Abstract
Oligonucleotide recombination allows the introduction of specific point mutations into the bacterial genome. Here we report the successful application of this technique to clinical isolates of Pseudomonas aeruginosa to allow subsequent investigations of the biological impact associated with resistance-conferring mutations.
Keywords: Recombination, Pseudomonas aeruginosa, fluoroquinolones, resistance mutations
Homologous recombination is a highly conserved process that facilitates genetic exchange between identical or nearly identical DNA molecules (Lovett et al., 2002). Swingle et al. (Swingle et al., 2010) demonstrated that bacterial recombination can be achieved experimentally by introducing high concentrations of single stranded synthetic oligonucleotides via electroporation. The authors successfully utilized this procedure to introduce site-specific mutations into the genomes of Pseudomonas syringae, Shigella flexneri, Escherichia coli, and Salmonella typhimurium. In the present study, we describe the use of this procedure to generate isogenic mutants from clinical isolates of Pseudomonas aeruginosa, an organism in which this technique has not been attempted previously. Pseudomonas aeruginosa is a leading cause of infection in the hospital setting, and resistance to the fluoroquinolone antibiotics has risen dramatically with their increase in use. (Linder et al., 2005, Neuhauser et al., 2003).
We recently showed in a large collection of clinical isolates that strains containing the gene that encodes for the exotoxin ExoU more readily acquire a second mutation in parC compared to exoS+ strains in addition to the fluoroquinolone resistance-conferring mutation in gyrA (Agnello and Wong-Beringer, 2012). Therefore, we sought to understand the biological effects of this specific point mutation in both exoU and exoS clinical isolates by utilizing the technique of oligonucleotide recombination to create isogenic parC mutants for multiple exoU and exoS clinical isolates.
Pseudomonas aeruginosa isolates were obtained from the respiratory tract of hospitalized patients with pneumonia and were stored at −80°C in 70% glycerol until ready for use. Seven isolates (3 exoU+ and 4 exoS+) were selected for recombination from our previous collection of 270 clinical isolates (Agnello and Wong-Beringer, 2012) based on the presence of the exoU or exoS gene and a mutation in gyrA conferring fluoroquinolone resistance. Our goal was to generate isogenic double mutants from these clinical strains. Oligos 22 nucleotides and 60 nucleotides in length were used and were designed based on the parC gene sequence of strain PAO1 (Winsor et al., 2011) (Table 2). The oligos were identical to the PAO1 sequence from nucleotides 249 to 270 and 230 to 289 respectively, save for the point mutation TCG→TTG at locations 12 and 31 of the oligos, corresponding to nucleotide 260 in the parC gene (Figure 1). This point mutation, which is the most common parC mutation observed among fluoroquinolone-resistant clinical strains, gives rise to the Ser87→Leu amino acid change in the ParC protein.
Table 2.
Oligos and Primers used
| Name | Use | Primer Sequence (5’-3’) | Referenc es |
|---|---|---|---|
| gyr A | PCR/ Sequencing |
Forward: ttatgccatgagcgagctgggcaacgact Reverse: aaccgttgaccagcaggttgggaatctt |
(Jalal and Wretlind, 1998) |
| par C | PCR/ Sequencing |
Forward: cgagcaggcctatctgaactat Reverse: gaaggacttgggatcgtccgga |
(Jalal and Wretlind, 1998) |
| parC*6 0 |
Recombinati on |
tgctcggcaagttccacccgcacggcgacttggcctgctacgaggccatggtgc tgatgg |
Present study |
| parC*2 2 |
Recombinati on |
gcacggcgacttggcctgctac | Present study |
Figure 1. Schematic of Recombination.
