Abstract
Early liver transplant (LT) for alcohol-associated disease (i.e. without a specific sobriety period) is controversial, but increasingly used. Using the multicenter American Consortium of Early Liver Transplantation for Alcoholic Hepatitis (ACCELERATE-AH) cohort, we aimed to develop a predictive tool to identify patients pre-transplant with low-risk for sustained alcohol use post-transplant to inform selection of candidates for early LT candidates. We included consecutive ACCELERATE-AH LT recipients between 2012–2017. All had clinically-diagnosed severe alcoholic hepatitis (AH), no prior diagnosis of liver disease or AH, and underwent LT without a specific sobriety period. Logistic and cox regression, classification and regression trees (CART), and LASSO regression were used to identify variables associated with sustained alcohol use post-LT. Among 134 LT recipients for AH with median abstinence pre-LT of 54 days, 74% were abstinent, 16% had slips only, and 10% had sustained alcohol use after median 1.6 (IQR 0.7–2.8) years follow-up post-LT. Four variables were associated with sustained use of alcohol post-LT, forming the SALT (Sustained Alcohol use post-LT) score (range, 0–11): >10 drinks/day at initial hospitalization (+4 points), multiple prior rehabilitation attempts (+4 points), prior alcohol-related legal issues (+2 points), prior illicit substance abuse (+1 point). C-statistic was 0.76 (95% CI, 0.68–0.83). SALT of ≥5 had 25% PPV (95% CI, 10%−47%) and 95% NPV (95% CI, 89%−98%) for sustained alcohol use post-LT. In internal cross-validation, the average c-statistic was 0.74.
Conclusion:
A novel prognostic score, the SALT score, using four objective pre-transplant variables identifies AH candidates for early LT at low-risk for sustained alcohol use post-transplant. This tool may assist in the selection of patients with AH for early LT or to guide risk-based interventions post-LT.
Keywords: transplantation, 6-month rule, accelerate-ah, recidivism, relapse
Traditionally, liver transplant (LT) centers adhere to a policy of offering LT in alcohol-associated liver disease (AALD) only after a specific period of abstinence (typically 6 months).1,2 However, some have advocated for exceptions to the “6-month rule”, with recognition that some patients with AALD can present acutely with such severe disease, either from alcoholic hepatitis (AH) or acute-on-chronic liver failure (ACLF), that a 6-month waiting period equates to almost certain death.1,2 The publication of a Franco-Belgian pilot study of early LT (i.e. without a specific period of abstinence) in 26 highly selected patients with severe AH, which showed a significant survival benefit with low incidence of post-LT alcohol use, led to changes in attitudes towards early LT for AH.3 In France, the proportion of LT centers offering early LT for AALD has increased from 31% to 100% between 2011–2016.4 In the United States, we recently reported retrospective results of the American Consortium of Early Liver Transplantation for Alcoholic Hepatitis (ACCELERATE-AH) cohort, which includes 12 LT centers from 8 UNOS regions performing early LT for AALD, which showed that the number of LT programs performing LT for AH has been increasing since 2012.5
Despite increasing application, early LT for AALD remains controversial due to concern for return to harmful drinking post-LT. Indeed, 25% of ACCELERATE-AH patients had evidence of any alcohol use post-LT, and 10% had sustained alcohol use after a median 1.6 years post-LT.5 Most importantly, sustained alcohol use post-LT was the strongest predictor of post-LT mortality.5 To minimize graft and patient losses in those transplanted for AH, additional objective pre-LT tools are needed to identify those at high or low risk for sustained alcohol use post-LT, to assist clinicians in selection of early LT candidates.
While some scoring systems have been developed to predict alcohol relapse in alcohol use disorder and subsequently applied to the LT population (e.g. High Risk Alcohol Relapse Scale -- HRAR), none have originated and been developed specifically in the LT or early LT population.6,7 We hypothesize early LT candidates (e.g. young, strong social support, imminently life-threatening disease) represent a unique and select subpopulation of alcohol use disorder requiring dedicated study, where existing tools to assess risk of alcohol relapse require validation, and novel tools targeted to this specific population may improve outcomes. We previously proposed the concept of a scoring system to predict those at risk for alcohol use after early LT for AH with the Hopkins Psychosocial Scale (HPSS), but this lacked robust statistical methods due to limitations of small sample size from a single-center experience.8,9 This current study’s aim was to leverage the large, multi-center ACCELERATE-AH cohort to develop a prognostic score from pre-LT variables that was predictive of low-risk of sustained alcohol use post-LT with the goal of improving long-term outcomes through more refined patient selection and/or provision of post-transplant interventions to minimize risk of harmful alcohol use.
