Abstract
Objective
To define the clinical phenotype of patients with myositis with anti-U1-ribonucleoprotein (RNP) autoantibodies.
Methods
In this longitudinal cohort study, the prevalence and severity of clinical features at disease onset and during follow-up in patients with anti-U1-RNP–positive myositis were compared to those with dermatomyositis (DM), immune-mediated necrotizing myopathy (IMNM), and the antisynthetase syndrome (AS).
Results
Twenty anti-U1-RNP–positive patients, 178 patients with DM, 135 patients with IMNM, and 132 patients with AS were included. Anti-U1-RNP–positive patients were younger (∼37 years) and more likely to be black (60%) than patients with AS, DM, or IMNM. Muscle weakness was a presenting feature in 15% of anti-U1-RNP–positive patients; 80% eventually developed weakness. Four of 7 anti-U1-RNP–positive patients had necrotizing muscle biopsies. Arthritis occurred in 60% of anti-U1-RNP–positive patients; this was increased compared to DM (18%) or IMNM (6%) (all p < 0.01). DM-specific skin features developed in 60% of anti-U1-RNP–positive patients. Interstitial lung disease (ILD) occurred in 45% of anti-U1-RNP–positive patients; fewer patients with DM (13%) and IMNM (6%) and more patients with AS (80%) developed ILD (all p < 0.01). Glomerulonephritis and pericarditis occurred in 25% and 40% of anti-U1-RNP–positive patients, respectively, but rarely in the other groups; these features occurred only in those with coexisting anti-Ro52 autoantibodies. No anti-U1-RNP patient had cancer-associated myositis or died during the study period.
Conclusions
Patients with anti-U1-RNP myositis typically present with proximal weakness and necrotizing muscle biopsies. Arthritis, dermatitis, and ILD are the most common extramuscular clinical features. Pericarditis and glomerulonephritis are uniquely found in patients with anti-U1-RNP–positive myositis.
The autoimmune myopathies are a heterogeneous family of diseases that affect both skeletal muscle and other organ systems. The most common forms of autoimmune myopathy include dermatomyositis (DM), immune-mediated necrotizing myopathy (IMNM), and the antisynthetase syndrome (AS). In addition, myositis may overlap with other autoimmune diseases.1
Myositis autoantibodies are associated with unique clinical phenotypes in patients with various forms of myositis, including overlap myositis. For example, autoantibodies recognizing U1-ribonucleoprotein (RNP) have been reported to occur in patients with myositis, including those who also have systemic lupus erythematosus, systemic sclerosis, or mixed connective tissue disease (MCTD). To date, the prevalence and severity of the muscular and extramuscular clinical features at disease onset and during follow-up have not been well-described in patients with myositis with anti-U1-RNP autoantibodies. Furthermore, no studies have directly compared the clinical features of patients with anti-U1-RNP–positive myositis to those with DM, IMNM, and AS.2
In the present study, we conducted a longitudinal cohort study of patients with myositis and anti-U1-RNP autoantibodies. The demographic, clinical, and laboratory features of these patients with myositis was compared to those with DM, IMNM, and AS.
Methods
Patients and autoantibody testing
All patients enrolled in the Johns Hopkins Myositis Center longitudinal cohort study between 2002 and 2017 were included if they were positive for anti-U1-RNP or myositis-specific autoantibodies as described below.
Patient sera were screened for anti-U1-RNP antibodies by ELISA at the Johns Hopkins laboratory or by S35-immunoprecipitation (IP), immunodiffusion, or RNA-IP at the Oklahoma Medical Research Foundation; positive samples were confirmed by RNA-IP at the NIH Muscle Disease Unit laboratory. Patient sera were screened for anti-Ro52 by EUROLINE myositis profile blot. Serum samples from anti-U1-RNP–positive patients were subsequently tested for (1) anti-dsDNA and anti-Sm autoantibodies by ELISA; (2) anti-centromere, anti-topoisomerase, anti-RNA polymerase III, and anti-U3-RNP autoantibodies using the EUROLINE systemic sclerosis profile; (3) and myositis-specific autoantibodies (as described for the comparison groups below).
The comparison groups included patients who were positive for myositis-specific autoantibodies by at least 2 different immunologic techniques from among the following: ELISA, in vitro transcription and translation IP, line blotting (EUROLINE myositis profile), IP from S35-labeled HeLa cell extracts, immunodiffusion, or RNA-immunoprecipitation as previously described.3 The AS group included all patients with an antisynthetase autoantibody. The DM group included all patients positive for anti-Mi2, anti-NXP2, anti-TIF1γ, or anti-MDA5 autoantibodies. The IMNM group included all patients positive for anti-SRP or anti-HMGCR autoantibodies.
Muscle strength was assessed by the examining physician using the Medical Research Council (MRC) scale; serial strength measurements for each patient were made by the same physician. The MRC scale was transformed to Kendall's 0–10 scale4 for analysis. The average of right- and left-side measurements for arm abduction and hip flexion strength was used for calculations (possible range 0–10).
