disease neuroimaging initiative cohort. J Neuroradiol 2014;41:350–357. [DOI] [PubMed] [Google Scholar]
- 44.Ware EB, Schmitz LL, Faul JD, et al. Heterogeneity in polygenic scores for common human traits. bioRxiv 2017:106062. [Google Scholar]
- 45.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wain LV, Verwoert GC, O'Reilly PF, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet 2011;43:1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okbay A, Beauchamp JP, Fontana MA, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 2016;533:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okbay A, Baselmans BM, De Neve JE, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 2016;48:624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for the primary and supplementary analyses are available from the HRS website (hrs.isr.umich.edu/data-products/access-to-public-data).