Abstract
Background & Aims:
Renal clearance is the major elimination pathway for sofosbuvir (SOF). We assessed the safety and efficacy of SOF-containing regimens in patients with varying baseline estimated glomerular filtration rate (eGFR).
Methods:
HCV-TARGET database is a multicentre, longitudinal ‘real-world’ treatment cohort.
Results:
A total of 1789 patients [genotypes 1 (72%), 2 (17%) 3 (9%), 4–6 (2%)] had baseline eGFR determination: 73 with eGFR≤45 (18 with eGFR≤30, 5 on dialysis) were compared to 1716 with eGFR>45 ml/min/1.73 m2. Patients with baseline eGFR≤45 vs. >45 differed in being female (55% vs. 36%), age ≥65 years (24% vs. 16%), Black race (22% vs. 12%), having cirrhosis with decompensation (73% vs. 24%) and being post-transplant (49% vs. 10%), all P < 0.05. All patients with eGFR≤45 were treated with SOF 400 mg/day (including those on haemodialysis) and had median starting ribavirin (RBV) dose of 800 mg (IQR: 400–1200). Sustained virologic response (SVR) frequencies were similar across eGFR groups, ranging from 82–83%. Patients with eGFR ≤45 more frequently experienced anaemia, worsening renal function and serious AEs (all P < 0.05), and these associations persisted when limiting analysis to RBV-free regimens. Patients with baseline eGFR≤30 and eGFR 31–45 had similar frequencies of efficacy and safety outcomes.
Conclusions:
Sustained viral clearance was achieved in 83% of patients with renal impairment (eGFR ≤45 ml/min/1.73 m2) treated with SOF-containing regimens. However, these patients had higher rates of anaemia, worsening renal dysfunction and serious adverse events regardless of use of RBV. Patient with renal impairment require close monitoring and should be treated by providers extensively experienced with SOF-containing regimens.
Keywords: decompensated cirrhosis, haemodialysis, liver transplantation, sustained virologic response
For several approved all-oral hepatitis C regimens, sofosbuvir (SOF) is the backbone of the combination therapy. SOF is extensively metabolized to the pharmacologically active metabolite GS-461203 with eventual dephosphorylation to the inactive metabolite GS- 331007 (1). Renal clearance is the major elimination pathway for SOF, via GS-331007, and compared to those with normal renal function, SOF AUC0–∞ was 170% higher and the GS-331007 AUC0–∞ was 450% higher in those with estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 (1). As a result, use of SOF is not recommended in patients on haemodialysis or with eGFR <30 ml/min/1.73 m2.
There is a significant unmet need for hepatitis C virus (HCV) treatment options in patients with renal dysfunction, including those on dialysis. Currently, the only FDA-approved all oral regimens for use in patients with severe renal dysfunction are elbasvir/grazoprevir and ombitasvir/paritaprevir/ritonavir with or without dasabuvir (2, 3). However, it is well recognized that HCV infection can directly, via glomerulonephritis and cryoglobulinemic vasculitis, or indirectly, via hepatic cirrhosis and associated complications of portal hypertension, cause renal dysfunction and large-scale community observational studies have shown that HCV infection increases the risk for incident chronic kidney disease (CKD) and progression to end-stage renal disease (4). As a result, because of the high need and limited alternatives, increasing off-label use of SOF in patients with moderate to severe renal dysfunction can be expected.
In this HCV-TARGET consortium study, we examined the real-world clinical experience with SOF-based therapy to assess the safety and efficacy of SOF containing regimens in HCV infected patients with varying baseline renal function.
Patients and methods
Study population and design
Hepatitis C virus-TARGET is a longitudinal, observational study of chronic hepatitis C patients from a consortium of academic (n = 39) and community (n = 15) centres from North America and Europe. Patients ≥18 years old were included if they underwent treatment with a SOF-containing regimen, including SOF/pegylated interferon (PEG)/ribavirin (RBV), SOF/RBV, SOF/simeprevir (SMV) or SOF/SMV/RBV. Treatment was chosen and administered per local standards at the study sites; the study protocol did not define specific treatment populations, regimens, dosing, duration or safety management guidelines.
Data were captured from sequentially enrolled patients using a common database that utilized novel, standardized source data abstraction previously described.(5) In brief, a centralized team of trained coders reviewed all redacted medical records obtained from participating sites for data entry. Throughout treatment and during post-treatment follow-up, demographic, clinical, adverse event and virological data were collected. Independent data monitors systematically reviewed the data entries for completeness and accuracy. All records were screened for extreme or unlikely values and verified/resolved with additional queries. The choice of and management of anaemia and renal complications was at the discretion of the investigators.
The protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The independent ethics committee at each participating study centre or a central Institutional Review Board approved the protocol if a local Institutional Review Board was not in place. All patients provided written informed consent for their participation.
Primary predictor and study outcomes
The primary predictor was baseline eGFR ≤45 ml/min/ 1.73 m2 compared to >45 mL/min/1.73 m2 as calculated by the modification of diet in renal disease study equation.(6) The primary predictor was selected after an exploratory analysis showed that outcomes were similar in patients with a baseline eGFR ≤30 ml/min/1.73 m2 (CKD class 4–5) vs. 31–45 ml/min/1.73 m2 (CKD class 3B) and that outcomes were similar in those with a baseline eGFR 46–59 ml/min/1.73 m2 (CKD class 3A) vs. ≥60 ml/min/1.73 m2 (CKD class 1–2). The efficacy endpoint was sustained virologic response (SVR) 12 weeks post-therapy (SVR12), defined as an undetectable plasma hepatitis C virus ribonucleic acid ≥12 weeks after treatment completion. The safety endpoints included early treatment discontinuation related and unrelated to adverse events; common SOF adverse events including fatigue, headache and nausea; anaemia adverse events, including requiring transfusion(s) and use of erythropoietin on treatment; need for RBV dose reductions or discontinuation; as well as worsening renal function, any serious adverse events, cardiac serious adverse events and death. The safety cohort included all patients who completed treatment and those who discontinued early while those on therapy at the time of data abstraction were excluded. The efficacy cohort included all patients who completed treatment and those that discontinued early while excluding those without 12 week follow-up post-treatment. In a subgroup analysis of patients with baseline eGFR ≤45 ml/min/1.73 m2, eGFR ≤30 ml/min/1.73 m2 was compared to 31–45 ml/min/1.73 m2.