At their initial visit to the Johns Hopkins Myositis Center, the presence or absence of clinical signs and symptoms at disease onset was established retrospectively based on a review of prior patient records and patient recollection. Interstitial lung disease (ILD) at the onset of the disease (often prior to the first visit at the Myositis Center) was assessed by retrospective chart review. At the first visit and on subsequent visits to the Johns Hopkins Myositis Center, the presence or absence of DM-specific rashes (i.e., heliotrope or Gotron rashes), SSc-specific skin involvement (i.e., sclerodactyly), esophageal symptoms (i.e., reflux and dysphagia), and AS-associated clinical symptoms (i.e., arthralgia) and signs, either observed by the clinician (i.e., mechanic’s hands and arthritis) or reported by the patient (i.e., fever and Raynaud phenomenon), were assessed prospectively. During follow-up, ILD was defined through a multidisciplinary approach as recommended by the American Thoracic Society (ATS).5 All patients with suspicion of pulmonary hypertension (PH) (compatible clinical and echocardiographic features) underwent a right heart catheterization. Those with a mean pulmonary arterial pressure ≥25 mm Hg at rest were considered as having PH.6 Pulmonary function testing (PFT) included spirometry, lung volumes measured by helium dilution, and diffusing capacity by single breath carbon monoxide (DLCO) based on ATS criteria.7 Muscle enzyme levels and PFTs were included for analysis if obtained within a period of 6 weeks before or after strength testing (except for peak, minimum, and mean values, where all available data were included). Glomerulonephritis was assessed by kidney biopsy and pericarditis by echocardiography.
The cumulative features recorded at all visits were used to classify the anti-U1-RNP–positive patients. Each patient was classified for myositis type based on the Bohan and Peter8 criteria and for MCTD using the Sharp et al.,2 Kasukawa et al.,9 Alarcon Segovia and Villareal,10 and Khan11 criteria. The patients were also classified using the 2013 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for systemic sclerosis12 and the 1997 ACR classification criteria for lupus.13
All available muscle biopsies were interpreted at the Johns Hopkins Neuromuscular Pathology Laboratory by pathologists blinded to autoantibody status. The pathologists consistently reported on the presence or absence of perifascicular atrophy, perivascular inflammation, primary inflammation (i.e., the invasion of non-necrotic fibers by mononuclear cells), and necrotizing myopathy (i.e., prominent myofiber necrosis in the absence of perifascicular atrophy or primary inflammation).
Standard protocol approvals and patient consents
This study was approved by the Johns Hopkins Institutional Review Board and written informed consent was obtained from each participant.
Statistical analysis
Dichotomous variables were expressed as percentage (count) and continuous variables as mean (SD). Bivariate comparisons of continuous variables were made using Student t test while bivariate comparisons of dichotomous variables were made either using χ2 test or Fisher exact test, as appropriate. CK, a highly positively skewed variable, was expressed as median, first, and third quartile for descriptive purposes and transformed through a base-10 logarithm for the statistical analysis. Each one of the study groups was compared to the sample of anti-U1-RNP patients.
To account for the different number of visits per patient, the evolution of the pulmonary function tests, CK levels, and muscle strength were studied using multilevel linear regression models with random slopes and random intercepts. The mean of hip flexor and arm abductor strength (range 0–10) was used as the strength outcome for regression analysis. Logistic regression was used to analyze dichotomous variables across groups adjusting by possible confounders.
The influence of nonmodifiable risk factors (sex, race, length of illness, and age at the onset of the first symptoms), the corticosteroid dose, and the administration of IV immunoglobulins, rituximab, methotrexate, azathioprine, and mycophenolate, were used as adjusting covariates. Other treatments administered to less than 10% of the cohort were not included in the analysis.
All statistical analyses were performed using Stata/MP 14.1. A 2-sided p value of 0.05 or less was considered significant with no correction for multiple comparisons.
Data availability
No unpublished data related to this study are publicly available.
Results
General features
Among 437 patients enrolled in the Johns Hopkins Myositis Center Longitudinal Cohort Study who underwent testing for anti-U1-RNP autoantibodies, 20 (4.6%) were positive. Of note, patients with inclusion body myositis, genetic muscle disease, toxic myopathies, and other nonmyositis diagnoses did not routinely undergo testing for anti-U1-RNP autoantibodies. The comparator groups included 178 patients with DM, 135 patients with IMNM, and 132 patients with AS.