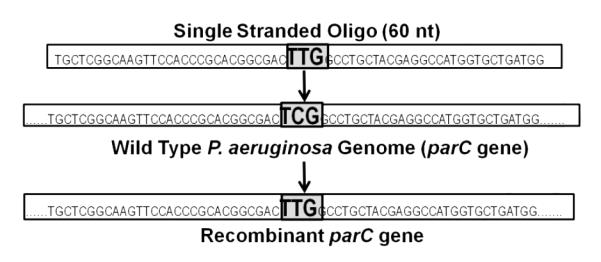
The successful single stranded oligonucleotide used was 60 base pairs in length and identical in sequence to a portion of the wild type parC gene, save for the point mutation indicated. The strains integrated the oligo into their genomes at the site of similarity within the parC gene.
For electroporation, a protocol for P. aeruginosa was adapted (Choi et al., 2006). An overnight culture was diluted 100 fold, incubated with shaking at 37°C and harvested at OD600= 0.3-0.5 by centrifugation at 3200 × g. Cells were resuspended twice in 300 mM sucrose at room temperature. Either the 22-nt or the 60 nt oligo (5-6 μg in 2-3 μl) was added to 40 μl of electrocompetent cells and transformed by electroporation at 2.5 kV in a .2 cm cuvette using a Micropulser (Bio-Rad). SOC medium (1 ml) was added immediately and the cells were outgrown overnight at 37°C on Pseudomonas Isolation Agar plus levofloxacin at concentrations 2, 4, 8, and 16 fold above the original minimum inhibitory concentration (MIC) of the isolates. For each isolate, a mock experiment was performed as a control in which cells underwent electroporation without the addition of any oligo.
Strains electroporated with the oligo 22 nucleotides in length did not grow on any selection plates. “Mock” control strains did not grow on any selection plate in 4 out of the 7 experiments. In the other 3 experiments, the mock cultures were able to grow on plates containing levofloxacin at a concentration 2 times higher than the strain’s MIC; however, subsequent sequencing of these colonies showed that no change had occurred in the parC gene, demonstrating that growth of the control strains on selection was not due to the presence of a spontaneous target site mutation.
Strains electroporated with the 60 nucleotide oligo grew on selection plates with a concentration of up to 16 times higher than their original MIC. Single colonies were selected from the highest concentration of levofloxacin-containing plates and used to inoculate 5 ml of LB broth, from which DNA was extracted using the DNeasy Mini Kit (Qiagen). Prior to sequencing, the PCR products were purified using the QIAquick PCR Purification Kit (Qiagen). Primers for PCR were previously published (Jalal and Wretlind, 1998) and are listed in Table 2. Sequencing results confirmed that all strains had incorporated the TCG→TTG mutation in the parC gene at nucleotide 260, and no extraneous recombination had occurred in the gyrA gene, despite the high degree of sequence similarity to parC.
Minimum inhibitory concentration (MIC) to levofloxacin was measured by broth microdilution according to guidelines recommended by CLSI (CLSI), 2007). MIC was also measured with the addition of an efflux pump inhibitor (Phe-Arg β-naphthylamide dihydrochloride, Sigma) at 20 μg/ml in order to more accurately reflect the resistance phenotype conferred by the specific point mutation introduced (Lomovskaya et al., 2001). The parC mutation increased the MIC in 5 out of the 7 isolates compared to the parent strains (Table 1).
Table 1.
Characteristics of Parent and Recombinant Strains
| Paren t Strain |
TTSS Genotype (exoU/exoS ) |
MIC/MI C + EP1a (μg/ml) |
Generatio n timeb (hrs) |
Recombinan t |
Recombinant MIC/MIC+EP Ia (μg /ml) |
Recombinan t Generation timeb (hrs) |
|---|---|---|---|---|---|---|
| U-1 | exoU+ | 16/1 | 3.0 | U-1parC* | 16/1 | 2.2 |
| U-2 | exoU+ | 2/2 | 2.7 | U-2parC* | 16/2 | 2.6 |
| U-3 | exoU+ | 16/1 | 2.6 | U-3parC* | 16/1 | 3.0 |
| S-1 | exoS+ | 16/1 | 2.6 | S-1 parC* | 16/8 | 2.8 |
| S-2 | exoS+ | 16/1 | 1.9 | S-2parC* | 16/1 | 1.7 |
| S-3 | exoS+ | 8/1 | 3.4 | S-3parC | 64/16 | 3.1 |
| S-4 | exoS+ | 2/.25 | 2.3 | S-4parC* | 8/4 | 3.1 |
The MIC of levofloxacin was measured with the addition of an efflux pump inhibitor in order to remove the contribution of efflux pump overexpression to the resistance phenotype observed.