Hepatitis C virus viral load levels were measured according to local practice, usually prior to treatment initiation, at weeks 4, 8 and at the end of treatment and 4 and 12 weeks after treatment discontinuation.
Definitions
Cirrhosis:
The presence of cirrhosis was defined by biopsy and/or a combination of clinical, laboratory and imaging criteria established a priori (5). Patients were determined to have cirrhosis if they had: (i) evidence of stage 4 fibrosis by liver biopsy any time prior to therapy, or (ii) evidence of stage 3 fibrosis by liver biopsy any time prior to therapy with any of the following criteria: platelet count <140 000 per μl, presence of oesophageal varices on oesophagogastroduodenoscopy, evidence of cirrhosis and/or portal hypertension and/or of ascites by imaging studies, FibroSure® (or equivalent) test, vibration-controlled transient elastography or equivalent compatible with stage 4 fibrosis (≥12.5 kPa) or (iii) in the absence of liver biopsy, any two of the following criteria: platelets count <140 000 per μl, presence of oesophageal varices, evidence of cirrhosis and/or portal hypertension and/or ascites by imaging studies, FibroSure® or equivalent test, elastography or equivalent compatible with stage 4 fibrosis.
Adverse events (AE):
(i) Any event that occurred on treatment was collected and reported regardless of the need or lack thereof for a prescription medication or a dose reduction or discontinuation of HCV treatment. AEs were reported in the patient note, identified by HCV-TARGET data abstractors, then entered into the database as text.
Anaemia:
Defined as the presence of at least one of the following: (i) a haemoglobin <10 g/dl or >2 g/dl drop if baseline haemoglobin was <10 g/dl; (ii) administration of erythropoiesis stimulating agents or (iii) need for blood transfusion.
Worsening renal function:
This outcome was abstracted from the HCV-TARGET database as reported by investigators. Worsening renal function included the following text terms: acute kidney failure, acute kidney injury, renal insufficiency, renal failure, azotaemia, acute renal failure, acute renal insufficiency and acute anuric renal failure.
Serious adverse event (SAE):
An AE that required hospitalization or met criteria for expedited reporting per FDA form MEDWATCH 3500.
Statistical analyses
The rate of SVR, relapse, treatment completion and frequency of AEs were calculated for the entire study population and for subpopulations. Unadjusted analyses were performed using either a chi square test for binary/ categorical variables, t-test for continuous variables or non-parametric trend tests for ordered variables (modified Wilcoxon-type rank sum) (7). A multivariable Poisson model reporting incident risk ratios for SVR12, worsening renal function and any SAE was used for analysis. The set of potential variables of interest were selected a priori based upon a consensus of clinical experts. Analyses were performed using STATA MP software version 13 (StataCorp LP, College Station, TX, USA).
Results
At the time of data abstraction, 1893 patients had started a SOF-containing treatment with 82 (4%) patients with 73 m2 (Fig. 1). A total of 104 patients who were still on HCV treatment were excluded from the safety and efficacy analyses (Fig. 1). All 1789 patients from the evaluable cohort who completed therapy were included in the safety cohort. Of the 1789 patients, 1559 (87%) were eligible for SVR12 and were included in efficacy analyses. Of the evaluable cohort (n = 1789), 36% were female, 16% were ≥65 years, 12% Black race and 7% Hispanic ethnicity (Table 1). The majority of patients were infected with genotype 1 HCV (72%) and 53% were HCV treatment experienced. Fifty-two percent of patients had cirrhosis at baseline, 24% with a history of decompensation and 39% with baseline model for end-stage liver disease (MELD) score ≥10. Compared to eGFR >45 ml/min/1.73 m2 patients, eGFR ≤45 ml/min/1.73 m2 patients were more frequently female and Black or African American race (Table 1). Cirrhosis, history of decompensation, prior liver or kidney transplant, use of immunosuppression, hepatocellular carcinoma and diabetes was more frequent among patients with lower baseline eGFR (Table 1). Compared to the eGFR >45 ml/min/1.73 m2 group, baseline median ALT and albumin were lower among eGFR ≤45 ml/min/1.73 m2 patients (Table 1).
Fig. 1.
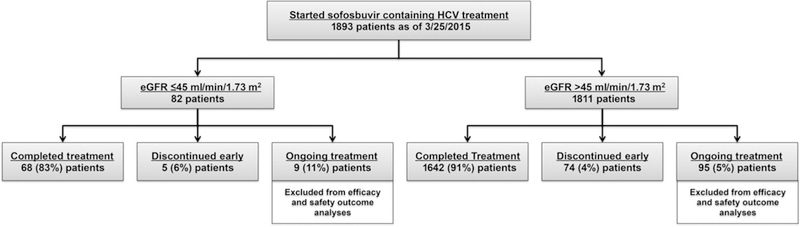
Disposition of patients. eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus.
Table 1.