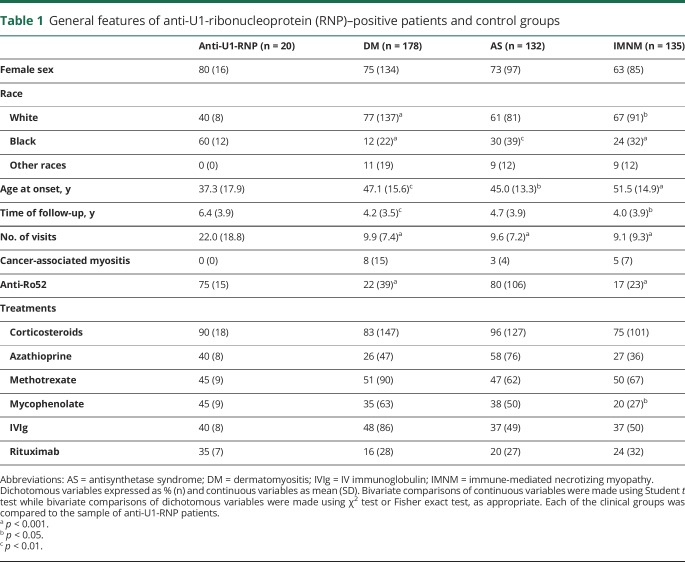
The mean follow-up time for the anti-U1-RNP–positive patient group was 6.4 years, which was longer than that for the DM or IMNM patient groups, who were followed for a mean time of 4.2 and 4.0 years, respectively (table 1). Anti-U1-RNP–positive patients had a mean of 22 visits per patient, which was more than double the mean number of visits for each of the other 3 groups (table 1). The median time between the onset of the disease and the first visit at Hopkins was 1 year (Q1–Q3: 0.6–6.4).
Table 1.
General features of anti-U1-ribonucleoprotein (RNP)–positive patients and control groups
Anti-U1-RNP–positive patients were younger (37.3 years old) at disease onset compared to patients with DM (47.1 years old; p < 0.01), IMNM (51.5 years old; p < 0.001), or AS (45.0 years old; p < 0.05). As in the other groups, there was a marked female predominance among anti-U1-RNP participants (80%). Of note, 60% of anti-U1-RNP–positive patients were black, which was greater than the one of black patients in the other groups; only 12% of patients with DM, 30% of patients with AS, and 24% of patients with IMNM were black (all p < 0.05 compared to the anti-U1-RNP–positive group). All groups were exposed to similar treatment modalities, although mycophenolate was more commonly used in anti-U1-RNP–positive patients (45%) compared to patients with IMNM (20%).
No anti-U1-RNP–positive patient died during the study period or developed any malignancy within 3 years of the onset of the first disease symptoms.
Muscle involvement
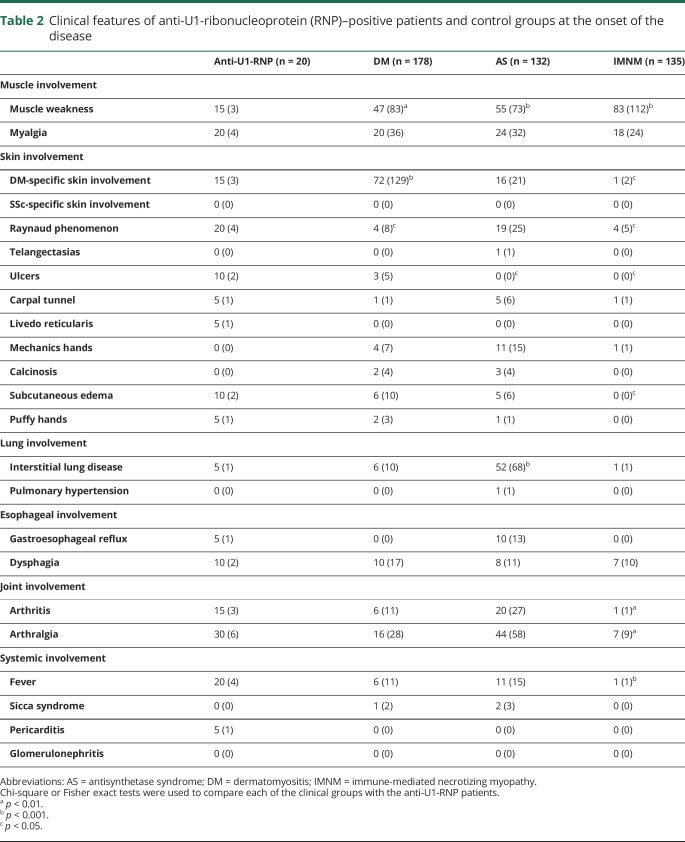
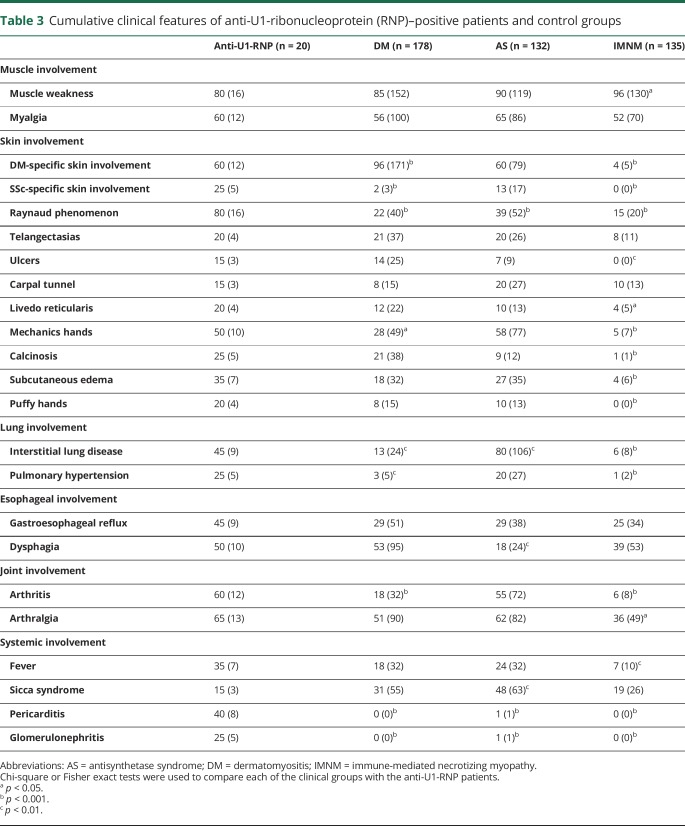