METHODS
Study Population
Twelve LT centers provided detailed retrospective data on consecutive patients transplanted with the indication of severe alcoholic hepatitis, as previously described.5 To summarize, all ACCELERATE-AH sites were asked about specific selection criteria the process of selection, and post-transplant care (Supplemental Table 1). Given the landmark Franco-Belgian study published in 2011,3 which strongly influenced practice across the United States in this indication for LT, only ACCELERATE-AH LT recipients from 2012 onwards were included in this analysis. Inclusion and exclusion criteria are thus similar to those used in this previously published European study.3 Specifically, inclusion criteria were age greater than 18 years, clinically-diagnosed severe acute AH (jaundice, prolonged INR, chronic and recent alcohol use) as indication for LT, no prior diagnosis of chronic liver disease or episodes of AH, and LT without a specific period of abstinence. To reflect real-life U.S. experience, liver biopsy was not required. Severity of the AH episode was based upon Maddrey’s discriminant function, with severe defined as a score of 32 or above. LT recipients had strong social support and did not have severe comorbid medical disorders. All patients were evaluated by a transplant social worker with detailed substance abuse evaluation prior to LT and, in all but one center, this evaluation is routinely supplemented by a separate evaluation by an addiction specialist. Exclusion criteria were other liver diseases (e.g. viral hepatitis), human immunodeficiency virus, and other contraindications to LT. Ten of 12 centers required full consensus agreement by the LT committee in the decision to list for early LT -- two centers did not utilize this as an inclusion criteria. No center strictly excluded patients with recent gastrointestinal bleeding or recent infection, and only two centers strictly excluded patients with history of co-morbid psychiatric disease, even if well-controlled.
Investigators from each center collected data regarding baseline characteristics and psychosocial profiles (e.g. illicit substance abuse history, family history of alcohol use disorder, history of alcohol-related legal issues, history of rehabilitation attempts) and quantification of pre-LT alcohol use from transplant social worker pre-LT evaluations, which were available in all patients. HRAR scores as previously described7 were calculated for all patients retrospectively.
Assessment of Alcohol Use Post-LT
LT recipients were questioned regarding any alcohol use and had documented responses at every post-LT visit. In addition to direct patient questioning, seven of 12 centers additionally monitored for alcohol use post-LT with routine urinary ethyl glucuronide or blood phosphatidylethanol testing and 3 additional centers selectively performed ethyl glucuronide or phosphatidylethanol testing at the discretion of the managing provider.10,11
There is no standard definition of alcohol relapse.12 Any alcohol use post-LT was captured with the average daily alcohol consumption based on patient report. To distinguish between a “slip” and sustained alcohol use, patients were assessed as to whether they were still drinking at most recent follow-up.12 Thus, alcohol use post-LT was categorized as none, alcohol use with recovered sobriety (“slip”), or sustained alcohol use. To distinguish between sustained alcohol use and a recent slip, sustained use was a minimum duration of 100 days.
Statistical Analysis
Demographic and clinical characteristics were described using means [SDs], medians [interquartile ranges (IQR)], and proportions as appropriate. Characteristics were compared by alcohol use post-LT using Wilcoxon rank sum and chi-square tests. The distribution of time from LT to first post-LT alcohol use was estimated using Kaplan-Meier plots. Abstinent patients were censored at last follow-up.
We first used unadjusted logistic models to identify factors associated with any or sustained post-LT alcohol use. We then used the least absolute shrinkage and selection operator (LASSO)13 to select multivariable models for each endpoint, beginning with factors with unadjusted p<0.1. Points for the SALT score were obtained by assigning 1 point to the weakest predictor retained by the LASSO, 4 points to the strongest, and then rounding the remaining coefficients appropriately in this interval. Exploratory analysis using cox proportional-hazard models was also performed.
Internal cross-validation of the overall LASSO model for sustained alcohol use post-LT was performed by randomly splitting the sample into ten groups. After leaving out each group in turn, the LASSO model was estimated using the other nine. Then if the LASSO model produced non-zero coefficients and the outcome varied in the omitted group, the predicted probability of sustained alcohol use post-LT was estimated for the omitted group, and the c-statistic calculated. Finally, the resulting c-statistics were averaged, and compared to the overall estimate.
Exploratory classification and regression trees (CART)14 were also examined. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC), Stata Version 15.0 (Stata Corp, College Station, TX), and R Version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria)
This study was approved by the Institutional Review Board at each participating center. University of California at San Francisco (UCSF) is the designated coordinating center for ACCELERATE-AH.
RESULTS
Cohort Characteristics
This study included 134 patients who underwent early LT for severe AH between January 2012 and March 2017 from 12 U.S. LT centers.5 Median post-LT follow-up was 1.6 years (IQR 0.7–2.8). Patients were 72% male, 82% Caucasian, 66% privately insured, with median pre-LT abstinence time of 54 days (IQR 36–88). At time of initial hospitalization, 65% were employed and 58% were married or with a stable companion. Baseline characteristics are summarized in Table 1. Complete records on pre-LT assessment of alcohol use disorder (AUD) by an addiction specialist or medical social worker were available in all patients. At initial hospitalization, median discriminant function was 78 (IQR 57–103) and MELD-Na was 34 (IQR 29–39). The median interval between time of last drink and admission date of the initial hospitalization for severe AH (i.e., diagnosis of AH) was 22.5 days (IQR 10–45). The range of this interval was 0–123 days, where 1 of 134 (0.7%) patients would not have met NIAAA criteria15 for the interval of alcohol intake prior to presentation with AH (i.e. onset of jaundice within prior 8 weeks, drinking within 60 days before the onset of jaundice). Median time between listing and LT was 6 days (IQR 3–11).
Table 1.