Generation time is an average of 3 independent experiments and was determined by calculating the number of generations during exponential phase
To measure growth rates, overnight cultures were diluted 10 fold and grown at 37°C with shaking. Cultures (150 μl) were sampled every 30 min for 8 hours and turbidity was measured in triplicate at OD600 using a microplate reader (Tecan Group Ltd., Switzerland). Results are an average of at least 3 independent experiments. Generation time during exponential phase was calculated by dividing the time interval over the number of generations. Interestingly, the growth rates of the recombinants were not significantly affected; the average difference between the generation times of the recombinants compared to the parents was less than 1 hour (Table 1).
The technique of oligonucleotide recombination described here that we have adapted for use in clinical isolates of Pseudomonas aeruginosa is an efficient and practical approach for inserting point mutations into specific sections of the bacterial genome. The threat of widespread increasing antibiotic resistance makes it imperative to gain a full understanding of the biological impact resistance mutations can have on the individual organism as well as the bacterial population as a whole. As laboratory-derived mutants do not reflect real world pathogenesis (Fux et al., 2005), this technique can prove useful in generating isogenic mutant strains from clinical isolates for in depth investigations.
Highlights.
We inserted specific point mutations into clinical isolates of P. aeruginosa.
We adapted the method of oligonucleotide recombination for use in P. aeruginosa.
We created isogenic, fluoroquinolone-resistant mutants from 7 clinical strains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (CLSI), C.L.S.I. Performance standards for antimicrobial susceptibility testing: 17th Informational supplement. Wayne, PA: 2007. pp. M100–S117. [Google Scholar]
- Agnello M, Wong-Beringer A. Differentiation in Quinolone Resistance by Virulence Genotype in Pseudomonas aeruginosa. PLoS One. 2012;7:e42973. doi: 10.1371/journal.pone.0042973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Kumar A, Schweizer HP. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods. 2006;64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Fux CA, Shirtliff M, Stoodley P, Costerton JW. Can laboratory reference strains mirror "real-world" pathogenesis? Trends Microbiol. 2005;13:58–63. doi: 10.1016/j.tim.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Jalal S, Wretlind B. Mechanisms of quinolone resistance in clinical strains of Pseudomonas aeruginosa. Microb Drug Resist. 1998;4:257–261. doi: 10.1089/mdr.1998.4.257. [DOI] [PubMed] [Google Scholar]
- Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med. 2005;118:259–268. doi: 10.1016/j.amjmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee VJ. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett ST, Hurley RL, Sutera VA, Aubuchon RH, Lebedeva MA. Crossing over between regions of limited homology in Escherichia coli. RecA-dependent and RecA-independent pathways. Genetics. 2002;160:851–859. doi: 10.1093/genetics/160.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289:885–888. doi: 10.1001/jama.289.7.885. [DOI] [PubMed] [Google Scholar]
- Swingle B, Markel E, Costantino N, Bubunenko MG, Cartinhour S, Court DL. Oligonucleotide recombination in Gram-negative bacteria. Mol Microbiol. 2010;75:138–148. doi: 10.1111/j.1365-2958.2009.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, Yu NY, Hancock RE, Brinkman FS. Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 2011;39:D596–600. doi: 10.1093/nar/gkq869. [DOI] [PMC free article] [PubMed] [Google Scholar]