At the onset of disease, muscle weakness was less prevalent among anti-U1-RNP–positive patients (15%) compared to those with DM (47%), AS (55%), or IMNM (83%) (p values all <0.01) (table 2). Weakness emerged in 80% of anti-U1-RNP–positive patients during the course of the disease, which was less frequent only in comparison to the patients with IMNM (80% vs 96%, p = 0.02) (table 3).
Table 2.
Clinical features of anti-U1-ribonucleoprotein (RNP)–positive patients and control groups at the onset of the disease
Table 3.
Cumulative clinical features of anti-U1-ribonucleoprotein (RNP)–positive patients and control groups
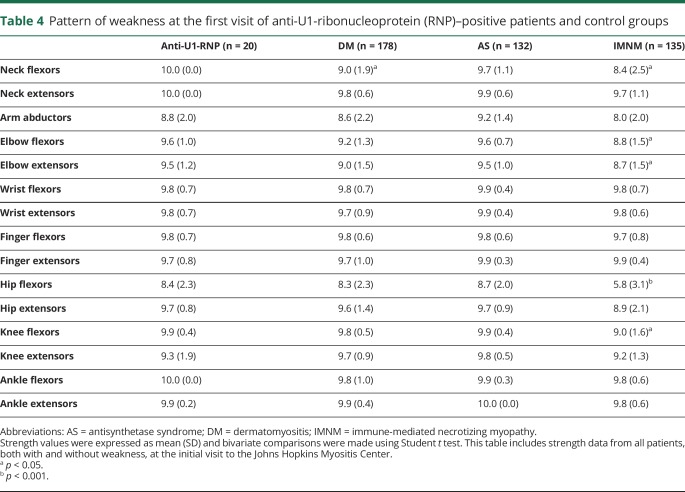
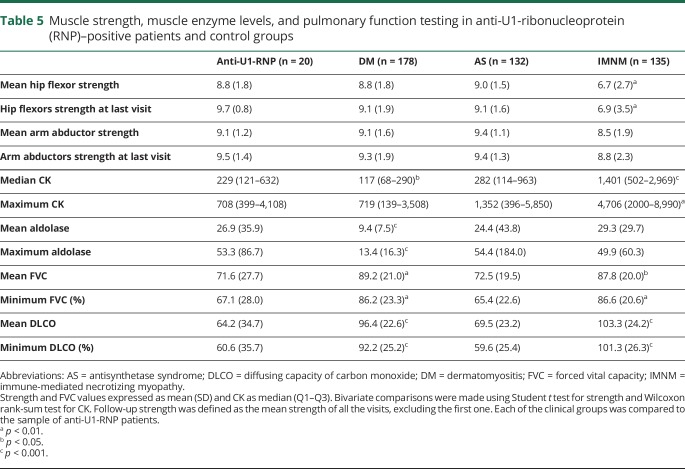
At their first visit to the Johns Hopkins Myositis Center, the severity of weakness in anti-U1-RNP–positive patients was similar to the patients with DM and patients with AS, with proximal weakness predominantly in hip flexors and arm abductors (table 4). At this point, 9 out of the 20 patients had measurable weakness in arm abductors or hip flexors but just 5 of the 20 maintained full strength over the follow-up period. Interestingly, U1-RNP–positive patients were the only group with no detectable neck weakness. Patients with anti-U1-RNP autoantibodies had higher median CK (229 vs 117 IU/L, p = 0.02) and aldolase levels (26.9 vs 9.4 IU/L, p < 0.001) compared to participants with DM (table 5). In contrast, anti-U1-RNP–positive patients had lower median CK levels compared to patients with IMNM (CK 229 vs 1,401 IU/L, p < 0.001) and were stronger, particularly in the hip flexors (mean Kendall score of 8.8 vs 6.7, p = 0.001) (table 5).
Table 4.