Patient Characteristics, n=134
| Characteristic | Value |
|---|---|
| Age – yr – median (IQR) | 42 (34–51) |
| Male, n (%) | 96 (72) |
| Race / Ethnicity, n (%) | |
| Caucasian | 110 (82) |
| African American | 8 (6) |
| Hispanic | 7 (5) |
| Asian | 5 (4) |
| Other | 4 (3) |
| Employed, n (%) | 87 (65) |
| Private Medical Insurance, n (%) | 97 (66) |
| Married / Stable Companion, n (%) | 78 (58) |
| History of Co-Morbid Psychiatric Disease, n (%) | 42 (31) |
| Substance Abuse History, n (%) | |
| Active Smoker | 23 (17) |
| Marijuana | 12 (9) |
| Non-Marijuana Illicit Substance | 16 (12) |
| History of Failed Rehabilitation Attempt, n (%) | |
| No Prior Attempt | 94 (70) |
| 1 Prior Attempt | 29 (22) |
| ≥2 Prior Attempts | 11 (8) |
| Family History of Alcohol Use Disordera, n (%) | |
| First Degree Relative | 31 (24) |
| Second Degree Relative Only | 17 (13) |
| History of Alcohol-Related Legal Issues, n (%) | |
| 1 Prior Episode | 27 (20) |
| ≥2 Prior Episodes | 13 (10) |
| Alcohol Consumption Immediately Prior to Hospitalizationb - units/day - median (IQR) | 9 (6–15) |
| Years of Heavy Drinkingc - median (IQR) | 15 (9–25) |
| Sodium*d- mg/dL - median (IQR) | 134 (129–137) |
| INR*e - median (IQR) | 2.0 (1.7–2.5) |
| Total Bilirubin* - mg/dL - median (IQR) | 26.2 (16.8–34.1) |
| Creatinine*f - mg/dL - median (IQR) | 1.80 (0.90–3.38) |
| MELD-Na Score* - median (IQR) | 34 (29–39) |
| Maddrey’s Score* -- median (IQR) | 78 (57–103) |
| Time between Last Drink and LT -- days -- median (IQR) | 54 (36–88) |
| Time between Listing and LT - days -- median (IQR) | 6 (3–11) |
Lab values at initial hospitalization
3 missing values
4 missing values
8 missing values
1 missing value
3 missing values
3 missing values
Post-Transplant Alcohol Use
Of 129 patients surviving to home discharge, 34 (26%) had evidence of any alcohol use post-LT (Table 2). 21 had slips only, whereas 13 had evidence of sustained alcohol use post-LT. Median time to first alcohol use post-LT was 154 days (IQR 74–320). Median time to first alcohol use post-LT was not significantly different amongst those with slips versus sustained alcohol use (150 vs. 201 days, p=0.74). Cumulative probability of any alcohol use post-LT at 1- and 3-years post-LT was 26% (95% CI, 19%−36%) and 33% (95%, 24%−44%) (Supplemental Figure 1). Cumulative probability of sustained alcohol use post-LT at 1- and 3-years post-LT was 10% (95% CI, 6%−18%) and 16% (95% CI, 9%−28%).
Table 2.
Factors Associated with Alcohol Use Post-LT in Unadjusted Logistic Regression
| Any Alcohol Use Post-LT (n=34) | Sustained Alcohol Use Post-LT (n=13) | |||
|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p |
| Sex, Male | 1.10 (0.46–2.67) | 0.83 | 1.32 (0.34–5.12) | 0.68 |
| Race, Caucasian | 0.61 (0.23–1.60) | 0.32 | 0.69 (0.18–2.75) | 0.60 |
| Age (per year) | 0.95 (0.91–0.99) | 0.02 | 0.98 (0.92–1.03) | 0.42 |
| Private Insurance | 1.07 (0.47–2.44) | 0.86 | 1.23 (0.38–4.02) | 0.73 |
| Married/Stable Companion | 0.52 (0.23–1.14) | 0.10 | 0.56 (0.18–1.78) | 0.33 |
| History of Co-Morbid Psychiatric Disease | 0.99 (0.43–2.28) | 0.98 | 0.59 (0.15–2.28) | 0.45 |
| History of Non-THC Illicit Substance Abuse | 1.31 (0.42–4.11) | 0.64 | 3.85 (1.03–14.4) | 0.04 |
| History of Failed | ||||
| Rehabilitation Attempt | ||||
| Any Prior Attempt | 1.30 (0.57–2.99) | 0.53 | 2.07 (0.65–6.60) | 0.22 |
| ≥2 Prior Attempts | 3.56 (0.73–9.00) | 0.14 | 6.92 (1.70–28.2) | 0.007 |
| Family History of Alcohol | 0.98 (0.43–2.22) | 0.95 | 1.63 (0.51–5.17) | 0.41 |
| Use Disorder | ||||
| Employed Immediately Prior to Hospitalization | 0.79 (0.36–1.74) | 0.56 | 0.98 (0.31–3.10) | 0.98 |
| History of Alcohol-Related | ||||
| Legal Issues | ||||
| Any Prior Episode | 2.07 (0.91–4.72) | 0.08 | 3.20 (1.00–10.2) | 0.05 |
| ≥2 Prior Episode |
3.18 (0.95–10.6) |
0.06 | 3.57 (0.83–15.3) | 0.09 |
| >10 Drinks/day at Presentation | 1.38 (0.61–3.12) | 0.44 | 5.37 (1.11–25.9) | 0.04 |
| Years of Heavy Drinking* | 0.97 (0.93–1.02) | 0.21 | 0.96 (0.90–1.03) | 0.29 |
| Days of Pre-Transplant Abstinence (per day) | 1.00 (0.99–1.01) | 0.89 | 1.00 (0.98–1.01) | 0.54 |
Duration of regular excessive alcohol use, defined as ≥20g of ethanol/day for women or ≥60g ethanol/day for men.