Baseline characteristics by baseline renal function
| Baseline characteristics | All Patients (N = 1789) | eGFR ≤45 (N = 73) | eGFR >45 (N = 1716) | P-value |
|---|---|---|---|---|
| Female, n(%) | 649 (36) | 40 (55) | 609 (36) | <0.001 |
| Age ≥65 years,n (%) Race, n (%) |
288(16) | 17 (24) | 271 (16) | 0.09 |
| White | 1428(80) | 52(71) | 1376 (80) | 0.06 |
| Black or African American | 218(12) | 16 (22) | 202 (12) | 0.01 |
| Asian / Pacific Islander | 45 (3) | 1 (1) | 44 (3) | 0.54 |
| Other | 86(5) | 3 (4) | 83 (5) | 0.79 |
| Missing | 12(1) | 1 (1) | 11 (1) | 0.46 |
| Hispanic ethnicity, n (%) HCV genotype, n (%) |
123(7) | 7(10) | 116 (7) | 0.36 |
| 1a | 806 (45) | 33 (45) | 773 (45) | 0.98 |
| 1b | 353 (20) | 15(21) | 339 (20) | 0.90 |
| 1 w/subtype not specified | 126(7) | 8(11) | 117 (7) | 0.10 |
| 2 | 295(17) | 11 (15) | 284 (17) | 0.73 |
| 3 | 161 (9) | 4(6) | 157 (9) | 0.28 |
| 4 | 36(2) | 1 (1) | 35 (2) | 0.71 |
| 5, 6 or other | 12(1) | 1 (1) | 11 (1) | 0.84 |
| Q80K polymorphism, n/N (%) | 53/114 (47) | 2/6 (33) | 51/108 (47) | 0.68 |
| Prior HCV treatment experience, no (%) | 950 (53) | 39(53) | 911 (53) | 0.96 |
| Prior 1st generation PI triple therapy experience, n (%) | 176(10) | 6 (8) | 170 (10) | 0.64 |
| Cirrhosis, n (%) | 930(52) | 47 (64) | 883 (52) | 0.03 |
| History of decompensation, n (%) | 437 (24) | 34(73) | 403 (24) | <0.001 |
| MELD ≥10, n (%) | 699(39) | 73(100) | 626 (36) | <0.001 |
| HIV, n (%) | 39(2) | 3 (4) | 36 (2) | 0.25 |
| Liver transplant, n (%) | 211 (12) | 36 (49) | 175 (10) | <0.001 |
| Kidney transplant*, n (%) Immunosuppression, n (%) | 22(1) | 6 (8) | 16 (1) | <0.001 |
| Tacrolimus | 181 (10) | 32 (44) | 149 (9) | <0.001 |
| Cyclosporine | 29(2) | 3 (4) | 26 (1) | 0.09 |
| Everolimus/sirolimus | 31 (2) | 7(10) | 24 (1) | <0.001 |
| Mycophenolate mofetil/mycophenolic acid | 109(6) | 23 (32) | 86 (5) | <0.001 |
| Hepatocellular carcinoma, n (%) | 186(10) | 15(21) | 171 (10) | 0.004 |
| Diabetes, n (%) | 415(23) | 32 (44) | 383 (22) | <0.001 |
| Haemodialysis, n (%) | 5 (<0.5) | 5 (7) | 0 (0) | <0.001 |
| Erythropoietin stimulating agent use at baseline, n (%) | 64 (4) | 6 (8) | 58 (3) | 0.03 |
| Total bilirubin (mg/dl), median (IQR) | 0.8 (0.5–1.2) | 0.7 (0.5–1.3) | 0.8 (0.5–1.2) | 0.44 |
| ALT (IU/L), median (IQR) | 65(41–110) | 50(31–79) | 66 (43–112) | <0.001 |
| Albumin (g/dl), median (IQR) | 4.0 (3.5–4.3) | 3.8 (3.3–4.2) | 4.0 (3.5 –4.3) | 0.02 |
| Haemoglobin (g/dl), median (IQR) | 14.2(13.1–15.3) | 12.1 (10.7–13.9) | 14.3 (13.2–15.4) | <0.001 |
| Platelets (× 103/μl), median (IQR) | 148 (95–208) | 138 (89–200) | 149 (96–208) | 0.21 |
| INR, median (IQR) | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 0.60 |
| HCV RNA (106 IU/ml), median (IQR) | 1.6 (0.5–4.3) | 1.9 (0.5–3.8) | 1.6 (0.5 –4.3) | 0.61 |
Eighteen patients with both liver and kidney transplants: 3 eGFR ≤45 ml/min/1.73 m2, 15 eGFR >45 ml/min/1.73 m2.
ALT, alanine aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INR, international normalized ratio; MELD, model for end-stage liver disease; PI, protease inhibitor.
Of the 73 evaluable patients with baseline eGFR ≤45 ml/min/1.73 m2, 18 had baseline eGFR ≤30 ml/ min/1.73 m2 including five patients on haemodialysis (none on peritoneal dialysis). When comparing patients with baseline eGFR ≤30 ml/min/1.73 m vs. eGFR 31–45 ml/min/1.73 m2, baseline characteristics were similar except for a lower frequency of males, cirrhosis and history of decompensation among those with eGFR ≤30 ml/min/1.73 m2 and (Table S1).
Treatment regimens
Of the evaluable cohort (N = 1789), SOF/SMV was used most frequently at 40%, followed by SOF/RBV at 30%, SOF/PEG/RBV at 18% and SOF/SMV/RBV at 11% (Fig. 2). Compared to patients with higher baseline eGFR, patients with lower baseline eGFR more frequently received the RBV-free regimen of SOF/SMV (53% vs. 40%, P = 0.02) and less frequently received peginterferon-containing therapy (7% vs. 19%, P = 0.008) (Figure S1). When comparing patients with eGFR ≤30 ml/min/1.73 m2 (n = 18) vs. eGFR 31–45 ml/min/1.73 m2 (n = 56), there were no significant differences in the treatment regimen selected. Four of the five patients receiving haemodialysis at baseline received SOF/SMV and one received SOF/RBV.
Fig. 2.