Pattern of weakness at the first visit of anti-U1-ribonucleoprotein (RNP)–positive patients and control groups
Table 5.
Muscle strength, muscle enzyme levels, and pulmonary function testing in anti-U1-ribonucleoprotein (RNP)–positive patients and control groups
Multilevel regression analysis showed that anti-U1-RNP–positive patients with higher CK levels were weaker than those with lower CK levels (β = −0.5, p = 0.03). This analysis also confirmed that, independent of the length of illness, age at onset, race, sex, or immunosuppressant treatment, IMNM was the only group weaker (β = −1.3, p < 0.001) and with higher CK levels (β = 0.7, p < 0.001) than anti-U1-RNP patients.
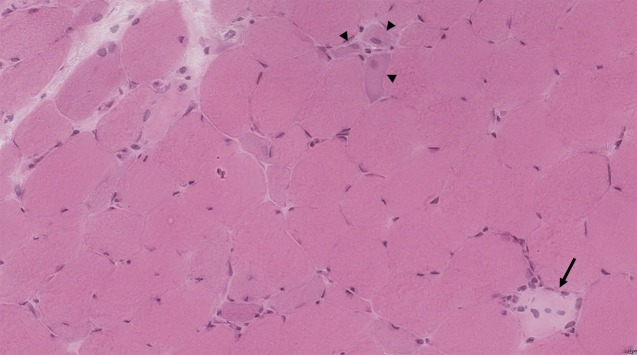
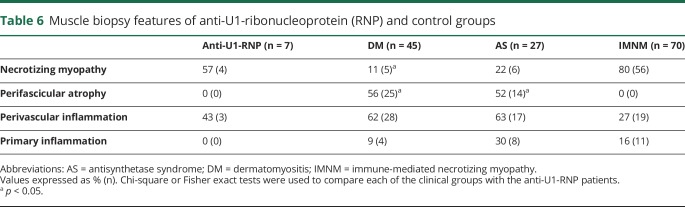
Of the 7 anti-U1-RNP patients with biopsies available for review at Johns Hopkins, 4 (57%) had a predominantly necrotizing pattern. The other 3 patients had biopsies revealing combinations of myofiber regeneration (in one biopsy), perimysial inflammation (in 2 biopsies), and perivascular inflammation (in 2 biopsies) (figure). The prevalence of a necrotizing muscle biopsy was not different in patients with AS (6/27, 22%, p = 0.2) or IMNM (56/70, 80%, p = 0.2), but was more common than in DM (5/45, 11%, p = 0.01). Perifascicular atrophy was not observed in any anti-U1-RNP patient or in IMNM, but was common in muscle biopsies from DM (25/45, 56%, p = 0.01) and AS (14/27, 52%, p = 0.03) patients. Similarly, lymphocytic invasion of non-necrotic muscle fibers was not found in any of the anti-U1-RNP–positive patient muscle biopsies. By contrast, muscle biopsies from 9% of patients with DM, 30% of patients with AS, and 16% of patients with IMNM showed this feature (table 6).
Figure. Muscle biopsy from an anti-U1-ribonucleoprotein–positive patient (hematoxylin & eosin).
The arrow shows a necrotic cell undergoing early-stage myophagocytosis. Regenerating myofibers, characterized by basophilic cytoplasm and enlarged nuclei, are indicated by the arrowheads (×200 magnification).
Table 6.
Muscle biopsy features of anti-U1-ribonucleoprotein (RNP) and control groups
Lung involvement
Although ILD was present in just 1 (5%) anti-U1-RNP patient at the onset of disease (table 2), 45% developed ILD during follow-up (table 3). In contrast, more patients with AS (80%, p = 0.002) and fewer patients with DM (13%, p = 0.002) or patients with IMNM (6%, p < 0.001) developed ILD during the course of disease. The mean forced vital capacity and DLCO was lower (71.6% and 64.2%) in anti-U1-RNP–positive patients compared to those with DM (89.2% and 96.4%, p < 0.005) or IMNM (87.7% and 103.3%, p < 0.02) (table 5).
While none of the 20 anti-U1-RNP–positive patients were noted to have PH at disease onset (table 2), 5 (25%) developed this during the course of their illness (table 3). The prevalence of PH was higher in anti-U1-RNP–positive patients than in DM (3%, p = 0.001) or IMNM (1%, p < 0.001), but was similar to that observed in AS (20%, p = 0.8). Three of 5 patients with PH had concomitant ILD. Of note, immunosuppressive therapy improved, but did not completely reverse, PH in all 3 anti-U1-RNP–positive patients with available longitudinal echocardiographic information.