Of the 13 patients with sustained alcohol use post-LT, 10 had evidence of binge (≥6 drinks for men, ≥4 drinks for women) or frequent (≥4 days of drinking per week) drinking. 7 had binge drinking, 8 had frequent drinking. 5 had both binge and frequent drinking.
In univariate analysis, only younger age (OR 0.95, 95% CI 0.91–0.99, p=0.02) was associated with evidence of any alcohol use post-LT. History of non-THC illicit substance abuse (OR 3.85, 95% CI 1.03–14.4, p=0.04), history of multiple failed rehabilitation attempts (OR 6.92, 95% CI 1.70–28.2, p=0.007), and greater than 10 drinks per day at initial presentation (OR 5.37, 95% CI 1.11–25.9, p=0.04) were associated with evidence of sustained alcohol use post-LT. Full results from unadjusted logistic regression of any alcohol use post-LT and sustained alcohol use post-LT are summarized in Table 2.
The median HRAR score was 2, and 18/129 (14.0%) had a score of 4 or greater. The proportion of LT recipients with HRAR score less than 4 was statistically similar amongst all 12 ACCELERATE-AH sites (Fisher’s exact p=0.26), with median proportion 90% (IQR 73–100%). HRAR scores were not predictive of sustained alcohol use post-LT (OR 1.16, 95% CI 0.76–1.77, p=0.49), and yielded a c-statistic of 0.56 (95% CI, 0.39–0.72).
Classification and Regression Tree (CART) Analysis
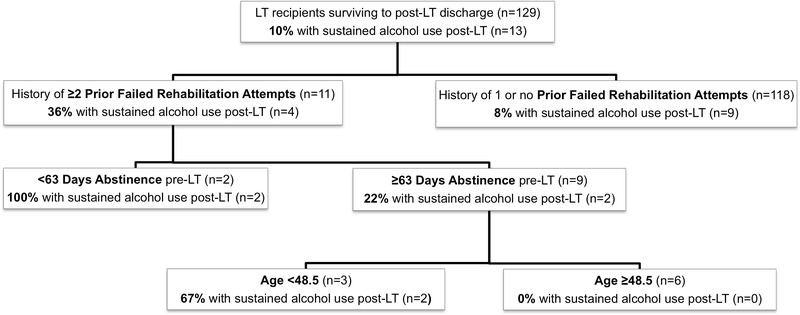
We performed a CART analysis for both any alcohol use post-LT and sustained alcohol use post-LT. The analysis for any alcohol use post-LT produced a tree that was too large to be clinically relevant (data not shown). CART analysis, however, was able to discriminate risk groups for sustained alcohol use post-LT. Those without history of multiple failed rehabilitation attempts were at low-risk (8%) of sustained alcohol use post-LT, whereas those with history of multiple failed rehabilitation attempts were at high risk (36%) (Figure 1). The CART tree discriminated further within the high-risk group by less than 63 days of abstinence pre-LT and less than 48.5 years of age, but the number of patients within these subgroups was small (Figure 1).
Figure 1. Classification and Regression Tree (CART) Analysis for Sustained Alcohol Use Post-LT.
CART analysis was able to discriminate those at high-risk for sustained alcohol use post-LT; those without history of multiple failed rehabilitation attempts were at low-risk (8%) of sustained alcohol use post-LT, whereas those with history of multiple failed rehabilitation attempts were at high risk (36%). The CART tree discriminated further within the high-risk group by less than 63 days of abstinence pre-LT and less than 48.5 years of age, but the number of patients within these subgroups was small.