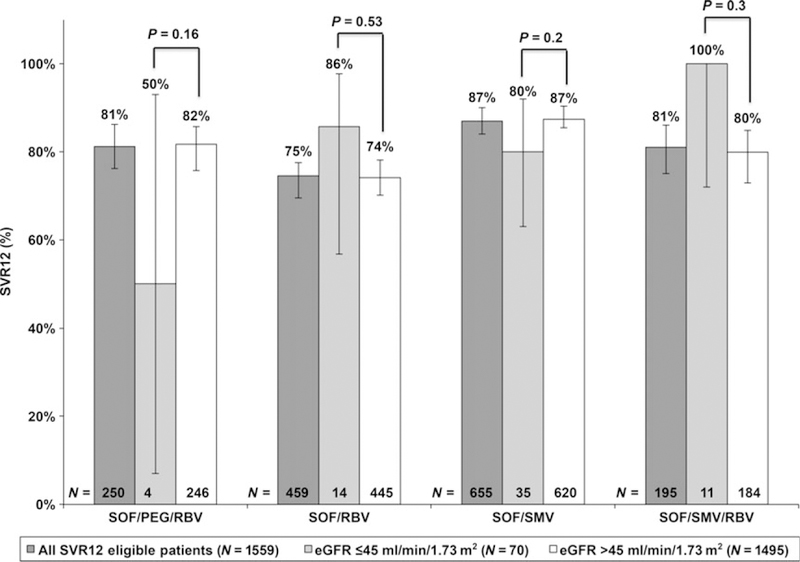
SVR12 rates with 95% confidence intervals by treatment regimen in total cohort and by baseline renal function. eGFR, estimated glomerular filtration rate; PEG, peg-interferon; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir; SVR12, sustained virologic response at 12 weeks.
Among the 1071 patients treated with RBV, the median initial total daily dose of RBV in patients with baseline eGFR ≤45 ml/min/1.73 m2 was 800 mg (IQR: 400–1200) compared to 1200 mg (IQR: 1000–1200) in patients with baseline eGFR >45 ml/min/1.73 m2s (P < 0.001) (Figure S2).
Efficacy outcome
SVR12 was achieved by 1273 of 1559 (82%, 95% CI: 80–84%) patients treated with SOF-containing regimens. SVR12 was achieved in 53 of 64 (83%, 95% CI: 71–91%) vs. 1220 of 1495 (82%, 95% CI: 80–84%) patients with baseline eGFR ≤45 ml/min/1.73 m2 vs. >45 ml/ min/1.73 m2 respectively (P = 0.81). By baseline eGFR group, SVR12 was similar regardless of treatment regimen used (Fig. 2) or cirrhosis status (Figure S3).
Sustained virologic response 12 was achieved in 15 of 17 (88%, 95% CI: 64–99%) and in 38 of 47 (81%, 95% CI: 64–99%) patients with baseline eGFR ≤30 ml/min/ 1.73 m2 and eGFR 31–45 ml/min/1.73 m2 respectively (P = 0.71). All five patients on HD at baseline achieved SVR12. SVR12 rates did not differ statistically among patients with eGFR ≤30 ml/min/1.73 m2 vs. eGFR 31–45 ml/min/1.73 m2, regardless of treatment regimen and cirrhosis status. SVR12 rates did not differ statistically among patients with eGFR ≤30 ml/min/1.73 m2 (88%, 95% CI: 64–99%) vs. eGFR >45 ml/min/1.73 m2 (82%, 95% CI: 80–84%) nor between patients with eGFR 31–45 (81%, 95% CI: 64–99%) ml/min/1.73 m2 vs. eGFR >45 ml/min/1.73 m2 (82%, 95% CI: 80–84%).
In univariate Poisson regression of SVR12, baseline eGFR ≤45 ml/min/1.73 m2 was not associated with SVR12 (IRR: 0.95, 95% CI: 0.81–1.12, P = 0.56) while genotype 1 (vs. genotype 2–6, other) and use of RBV-free regimen were associated with achieving SVR12 (Table 2). Male sex (vs. female), HCV treatment naïve (vs. prior HCV treatment), cirrhosis (vs. no cirrhosis) were negatively associated with SVR12 (Table 2). In multivariate regression analysis, baseline eGFR ≤45 ml/ min/1.73 m2 remained non-significant as predictor of SVR12 (IRR: 0.95, 95% CI: 0.80–1.12, P = 0.52) (Table 2).
Table 2.
Association between baseline variables and SVR12
| Baseline characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| IRR (95% CI) | P-value | IRR (95% CI) | P-value | |
| eGFR ≤45 (vs. eGFR >45) | 0.95(0.81–1.12) | 0.56 | 0.95(0.80–1.12) | 0.52 |
| Male (vs. female) | 0.90(0.85–0.96 | 0.001 | 0.92 (0.87–0.98) | 0.01 |
| Black or AA race (vs. non-Black or non-AA) | 0.92(0.83–1.02) | 0.13 | 0.89 (0.80–0.98) | 0.02 |
| Genotype 1 (vs. non-1) | 1.08(1.01–1.17) | 0.03 | 1.02 (0.94–1.12) | 0.60 |
| Prior HCV treatment (vs. treatment naïve) | 0.93(0.87–0.99) | 0.01 | 0.93 (0.88–0.99) | 0.02 |
| Cirrhosis (vs. no cirrhosis) | 0.87(0.82–0.93) | <0.001 | 0.86 (0.80–0.91) | <0.001 |
| Any transplant* (vs. non-transplant) | 0.95(0.86–1.05) | 0.33 | 0.95 (0.86–1.06) | 0.36 |
| RBV-free regimenf† (vs. RBV-containing regimens) | 1.20(1.13–1.27) | <0.001 | 1.22 (1.13–1.31) | <0.001 |
Includes liver alone transplant, kidney alone transplant and simultaneous liver–kidney transplant.
Includes patients treated with SOF/SMV vs. those treated with SOF/PEG/RBV, SOF/RBV, SOF/SMV/RBV.
AA, African American; HCV, hepatitis C virus; IRR, incident rate ratio; RBV, ribavirin.