Logistic regression confirmed that, independent of the age at onset, length of illness, sex, or race, ILD occurred less frequently in anti-U1-RNP–positive patients than in patients with AS (odds ratio [OR] 0.2, p < 0.001) and more frequently in anti-U1-RNP–positive patients than in patients with DM (OR 4, p = 0.01) or IMNM (OR 13, p < 0.001). Moreover, logistic regression showed that PH was more common in anti-U1-RNP–positive patients compared to those with DM (OR 10.2, p = 0.001) or IMNM (OR 23, p = 0.01). There was no difference in the prevalence of PH between those with anti-U1-RNP autoantibodies and those with AS (OR 1.1, p = 0.8). Multilevel regression models also confirmed that, independent of the abovementioned confounding variables and treatments received, DLCO in patients with anti-U1-RNP autoantibodies was similar to patients with AS (DLCO β = 13%, p = 0.1), but was more severe when compared to those with IMNM (DLCO β = 33%, p = 0.006) or DM (DLCO β = 34%, p < 0.001).
Skin involvement
Heliotrope rashes or Gottron sign, the characteristic cutaneous features of DM, were present in only 15% of patients with anti-U1-RNP autoantibodies at the onset of disease (table 2). However, the cumulative presence of these features rose to 60% during the follow-up period (table 3). Sclerodactyly, a typical skin feature of systemic sclerosis, was not present in any anti-U1-RNP–positive patient at the onset of the disease (table 2), but ultimately affected 25% of these patients (table 3). Raynaud phenomenon and mechanic's hands, characteristic cutaneous manifestations of AS, were present during the course of disease in 80% and 50% of U1-RNP–positive patients, respectively; both of these features were less common in DM (22% and 28%, respectively; both p < 0.04) (table 3). Of note, Raynaud phenomenon occurred more often in anti-U1-RNP–positive patients than in those with AS (39%, p < 0.001) (table 3). As expected, compared to anti-U1-RNP–positive patients, those with IMNM had a markedly lower prevalence of skin involvement.
Other extramuscular involvement
Glomerulonephritis was not present in any of the 20 anti-U1-RNP–positive myositis patients at the onset of disease and pericarditis was initially present in just 1 (5%) patient (table 2). However, glomerulonephritis occurred in 5 (25%) patients during the course of disease (table 3) and renal biopsies revealed membranous glomerulonephritis in 4 (80%) of these cases. Similarly, pericarditis eventually complicated the clinical course in 8 (40%) of those with anti-U1-RNP autoantibodies (table 3). In contrast, aside from a single patient with AS, neither glomerulonephritis nor pericarditis were diagnosed in patients with other forms of myositis.
The prevalence of gastroesophageal reflux or dysphagia was uncommon at the onset of the disease (5%–10%) (table 2) but eventually affected ∼50% of the anti-U1-RNP–positive patients (table 3). Dysphagia was more prevalent among those with anti-U1-RNP autoantibodies when compared to the patients with AS (18%, p < 0.01) (table 3).
At the onset of disease, arthritis and arthralgia were present in 15% and 30% of anti-U1-RNP–positive patients, respectively (table 2); the prevalence of joint involvement increased during the course of the disease, eventually affecting 60%–65% of patients (table 3). Arthritis was more common in anti-U1-RNP–positive patients compared to those with DM or IMNM (18% and 6%, both p < 0.001) (table 3).
Coexisting anti-Ro52 autoantibodies
Since anti-Ro52 autoantibodies are commonly found in patients with myositis and may be associated with more severe disease,14 all patients were tested for these autoantibodies. Anti-Ro52 autoantibodies were more frequent in anti-U1-RNP–positive patients (75%) than in patients with DM (22%) or IMNM (17%) (both p < 0.001). Coexisting anti-Ro52 autoantibodies were found in 80% of patients with AS, which was similar when compared to the prevalence of these autoantibodies in anti-U1-RNP–positive patients (table 1).
Muscle weakness was not significantly different among anti-U1-RNP–positive patients with and without anti-Ro52 autoantibodies. Similarly, the severity of ILD was not significantly different among anti-U1-RNP–positive patients with and without anti-Ro52 autoantibodies. Despite that, the prevalence of ILD was nonsignificantly increased in anti-Ro52 positive patients (53% vs 20%, p = 0.3). Of note, 5 of 15 (33%) patients who had both anti-U1-RNP and anti-Ro52 autoantibodies were found to have PH, whereas no anti-U1-RNP–positive patient without anti-Ro52 autoantibodies was diagnosed with PH (p = 0.3).
Interestingly, glomerulonephritis and pericarditis occurred also only in patients with both anti-U1-RNP and anti-Ro52 autoantibodies. Glomerulonephritis occurred in 5 of 15 (42%) patients with both anti-U1-RNP and anti-Ro52 autoantibodies (p = 0.3). Pericarditis occurred in 8 of 12 (67%) patients with these 2 autoantibodies; this was increased compared to anti-U1-RNP–positive patients without anti-Ro52 autoantibodies (p = 0.05).
Coexisting autoantibodies associated with scleroderma, lupus, and myositis
As some patients with anti-U1-RNP autoantibodies have clinical features of scleroderma or lupus, we tested for autoantibodies classically associated with these diseases. No anti-U1-RNP–positive patient had anti-topoisomerase, anti-centromere, or anti-polymerase III autoantibodies (i.e., scleroderma-associated autoantibodies). Coexisting autoantibodies recognizing Sm, dsDNA, and U3-RNP (i.e., lupus-associated autoantibodies) were found in 5 (25%), 3 (15%), and 1 (5%) of the anti-U1-RNP–positive patients, respectively.