LASSO Regression Models and SALT Score
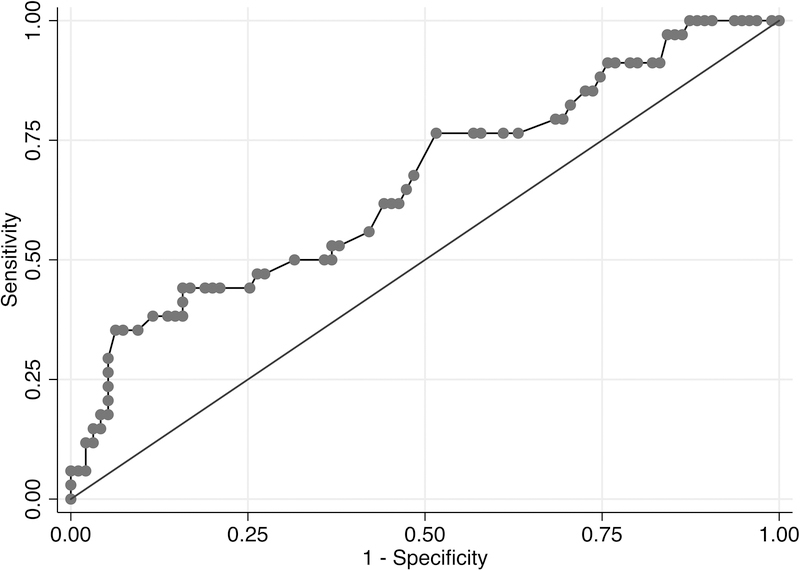
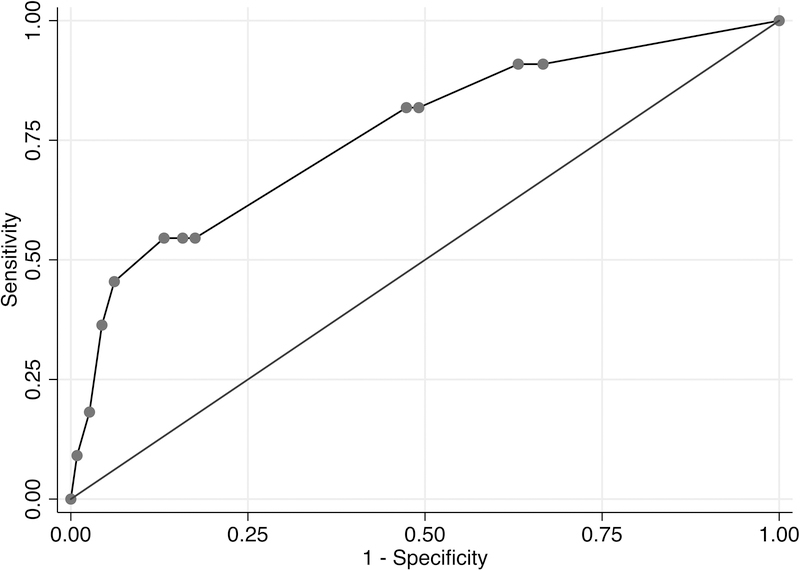
A LASSO logistic model for any alcohol use post-LT, which incorporated age, history of multiple alcohol-related legal issues, and history of any alcohol-related legal issues, had a c-statistic estimate of 0.66 (95% CI, 0.57–0.74) (Figure 2a). Given the relatively low c-statistic, a point-score assignment was not attempted to predict any alcohol use post-LT. For sustained alcohol use post-LT, a LASSO logistic model, including greater than 10 drinks per day at initial hospitalization, history of illicit substance abuse, history of any alcohol-related legal issues, and history of multiple rehabilitation attempts yielded a c-statistic estimate of 0.76 (95% CI, 0.68–0.83) (Figure 2b).
Figure 2a. AUROC Curve of Full LASSO Model for Any Alcohol Use Post-LT.
A LASSO logistic model for any alcohol use post-LT, which incorporated age, history of multiple alcohol-related legal issues, and history of any alcohol-related legal issues as an ordinal variable, had a c-statistic estimate of 0.66 (95% CI, 0.57–0.74).
Figure 2b. AUROC Curve of Full LASSO Model for Sustained Alcohol Use Post-LT.
A LASSO logistic model for sustained alcohol use post-LT, which incorporated greater than 10 drinks per day at initial hospitalization, history of illicit substance abuse, history of any alcohol-related legal issues, and history of multiple rehabilitation attempts had a c-statistic estimate of 0.76 (95% CI, 0.68–0.83).
Cut-points of 9 and 11 drinks per day were examined and found to be less useful as predictors. Using a cutoff of 9 drinks/day, only 2 patients were reclassified as exposed, and the C-statistic for the sustained alcohol use model declined slightly to 0.75 (95% CI, 0.67–0.83), with minimal effects on sensitivity and specificity at various cut-points. Using a cutoff of 11, 14 patients were reclassified as unexposed. This predictor was only very weakly associated with sustained relapse in unadjusted analysis (p=0.23) and was thus not useful as a predictor. This sensitivity analysis suggests that the cutoff of 10 units/day is robust.
Using point-scores from the shrunken LASSO coefficients for predictors of sustained alcohol use post-LT, we generated the SALT (Sustained Alcohol use post-LT) score (range, 0–11) (Table 3). Using a cutoff of ≥5, the SALT score had a positive predictive value of 25% (95% CI, 10%−47%), negative predictive value of 95% (95% CI, 89%−98%), and specificity of 84% (95% CI, 76%−90%) for sustained alcohol use post-LT (Table 4). Setting the cutoff at the maximum score of 11, the SALT score had a positive predictive value of 50% (95% CI, 1%−99%), negative predictive value of 92% (95% CI, 86%−96%), and specificity of 99% (95% CI, 95%−100%). 104 of 134 (78%) patients had SALT score of 5 or less.
Table 3.
SALT Score* to Predict Sustained Alcohol Use Post-LT
| Variable | Points |
|---|---|
| >10 Drinks/day at Presentation | +4 |
| ≥2 Prior Failed Rehabilitation Attempts | +4 |
| Any History of Prior Alcohol-Related Legal Issues | +2 |
| History of Non-THC Illicit Substance abuse | +1 |
The SALT Score was generated from a full LASSO logistic point-score model to predict sustained alcohol use post-LT. The score assigns points to variables which were associated with sustained alcohol use post-LT, and ranges 0–11. Using a cutoff of 5, the SALT score had a c-statistic estimate of 0.76 to predict sustained alcohol use post-LT.
Table 4.