Bold text signifies results for primary predictor and for statistically significant covariates in multivariate model.
Safety outcomes
Of the 1789 patients included in the safety analysis, 79 (4%) discontinued treatment early, approximately half (46/79) doing so as a result of an adverse event (Table 3). Frequency of early treatment discontinuation (complete treatment discontinuation, not discontinuation of just a component of treatment like RBV) was similar between patients with baseline eGFR ≤45 ml/min/1.73 m2 and patients with baseline eGFR >45 ml/min/1.73 m2 (Table 3). Reasons for early treatment discontinuations are provided in Table S2.
Table 3.
Safety outcomes by baseline renal function
| Safety outcome | All patients, N = 1789 | eGFR ≤45, N = 73 | eGFR >45, N = 1716 | P-value |
|---|---|---|---|---|
| Early treatment discontinuation, n (%) | 79(4) | 5 (7) | 74 (4) | 0.25 |
| Early treatment discontinuation because of AE, n (%) | 46 (3) | 3 (4) | 43 (3) | 0.43 |
| Common AEs, n (%) | ||||
| Fatigue | 621 (35) | 22 (30) | 599 (35) | 0.40 |
| Headache | 303 (17) | 10(14) | 293 (17) | 0.45 |
| Nausea | 291 (16) | 11 (15) | 280(16) | 0.78 |
| Anaemia AE, n (%) | 295 (16) | 22 (30) | 273 (16) | 0.001 |
| Required transfusion(s) | 41 (2) | 7(10) | 34 (2) | 0.001 |
| Erythropoietin stimulating drugs | 73 (4) | 9(12) | 64 (4) | 0.002 |
| started on treatment, n (%) | ||||
| Reduction in RBV due to anaemia*, n/N (%) | 229/1071 (21) | 11/34 (32) | 218/1071 (21) | 0.11 |
| RBV discontinuation*, n/N(%) | 17/1071 (2) | 4/34 (12) | 13/1037(1) | 0.002 |
| Worsening renal function †n(%) | 29 (2) | 11 (15) | 18(1) | <0.001 |
| Any serious AEs, n (%) | 124 (7) | 16 (22) | 108(6) | <0.001 |
| Cardiac serious AEs, n (%) | 64 (4) | 3(4) | 61 (4) | 0.74 |
| Death, n (%) | 13(1) | 1 (1)‡ | 12(1) | 0.42 |
Among patients treated with RBV.
Outcome abstracted from HCV TARGET database as reported by investigators; includes test terms of acute kidney failure, acute kidney injury, renal failure acute, renal insufficiency, renal failure, azotemia, azotaemia, acute renal failure, acute renal failure, anuric renal failure and impaired renal function.
eGFR ≤45 patient that died: Liver transplant recipient with baseline MELD of 26 who died from worsening renal failure and hepatic decompensation.
AE, adverse event; HCV, hepatitis C virus; RBV, ribavirin.
Bold text signifies statistically significant differences.
Patients with baseline eGFR ≤45 ml/min/1.73 m2 more frequently experienced anaemia-related adverse events, required transfusion(s), started erythropoietin stimulating drugs on treatment and required RBV discontinuation compared to patients with baseline eGFR >45 ml/min/1.73 m2 (Table 3). Further, patients with baseline eGFR ≤45 ml/min/1.73 m2 more frequently reported an AE related to worsening renal function and had more SAEs (Table 3). The frequency of safety outcomes was similar between patients with baseline eGFR ≤30 ml/min/1.73 m2 vs. eGFR 31–45 ml/min/1.73 m2 (Table S3). The on-treatment trend of eGFR among patients with baseline eGFR ≤45 ml/min/1.73 m2 who experienced worsening renal function is shown in Fig. 3. Of the patients with worsening renal function in the eGFR ≤45 ml/min/1.73 m2 group, 91% still went on to achieve SVR. Twenty percent of patients who had worsening renal failure were in the eGFR ≤30 ml/min/1.73 m2 group and 100% of these patients achieved SVR.
Fig. 3.
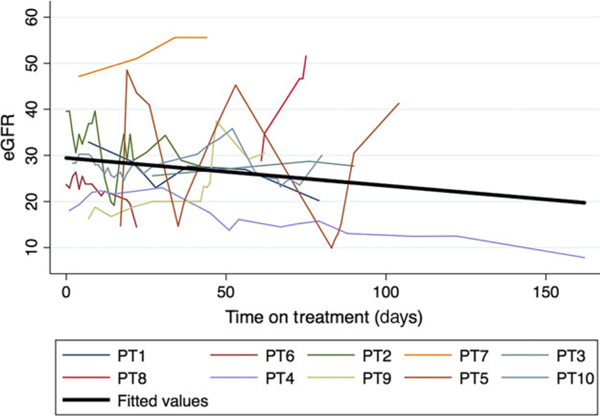
On treatment eGFR trend among patients with baseline eGFR ≤45 who experienced worsening renal function. One patient with baseline eGFR <45 and worsening renal failure was excluded from this figure because of lack of longitudinal eGFR submitted.
In a subgroup analysis focusing on patients treated with the RBV-free regimen SOF/SMV, patients with eGFR ≤45 ml/min/1.73 m2 (vs. eGFR >45 ml/min/ 1.73 m2) more frequently experienced anaemia AEs, required transfusion(s), experienced worsening renal function and experienced SAEs (Table 4). There was no impact of RBV use as a predictor of safety outcomes in genotype 1-infected patients treated with SOF/SMV with or without RBV. Further, in a subgroup analysis of patients who did not receive the protease-inhibitor SMV, patients with baseline eGFR ≤45 ml/min/1.73 m2 again more frequently experienced anaemia AEs, required transfusion(s), experienced worsening renal function and experienced SAEs (Table S4).
Table 4.