Anti-U1-RNP–positive patients with coexisting anti-dsDNA autoantibodies had more pericarditis (100% vs 29%, p = 0.05), glomerulonephritis (100% vs 12%, p = 0.009), and subcutaneous edema (100% vs 24%, p = 0.03) than those without anti-dsDNA autoantibodies. We did not identify other significant clinical differences between anti-U1-RNP–positive patients with and without anti-Sm or anti-dsDNA autoantibodies.
Three anti-U1-RNP–positive patients had coexisting anti-Jo1 autoantibodies; these patients were excluded from the AS group for the purposes of this study. Otherwise, no patient with anti-U1-RNP autoantibodies had a myositis-specific autoantibody.
Clinical classification of anti-U1-RNP–positive myositis
All 20 anti-U1-RNP–positive patients fulfilled the Bohan and Peter8 criteria for either DM (60%) or polymyositis (40%). Given their diverse clinical manifestations, most of these patients also fulfilled diagnostic criteria for one or more other systemic autoimmune diseases. For example, 9 (45%) met the 2013 ACR/EULAR classification criteria for scleroderma and 11 (55%) met the 1997 ACR classification criteria for lupus. Ninety percent of anti-U1-RNP–positive patients met at least one set of criteria for MCTD: 18 (90%) met the Kasukawa et al.9 criteria, 16 (80%) met the Khan11 criteria, 14 (70%) met the Alarcon Segovia and Villareal10 criteria, and 5 (25%) met the Sharp et al.2 criteria.
Discussion
In this study, we have defined the distinctive clinical phenotype of patients with myositis with anti-U1-RNP autoantibodies. These patients are younger and more likely to be black then those with DM, IMNM, or AS. This is consistent with a prior report that a high proportion of anti-U1-RNP–positive patients are black.15 Like the other myositis groups, those with anti-U1-RNP autoantibodies have proximal pattern of muscle weakness. However, the neck muscles are spared only in those with anti-U1-RNP autoantibodies. Anti-U1-RNP–positive patients are also notable for their prominent extramuscular manifestations. These include Raynaud phenomenon (80%), arthralgia/arthritis (60%), DM skin features (60%), necrotizing muscle biopsies (57%), mechanic's hands (50%), and dysphagia (50%). Other common clinical manifestations in these patients include ILD (45%), pericarditis (40%), subcutaneous edema (35%), fever (35%), glomerulonephritis (25%), pulmonary hypertension (25%), sclerodactyly (25%), and calcinosis (25%).
The unique clinical phenotype of anti-U1-RNP–positive patients can be further appreciated by comparing them to each of the 3 other myositis groups separately. Compared to patients with DM, those with anti-U1-RNP autoantibodies are more likely to have sclerodactyly, Raynaud phenomenon, mechanic's hands, ILD, arthritis, pericarditis, and glomerulonephritis; as expected, they are less likely to have heliotrope or Gottron sign. Compared with patients with AS, anti-U1-RNP–positive patients are more likely to have Raynaud phenomenon, dysphagia, pericarditis, and glomerulonephritis; they are less likely to have ILD and sicca syndrome. Finally, anti-U1-RNP–positive patients are more likely to have all of the studied extramuscular manifestations of disease compared to those with IMNM. In contrast, significantly more patients with IMNM have weakness and weakness is more severe in patients with IMNM.
Given that patients with IMNM seem to have the least in common with anti-U1-RNP–positive patients, it may be surprising to find that muscle biopsies from both groups can be strikingly similar, with prominent myofiber necrosis and scant lymphocytic infiltration. Of note, others have also reported myofiber necrosis and regeneration in muscle biopsies from anti-U1-RNP–positive patients.16,17 Since the prognosis of patients with anti-U1-RNP autoantibodies is different from those with anti-HMGCR myopathy or anti-SRP myopathy, testing for each of these autoantibodies is indicated in patients presenting with a necrotizing muscle biopsy.
Pericarditis with or without glomerulonephritis occurred in 40% of patients with myositis with anti-U1-RNP autoantibodies; these complications were exceedingly rare in the other myositis groups. Of note, all 8 anti-U1-RNP–positive myositis patients with pericarditis/glomerulonephritis had coexisting anti-Ro52 autoantibodies. Similarly, PH was detected only in RNP-positive patients who were also positive for anti-Ro52 autoantibodies. Taken together, 60% of anti-U1-RNP–positive patients with coexisting anti-Ro52 antibodies developed PH, pericarditis, or glomerulonephritis, while no patient without anti-Ro52 developed any of these manifestations (p = 0.04). Although it requires confirmation in other cohorts, based on these observations, clinicians could consider testing anti-U1-RNP–positive myositis patients for anti-Ro52 to identify those patients most at risk for developing these serious extramuscular manifestations of disease.