Sensitivity, Specificity, Positive and Negative Predictive Values of SALT Score to Predict Sustained Alcohol Use Post-LT
| SALT Score Cutoff | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) |
|---|---|---|---|---|
| ≥5 | 55% (23–83%) | 84% (76–90%) | 25% (10–47%) | 95% (89–98%) |
| ≥6 | 55% (23–83%) | 87% (79–92%) | 29% (11–52%) | 95% (89–98%) |
| ≥7 | 45% (17–77%) | 94% (88–97%) | 42% (15–72%) | 95% (89–98%) |
| ≥8 | 27% (6–61%) | 96% (90–99%) | 38% (9–76%) | 93% (87–97%) |
| ≥9 | 18% (2–52%) | 97% (93–99%) | 40% (5–85%) | 93% (86–97%) |
| ≥10 | 18 (2–52%) | 97% (93–99%) | 40% (5–85%) | 93% (86–97%) |
| 11 | 9 (0–41%) | 99% (95–100%) | 50% (1–99%) | 92% (86–96%) |
In internal cross-validation of the LASSO model for sustained alcohol use post-LT, the average c-statistic was 0.74, only slightly smaller than the full-data estimate. A LASSO cox proportional-hazard model with the same four variables had a similar c-index estimate of 0.73. A LASSO logistic model using only three variables (greater than 10 drinks per day at initial hospitalization, history of any alcohol-related legal issues, and history of multiple rehabilitation attempts) had a similar c-statistic estimate of 0.76, but inferior PPV at the maximal score (38%).
DISCUSSION
Return to sustained drinking post-LT has important ethical and clinical implications for any LT patient with AALD, but may be especially true for those transplanted without a period of abstinence, as is the case in patients with AH. This is justifiably a major concern, as sustained alcohol use post-LT has been associated with a five-fold increased risk of overall death compared to abstinent LT recipients.5 In the early LT population, where acuity and severity of disease precludes the ability to mandate pre-LT treatment for alcohol use disorder, careful selection of candidates for LT is imperative. By leveraging the large sample size in our multi-center cohort, we were able to develop a novel point-score, the SALT score, to aid in identifying those at low-risk for sustained alcohol use after early LT with the indication of severe AH. The SALT score incorporates 4 variables (greater than 10 drinks per day at initial hospitalization, history of illicit substance abuse, history of any alcohol-related legal issues, and history of multiple rehabilitation attempts), which are routinely collected during pre-LT assessment, and most importantly are objective, and less prone to bias. The SALT score can easily be applied by any transplant provider as a tool to help guide their current selection process for early LT candidacy.
The LASSO13 regression method used to develop the SALT score controls variable selection and coefficient shrinkage via cross-validation, which on average prevents overfitting and improves prediction in new samples, and is better suited than other regression methods when the number of events is low, such as this study. In contrast to some competitive methods, including neural nets, the LASSO method tends to reduce the number of retained predictors, enhancing clinical utility.13 It also produces coefficients, allowing for the development of a clinically interpretable scoring system, which can easily be understood and applied by clinicians.13 The SALT score’s c-index of 0.76 was superior to the HRAR’s c-index of 0.56. A SALT score of 5 or greater, which has a positive predictive value of 25% and negative predictive value of 95%, and a positive predictive value up to 50% and negative predictive value of 92% at the maximal score of 11, can be used by transplant providers to identify LT candidates at low risk for sustained alcohol use. However, given the low PPV of the SALT score, a high SALT score requires further evaluation and should not necessarily preclude early LT -- other patient, family and center-specific factors should be considered in making the decision for LT among patients with high SALT scores, or perhaps such patients should be targeted for more intensive post-LT medical management of alcohol use disorder.
There is no standard definition for alcohol relapse in the transplant literature.12,16 A prior prospective study17 described five separate “trajectories” of alcohol use post-LT for AALD, where all deaths from recurrent AALD were LT recipients with sustained moderate or heavy alcohol use post-LT. While our retrospective study design does not allow for the granularity to discriminate these five trajectories in our cohort, our data supports the concept of using pre-LT variables to predict different risk groups for alcohol use post-LT and this information may help both in selection of candidates and for design of targeted interventions to prevent harmful alcohol use post-LT. Specifically, while there were some similarities in patients with any alcohol use and sustained alcohol use post-LT (e.g. history of alcohol-related legal issues), there were some characteristics specific to low versus non-low risks of sustained alcohol use, namely >10 drinks per day at presentation and history of non-THC illicit substance abuse. Indeed, in the addiction literature, alcohol use disorder has been found to stratify into 2 groups: Type I alcoholism versus Type II alcoholism, with Type II defined as addiction to two or more substances, not including nicotine.18,19 These two types of alcoholism have been found to have different CNS-biochemical and physical phenotypes, natural history of disease, and responses to treatment.18 For example, Type II alcoholism compared to Type I, has onset of disease at a younger age, includes a higher proportion of men, is more likely to have alcohol-related arrests, less likely to achieve natural remission, and less likely to respond to rehabilitation therapies.18,19 These features of Type II alcoholism seem to encompass the many of the significant risk factors for return to drinking post-LT in our cohort, and highlight the importance of a careful psychosocial evaluation pre-LT by an addiction specialist.