Safety outcomes by baseline renal function in RBV-free regimen (SOF/SMV)
| Safety outcome | All patients, N = 718 | eGFR ≤45, N = 39 | eGFR >45, N = 679 | P-value |
|---|---|---|---|---|
| Early treatment discontinuation, n (%) | 37(5) | 3 (8) | 34(5) | 0.45 |
| Early treatment discontinuation because of AE, n (%) Common AEs, n (%) | 21 (3) | 3 (8) | 18 (3) | 0.10 |
| Fatigue | 76(25) | 9 (23) | 167(25) | 1.00 |
| Headache | 112(16) | 4(11) | 108(16) | 0.50 |
| Nausea | 92(13) | 5(13) | 87(13) | 1.00 |
| Anaemia AE, n (%) | 121 (17) | 12(31) | 109 (16) | 0.03 |
| Required transfusion(s) | 9 (1) | 3 (8) | 6 (1) | 0.01 |
| Erythropoietin stimulating drug started on treatment, n (%) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Worsening renal function*, n (%) | 13(2) | 4(10) | 9 (1) | 0.004 |
| Any serious AEs, n (%) | 41 (6) | 7(18) | 34(5) | 0.005 |
| Cardiac serious AEs, n (%) | 2 (<0.5) | 0 (0) | 2 (<0.5) | 0.73 |
| Death, n (%) | 6 (1) | 1 (3)† | 5 (1) | 0.28 |
Outcome abstracted from HCV TARGET database as reported by investigators; includes test terms of acute kidney failure, acute kidney injury, renal insufficiency, renal failure, azotemia, acute renal failure, acute renal failure, anuric renal failure and impaired renal function.
The patient with eGFR ≤45 who died was a liver transplant recipient with baseline MELD of 26 who died from worsening renal failure and hepatic decompensation.
Bold text signifies statistically significant differences.
AE, adverse event; HCV, hepatitis C virus; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir.
In univariate Poisson regression, baseline eGFR ≤45 ml/min/1.73 m2 was associated with both worsening renal function and any SAE (Table 5). In multivariate regression analysis, baseline eGFR ≤45 ml/min/1.73 m2 remained a significant predictor of worsening renal function (IRR: 4.71, 95% CI: 1.85–12.0, P = 0.001) but not of any SAE (IRR: 1.59, 95% CI: 0.93–2.73, P = 0.09) (Table 5).
Table 5.
Predictors of safety outcomes
| Baseline characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| IRR (95% CI) | P-value | IRR (95% CI) | P-value | |
| Safety outcome: worsening renal function* | ||||
| eGFR ≤45 (vs. eGFR >45) | 13.6(6.5–28.7) | <0.001 | 4.71 (1.85–12.0) | 0.001 |
| Male (vs. female) | 0.83 (0.38–1.78) | 0.63 | 1.47 (0.68–3.17) | 0.33 |
| Age≥65 (vs. age<65) | 1.18(0.45–3.09) | 0.73 | 1.21 (0.41–3.57) | 0.73 |
| Black or AA race (vs. non-Black or non-AA) | 0.90 (0.27–2.95) | 0.86 | 0.78(0.20–3.14) | 0.73 |
| Genotype 1 (vs. non-1) | 0.67 (0.31–1.45) | 0.31 | 0.45(0.20–1.02) | 0.06 |
| Prior HCV treatment (vs. treatment naive) | 0.52 (0.24–1.13) | 0.10 | 0.50(0.23–1.07) | 0.07 |
| Cirrhosis (vs. no cirrhosis) | 3.23 (1.48–2.55) | 0.01 | 1.98(0.56–6.95) | 0.29 |
| MELD ≥ 10 (vs. MELD < 10) | 0.46 (0.22–0.98) | 0.05 | 1.11 (0.45–2.77) | 0.82 |
| Any transplant† (vs. no transplant) | 1.70(0.35–8.31) | 0.52 | 1.28(0.53–3.10) | 0.58 |
| Diabetes (vs. no diabetes) | 4.14(1.95–8.77) | <0.001 | 2.07(0.87–4.90) | 0.10 |
| Haemoglobin (per g/dL) | 0.52 (0.44–0.62) | <0.001 | 0.61 (0.50–0.75) | <0.001 |
| Safety outcome: any serious adverse event | ||||
| eGFR ≤ 45 (vs. eGFR > 45) | 3.43 (2.14–5.50) | <0.001 | 1.59(0.93–2.73) | 0.09 |
| Male sex (vs. female) | 0.76(0.54–1.08) | 0.12 | 1.04 (0.74–1.46) | 0.83 |
| Age ≥ 65 (vs. age < 65) | 0.99 (0.62–1.59) | 0.96 | 0.96(0.62–1.50) | 0.86 |
| Black or AA race (vs. non-Black or non-AA) | 0.84 (0.41–1.47) | 0.54 | 0.89(0.51–1.54) | 0.67 |
| Genotype 1 (vs. non-genotype 1) | 0.86 (0.59–1.23) | 0.40 | 0.69 (0.48–0.99) | 0.05 |
| Prior HCV treatment (vs. treatment naïve) | 0.86 (0.61–1.20) | 0.37 | 0.80(0.57–1.12) | 0.19 |
| Cirrhosis (vs. no cirrhosis) | 3.17 (2.10–4.77) | <0.001 | 1.94 (1.13–3.36) | 0.02 |
| MELD ≥ 10 (vs. MELD < 10) | 0.43 (0.31–0.61) | <0.001 | 0.74(0.48–1.14) | 0.17 |
| Any transplant† (vs. no transplant) | 2.10(1.11–4.01) | 0.02 | 0.95(0.62–1.46) | 0.81 |
| Diabetes (vs. no diabetes) | 1.46(1.02–2.11) | 0.04 | 1.02(0.71–1.46) | 0.93 |
| Haemoglobin (per g/dL) | 0.65 (0.60–0.70) | <0.001 | 0.69 (0.64–0.76) | <0.001 |
Genotype was excluded as would not converge, not enough variation to examine this predictor.