This study has several limitations. First, most of the conclusions are based on signs and symptoms that were recorded prospectively from the beginning of the study in 2002. Consequently, we could not include activity and damage tools that were not available when the study started. Second, data used in this study are based on patients presenting to a multidisciplinary myositis center and may be biased towards including patients with active muscle and lung disease. Third, due to the relatively small sample size of our anti-U1-RNP–positive population, our study may have been underpowered to detect differences in some key features like the association between ILD and anti-Ro52 autoantibodies. Future studies including larger numbers of anti-U1-RNP–positive patients will be of value.
These limitations notwithstanding, we have shown that patients with anti-U1-RNP autoantibodies appear to have a unique syndrome different from patients with DM, AS, or IMNM. This syndrome is characterized by proximal muscle weakness, necrotizing muscle biopsies, and frequent extramuscular manifestations. Glomerulonephritis, pericarditis, and PH are relatively common in anti-U1-RNP–positive patients with coexisting anti-Ro52 autoantibodies but rare in the other myositis groups. We propose that testing for anti-Ro52 autoantibodies may be useful to determine which anti-U1-RNP–positive patients are at most risk for these complications.
Glossary
- ACR
American College of Rheumatology
- AS
antisynthetase syndrome
- ATS
American Thoracic Society
- DLCO
diffusing capacity of carbon monoxide
- DM
dermatomyositis
- EULAR
European League Against Rheumatism
- ILD
interstitial lung disease
- IMNM
immune-mediated necrotizing myopathy
- IP
immunoprecipitation
- MCTD
mixed connective tissue disease
- MRC
Medical Research Council
- OR
odds ratio
- PFT
pulmonary function testing
- PH
pulmonary hypertension
- RNP
ribonucleoprotein
Appendix. Authors
Study funding
This work was financially supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH. I.P.F. was supported by a fellowship from The Myositis Association. E.T. was supported by the Scientist Development Award from the Rheumatology Research Foundation and the Jerome-Greene Foundation. The Johns Hopkins Rheumatic Disease Research Core Center, which processed and banked the sera and performed some of the autoantibody assays, is supported by NIH grants P30-AR-053503 and P30-AR-070254.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;372:1734–1747. [DOI] [PubMed] [Google Scholar]
- 2.Sharp GC, Irvin WS, Tan EM, Gould RG, Holman HR. Mixed connective tissue disease: an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med 1972;52:148–159. [DOI] [PubMed] [Google Scholar]
- 3.De Lorenzo R, Pinal-Fernandez I, Huang W, et al. Muscular and extramuscular clinical features of patients with anti-PM/Scl autoantibodies. Neurology 2018;90:e2068–e2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rider LG, Werth VP, Huber AM, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res 2011;63(suppl 11):S118–S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 7.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–735. [DOI] [PubMed] [Google Scholar]
- 8.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–407. [DOI] [PubMed] [Google Scholar]
- 9.Kasukawa R, Tojo T, Miyawaki S. Preliminary diagnostic criteria for classification of mixed connective tissue disease. In: Kasukawa R, Sharp G, eds. Mixed Connective Tissue Disease and Antinuclear Antibodies. Amsterdam: Elsevier; 1987:41. [Google Scholar]
- 10.Alarcon Segovia D, Villareal M. Classification and diagnostic criteria for mixed connective tissue disease. In: Kasukawa R, Sharp G, eds. Mixed Connective Tissue Disease and Anti-nuclear Antibodies. Amsterdam: Elsevier; 1987:33. [Google Scholar]
- 11.Kahn M Syndrom de Sharp. In: Kahn M, Peltier A, Meyer O, Piette J, eds. Les Maladies Systemiques, 3rd ed. Amsterdam: Elsevier; 1991:545. [Google Scholar]
- 12.van den Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum 2013;65:2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 14.Marie I, Hatron PY, Dominique S, et al. Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody. Semin Arthritis Rheum 2012;41:890–899. [DOI] [PubMed] [Google Scholar]
- 15.Coppo P, Clauvel JP, Bengoufa D, Oksenhendler E, Lacroix C, Lassoued K. Inflammatory myositis associated with anti-U1-small nuclear ribonucleoprotein antibodies: a subset of myositis associated with a favourable outcome. Rheumatology 2002;41:1040–1046. [DOI] [PubMed] [Google Scholar]
- 16.Oxenhandler R, Hart M, Corman L, Sharp G, Adelstein E. Pathology of skeletal muscle in mixed connective tissue disease. Arthritis Rheum 1977;20:985–988. [DOI] [PubMed] [Google Scholar]
- 17.Vianna MA, Borges CT, Borba EF, Caleiro MT, Bonfá E, Marie SK. Myositis in mixed connective tissue disease: a unique syndrome characterized by immunohistopathologic elements of both polymyositis and dermatomyositis. Arq Neuropsiquiatr 2004;62:923–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No unpublished data related to this study are publicly available.