Both the LASSO and CART analyses performed relatively poorly in predicting any alcohol use post-LT. Slips are generally not thought to have the same clinical consequences post-LT as sustained heavy use.12,17,20,21 We previously reported that the majority of patients with any alcohol use post-LT had “harmful” patterns of binge or frequent drinking,5 and our retrospective design lacks the granularity to discriminate between rare versus frequent slips. Whether slips are causal to poor outcomes in the post-LT AH population should be studied further with prospective, longitudinal data. For the time being, we maintain that monitoring for any alcohol use post-LT is important in patients with a history of AALD, and intervened upon aggressively when identified.
SALT scores <5 had a very high negative predictive value as well as substantial specificity for sustained alcohol use post-LT, indicating that it is best used to identify those at lowest risk for this event. The lower and less precisely estimate positive predictive value and sensitivity of SALT scores ≥5 both reflect the rarity of sustained alcohol use post-LT, even in a high-risk population such as AH without a period of pre-LT sobriety, which makes prediction models challenging to develop. Albeit a rare event, the consequences of sustained drinking post-LT are significant both for patient outcomes and the morale of transplant providers.5,8 Thus, even as we gain a better understanding of risk factors for harmful drinking patterns post-LT and refinement of pre-LT selection process, there is concomitant need for improved post-LT management of alcohol use disorder to prevent poor outcomes. Poor outcomes related to post-LT alcohol use should be considered avoidable in any transplant recipient, and prospective studies need to address how best to monitor for alcohol use post-LT, and which interventions are successful to prevent and treat harmful post-LT drinking patterns.
There are some limitations to our study. First, the study is retrospective and methods of monitoring for alcohol use post-LT were not harmonized. While all centers queried every LT recipient for alcohol use post-LT at each visit, not all used biochemical screening for alcohol use post-LT. There is need for prospective studies with a uniform protocol to monitor alcohol use post-LT to build upon our findings. Second, we were unable to perform external validation of our SALT score and our model is thus at risk for over-optimism. Due to the rarity of outcome (sustained alcohol use post-LT) and lack of other large cohorts in early LT for AH, external validation is not feasible at this time, but should be pursued as the number of LTs for this indication increases and sufficient follow-up has been achieved. A recent meta-analysis evaluating LT for AH,22 and a recent UNOS analysis,23 would imply that ACCELERATE-AH encompasses the vast majority of early LT for AH performed in the United States, and an ongoing clinical trial limits access to data from the Franco-Belgian cohort. However, we were able to perform internal cross-validation amongst 10 random groups, which did provide a similar c-statistic to the regular estimate (0.74 vs. 0.76), suggesting that the SALT score may be robust. Third, this score was developed in a select population of early LT for clinically-diagnosed severe AH as first liver decompensation – whether this can be applied to different populations of AALD, including ACLF with multiple decompensations, remains unknown. Remarkably, our CART analysis for sustained alcohol use post-LT bears striking resemblance to a recently published CART prediction tree for alcohol relapse post-hospitalization in a non-LT population of AH from Spain,24 including risk stratification by history of failed rehabilitation attempts, and age <48 (the exact age dichotomy of our CART analysis), which suggests the importance of these psychosocial characteristics, and potential generalizability of our findings, which should be confirmed in larger prospective cohorts.
In conclusion, we developed the SALT score, which is a novel, simple point-score using four objective pre-transplant variables that may assist in identifying patients at low-risk of sustained alcohol use after early LT for AH. Stratification of potential LT candidates into high and low risk groups may help with candidate selection and post-LT interventions to prevent sustained alcohol use.
Supplementary Material
Acknowledgments
Financial Support
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases: UCSF Liver Center P30 DK026743 and T32 DK060414 [Dr. Lee].
List of Abbreviations
- AH
alcoholic hepatitis
- ALC
alcoholic cirrhosis
- AALD
alcohol-associated liver disease
- LT
liver transplantation
- MELD
Model of End-Stage Liver Disease
Contributor Information
DR. Brian P. Lee, Department of Gastroenterology, University of California, San Francisco, San Francisco, CA, USA.
Eric Vittinghoff, Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA, USA.
Christine Hsu, Department of Gastroenterology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Hyosun Han, Department of Gastroenterology, Keck School of Medicine of USC, Los Angeles, CA, USA.
DR. George Therapondos, Department of Gastroenterology, Ochsner Medical Center, Jefferson, LA, USA.
Oren K. Fix, Department of Gastroenterology, Swedish Medical Center, Seattle, WA, USA.
David W. Victor, Department of Gastroenterology, Houston Methodist Hospital, Houston, TX, USA.
Deepti Dronamraju, Department of Gastroenterology, University of Maryland School of Medicine, Baltimore, MD, USA.
DR. Gene Y. Im, Department of Liver Diseases, Icahn School of Medicine at Mount Sinai, New York City, NY, USA.
Michael D. Voigt, Department of Gastroenterology, University of Iowa Carver College of Medicine, Iowa City, IA, USA.
John P. Rice, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Michael R. Lucey, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Sheila Eswaran, Department of Gastroenterology, Rush Medical College, Chicago, IL, USA.
Po-Hung Chen, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Zhiping Li, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Haripriya Maddur, Department of Gastroenterology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Norah A. Terrault, Department of Gastroenterology, University of California, San Francisco, San Francisco, CA, USA.