Includes liver alone transplant, kidney alone transplant and simultaneous liver–kidney transplant.
AA, African American; HCV, hepatitis C virus; RBV, ribaviri.
Bold text signifies results for primary predictor and for statistically significant covariates in multivariate model.
Discussion
Drawing from a large, multinational experience of SOF-containing regimens in patients with chronic hepatitis C, we evaluated the association between baseline renal dysfunction and key treatment and safety outcomes. We found that SVR12 rates did not vary significantly by baseline renal dysfunction but reported safety outcomes of worsening renal function and SAEs were at least 3.5 times more frequent in patients with baseline eGFR ≤45 ml/min/1.73 m2 vs. >45 ml/min/1.73 m2. Patients with baseline eGFR ≤30 and eGFR 31–45 had similar frequencies of safety outcomes. Overall, these results show that SOF-containing treatments remain highly efficacious among patients with renal dysfunction but that their use in these patients necessitates careful monitoring for and aggressive management of AEs during treatment.
New or worsening renal impairment has not been reported as a safety signal in clinical trials with SOF-containing therapies.(1, 8, 9) This may be because of the selected patient population in clinical trials with almost universal exclusion of patients with significant baseline renal dysfunction. In the published literature, heterogenous results are reported. In a study of six patients with baseline eGFR ≤30 ml/min/1.73 m2 undergoing SOF-containing treatment with full-dose SOF (400 mg daily), one patient (17%) experienced worsening renal function that was deemed unrelated to treatment (10). In a study examining full-dose SOF in 10 patients with baseline eGFR ≤30 ml/min/1.73 m2, one patient (10%) required haemodialysis initiation (11). In contrast, among a total of 16 patients with end-stage renal disease treated with half-dose SOF plus simeprevir, no renal events were reported (12, 13). The underlying aetiology or pathophysiology for the reported worsening renal impairment in patients treated with SOF-containing therapies remains unclear. Indeed, in the absence of a contemporaneous control group of untreated HCV patients with baseline impaired renal function, worsening renal impairment cannot be directly attributed to HCV therapy.
Ribavirin-induced anaemia is a well described phenomenon (14) and perhaps the induced anaemia combined with the relatively lower baseline haemoglobin noted in patients with renal dysfunction contribute to the observed worsening renal impairment. Further, first-generation protease inhibitors have been shown to increase blood RBV concentrations possibly potentiating the anaemia effect (15, 16). However, subgroup analysis examining safety outcomes among patients not exposed to RBV or peg-IFN did not result in elimination of the worsening renal impairment noted in patients with reduced baseline renal function, suggesting that the observed safety signal is not related to either RBV or peg-IFN.
The natural history of cirrhosis includes development of portal hypertension, which eventually can result in renal impairment (17). Furthermore, decompensated cirrhosis with ascites often necessitates treatment with diuretic medications, which can independently worsening renal function. Multivariate analysis showed that eGFR ≤45 ml/min/1.73 m2 was an independent risk factor for worsening renal function, even when controlling for the presence of cirrhosis and MELD ≥ 10, suggesting that cirrhosis itself is less likely to be the main aetiology for the observed safety signal. However, the use of diuretics concomitant with complications of cirrhosis could account for the observed worsening renal function.
About half of the patients with baseline eGFR ≤45 ml/min/1.73 m2 treated with SOF-containing regimens were previous transplant recipients. Exposure to calcineurin inhibitors, as well as a high prevalence of metabolic risk factors such as diabetes mellitus in the post-transplant setting, contribute to post-transplant renal impairment and end-stage renal disease (18). This may explain why so many of the treated post-transplant patients had baseline eGFR ≤45 ml/min/1.73 m2. However, multivariate analysis showed that eGFR ≤45 ml/ min/1.73 m2 was an independent risk factor for worsening renal function, even after adjusting for transplant status.
The first-generation protease inhibitors, telaprevir and boceprevir, have been associated with renal impairment (19, 20). While renal impairment as a result of the second-generation protease inhibitor, SMV, has not been reported (21), it is possible that a protease inhibitor class effect that could explain the worsening renal impairment seen in this study. However, in a population of patients with normal baseline renal function (baseline mean eGFR of 87 ml/min/1.73 m2), there is evidence that SMV is not associated with decreased eGFR or renal events (22). Further, in subgroup analysis, we found that excluding patients exposed to SMV did not eliminate of the worsening renal impairment noted in patients with reduced baseline renal function, again suggesting that the observed safety signal is not related to SMV.
There are some limitations of our study. First, the definition of worsening renal function was not standardized. The outcome was abstracted from treatment documentation and therefore was not standardized. Second, we did not analyse safety results beyond the HCV treatment period. A strong argument for SOF directly causing the worsening renal function would have been bolstered if the worsening renal function were reversed with discontinuation of SOF. Thus, future longitudinal studies are critical to evaluate for reversibility. Third, because all patients were treated as standard of care based on local practice, differences among patient populations or among sites, including academic and community sites, may have affected our results. However, HCV-TARGET represents the largest prospective cohort of HCV-treated patients in the United States, and allows an indepth analysis of ‘real-life’ experience of HCV treatment.
In summary, we show that in HCV-infected patients treated with SOF-containing regimens, SVR rates are not significantly influenced by baseline renal dysfunction, but more renal safety events occur. Whether this is a direct SOF effect or not remains to be determined. Additional longitudinal studies, preferably with untreated controls, would help to clarify the risk groups. Regardless, given the frequent use of SOF in currently approved HCV combination therapies, our results highlight the need for clinicians to discuss potential risks and benefits of SOF-based regimens with patients with impaired renal function, and the need for close monitoring for renal safety events during treatment.
Supplementary Material
Key points.
Use of sofosbuvir (SOF) is not recommended if the estimated glomerular filtration rate (eGFR) ≤30 ml/min/1.73 m2, but real-world data are available from HCV-TARGET on use of SOF in those with low eGFR.
Sustained virologic responses (SVR) rates with SOF-containing regimens were 88% and 81% in patients with baseline eGFR ≤30 and eGFR 31–45 mL/min/1.73m2 respectively.
Patients with reduced baseline renal function more frequently experienced anaemia, worsening renal function and serious AEs on treatment with SOF- containing regimens.
Patients with renal impairment warrant close monitoring during treatment and should be treated by providers extensively experienced with SOF-containing regimens.
Acknowledgements
Financial support: HCV-TARGET is an investigator-initiated study jointly sponsored by The University of Florida, Gainesville, FL (PI: Nelson), and The University of North Carolina at Chapel Hill, Chapel Hill, NC (PI: Fried). It was funded in part by AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, and Vertex. Funded in part by CTSA UF UL1TR000064. Dr Fried was funded in part by NIH Mid-Career Mentoring Award K24 DK066144. The author V. S. was supported in part by an NIH-funded postdoctoral research training program grant (T32 DK060414). N. T. is UCSF Liver Center member (NIH P30 DK 026743).
Abbreviations
- AE
adverse event
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- HCV RNA
hepatitis C virus ribonucleic acid
- MELD
model of end-stage liver disease
- PEG
pegylated interferon
- RBV
ribavirin
- SAE
serious adverse event
- SMV
simeprevir
- SOF
sofosbuvir
- SVR
sustained virologic response
Footnotes
Conflict of interest: J. L.: BMS, Gilead, Janssen, Holo-gic, Merck; M. S.: Gilead; M. S.: Merck: R. C.: Gilead, Abbvie, Merck, BMS, Mass Biologics, Janssen; D. N.: AbbVie, Gilead, BMS, Janssen, Merck, GSK; M. F.: Genentech/Roche, Merck, Vertex, Janssen, Gilead, Bristol Myers Squibb, AbbVie, Glaxo; N. T.: Gilead, AbbVie, Merck, Eisai, Biotest.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1111/liv.13102/suppinfo
Trial Registration Number: NCT01474811.
References
- 1.Gilead Sciences. Sofosbuvir [package insert]. Foster City, CA: Gilead Sciences, 2015. [Google Scholar]
- 2.AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis C. Available at http://www.hcvguidelines.org. Accessed 5 March 2015.
- 3.Merck & Co., Inc. Elbasvir and Grazoprevir [package insert]. Whitehouse Station, NJ: Merck & Co., Inc, 2016. [Google Scholar]
- 4.Tsui JI, Vittinghoff E, Shlipak MG, et al. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med 2007; 167: 1271–6. [DOI] [PubMed] [Google Scholar]
- 5.Gordon SC, Muir AJ, Lim JK, et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J Hepatol 2015; 62: 286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–70. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J A Wilcoxon-type test for trend. Stat Med 1985; 4: 87–90. [DOI] [PubMed] [Google Scholar]
- 8.Sciences Gilead. Ledipasvir and Sofsobuvir [Package Insert]. Foster City, CA: Gilead Sciences, 2015. [Google Scholar]
- 9.Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015; 61: 1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hundemer GL, Sise ME, Wisocky J, et al. Use of sofosbu-vir-based directacting antiviral therapy for hepatitis C viral infection in patients with severe renal insufficiency. Infect Dis (Lond) 2015; 47: 924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin P, Gane EJ, Ortiz-Lasanta G, et al. Safety and Efficacy of Treatment with Daily Sofosbuvir 400mg + Ribavirin 200mg for 24 weeks in Genotype 1 or 3 HCV-Infected Patients with Severe Renal Impairment. Program and abstracts of the 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 13–17 2015; San Francisco, Abstract 1128. [Google Scholar]
- 12.Kalyan Ram B, Frank C, Adam P, et al. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol 2015; 63: 763–5. [DOI] [PubMed] [Google Scholar]
- 13.Perumpail RB, Wong RJ, Ha LD, et al. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis 2015; 17: 275–8. [DOI] [PubMed] [Google Scholar]
- 14.Canonico PG, Kastello MD, Spears CT, et al. Effects of ribavirin on red blood cells. Toxicol Appl Pharmacol 1984; 74: 155–62. [DOI] [PubMed] [Google Scholar]
- 15.Karino T, Ozeki I, Hige S, et al. Telaprevir impairs renal function and increases blood ribavirin concentration during telaprevir/pegylated interferon/ribavirin therapy for chronic hepatitis C. J Viral Hepat 2014; 21: 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menna P, Gallo P, Vespasiani Gentilucci U, et al. Telapre- vir raises the plasma/whole blood ribavirin ratio: trying to come full circle on a dangerous relationship. J Viral Hepat 2014; 21: e136–7. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008; 48: 2064–77. [DOI] [PubMed] [Google Scholar]
- 18.Trotter JF, Grafals M, Alsina AE. Early use of renal-sparing agents in liver transplantation: a closer look. Liver Transpl 2013; 19: 826–42. [DOI] [PubMed] [Google Scholar]
- 19.Hezode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS C020-CUPIC) - NCT01514890. J Hepatol 2013; 59: 434–41. [DOI] [PubMed] [Google Scholar]
- 20.Mauss S, Hueppe D, Alshuth U. Renal impairment is frequent in chronic hepatitis C patients under triple therapy with telaprevir or boceprevir. Hepatology 2014; 59: 46–8. [DOI] [PubMed] [Google Scholar]
- 21.Janssen Therapeutics. Simeprevir [package insert]. Titusville, NJ: Janssen Therapeutics, 2015. [Google Scholar]
- 22.Mauss S, Butí M, Moreno C, et al. Renal function in HCV genotype 1-infected treatment-naïve patients receiving simeprevir in combination with Peg-IFN and ribavirin: a post-hoc analysis. J Viral Hepatitis 2014; 21: 29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