References
- 1.Donckier V, Lucidi V, Gustot T, Moreno C. Ethical considerations regarding early liver transplantation in patients with severe alcoholic hepatitis not responding to medical therapy. J Hepatol 2014;60(4):866–71. [DOI] [PubMed] [Google Scholar]
- 2.Lucey MR. Liver transplantation for severe alcoholic hepatitis – The PRO view. Liver Int 2017;(October 2016):343–4. [DOI] [PubMed] [Google Scholar]
- 3.Mathurin P, Moreno C, Samuel D, et al. Early Liver Transplantation for Severe Alcoholic Hepatitis. N Engl J Med 2011;(365):1790–800. [DOI] [PubMed] [Google Scholar]
- 4.Mathurin P, Lucey MR. Alcohol, liver disease, and transplantation. Curr Opin Organ Transplant 2018;1. [DOI] [PubMed] [Google Scholar]
- 5.Lee BP, Mehta N, Platt L, et al. Outcomes of Early Liver Transplantation for Patients with Severe Alcoholic Hepatitis. Gastroenterology 2018;155(2):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates WR, Booth BM, Reed DA, Brown K, Masterson BJ. Descriptive and predictive validity of a high-risk alcoholism relapse model. J Stud Alcohol 1993;54(6):645–51. [DOI] [PubMed] [Google Scholar]
- 7.De Gottardi A A simple score for predicting alcohol relapse after liver transplantation. Arch Intern Med 2007;167:1183–8. [DOI] [PubMed] [Google Scholar]
- 8.Lee BP, Chen P-H, Haugen C, et al. Three-year Results of a Pilot Program in Early Liver Transplantation for Severe Alcoholic Hepatitis. Ann Surg 2017;265(1):20–9. [DOI] [PubMed] [Google Scholar]
- 9.Weeks SR, Sun Z, McCaul ME, et al. Liver Transplantation for Severe Alcoholic Hepatitis, Updated Lessons from the World’s Largest Series. American College of Surgeons; 2018. [DOI] [PubMed] [Google Scholar]
- 10.Piano S, Marchioro L, Gola E, et al. Assessment of Alcohol Consumption in Liver Transplant Candidates and Recipients: The Best Combination of the Tools Available. Liver Transplant 2014;20:815–22. [DOI] [PubMed] [Google Scholar]
- 11.Staufer K, Andresen H, Vettorazzi E, Tobias N, Nashan B, Sterneck M. Urinary ethyl glucuronide as a novel screening tool in patients pre- and post-liver transplantation improves detection of alcohol consumption. Hepatology 2011;54(5):1640–9. [DOI] [PubMed] [Google Scholar]
- 12.Lucey MR. Liver transplantation for alcoholic liver disease. Nat Rev Gastroenterol Hepatol 2014;11(5):300–7. [DOI] [PubMed] [Google Scholar]
- 13.Tibshirani R Regression Shrinkage and Selection via the Lasso. J R Stat Soc B 1996;58(1):267–88. [Google Scholar]
- 14.Breiman L, Friedman J, J.Stone C, Olshen RA. Classification Algorithms and Regression Trees. Wadsworth Stat 1984;246–80. [Google Scholar]
- 15.Crabb DW, Bataller R, Chalasani NP, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients with Alcoholic Hepatitis: Recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150(4):785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ursic-bedoya J, Faure S, Donnadieu-rigole H, Pageaux G. Liver transplantation for alcoholic liver disease : lessons learned and unresolved issues. World J Gastroenterol 2015;21(39):10994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimartini A, Dew MA, Day N, et al. Trajectories of alcohol consumption following liver transplantation. Am J Transplant 2010;10(10):2305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloninger RC, Sigvardsson S, Bohman M. Type I and type II alcoholism: An Update. Alcohol Health Res World 1996;20(18–23):833. [PMC free article] [PubMed] [Google Scholar]
- 19.Beresford TP, Wongngamnit N, Temple B. Alcoholism: diagnosis and natural history in the context of medical disease. In: Alcohol Abuse and Liver Disease. 2015. p. 23–34. [Google Scholar]
- 20.Dumortier J, Dharancy S, Cannesson A, et al. Recurrent Alcoholic Cirrhosis In Severe Alcoholic Relapse After Liver Transplantation : A Frequent and Serious Complication. Am J Gastro 2015;110(May):1160–6. [DOI] [PubMed] [Google Scholar]
- 21.Rice JP, Eickhoff J, Agni R, Ghufran A, Brahmbhatt R, Lucey MR. Abusive Drinking After Liver Transplantation Is Associated With Allograft Loss and Advanced Allograft Fibrosis. Liver Transplant 2013;19:1377–86. [DOI] [PubMed] [Google Scholar]
- 22.Marot A, Dubois M, Trépo E, Moreno C, Deltenre P. Liver transplantation for alcoholic hepatitis: A systematic review with meta-analysis. PLoS One 2018;13(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholankeril G, Liu A, Sandhu J, et al. Increasing Acceptance of Severe Acute Alcoholic Hepatitis as an Indication for Liver Transplantation with Outcomes comparable to Fulminant Hepatic Failure. Hepatology 2017;66(1):17A. [Google Scholar]
- 24.Altamirano J, López-Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology 2017;66(6):1842–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.