Introduction
Sarcoidosis is a chronic multisystem inflammatory disorder characterized by the formation of immune granulomas that may lead to serious, often irreversible, disability and significant disease-associated mortality.1 Current understanding suggests that sarcoidosis represents a helper T cell 1–mediated granulomatous immune response to an unidentified antigen, but the exact pathogenesis remains unknown.1 Polycythemia vera (PV) is an acquired myeloproliferative neoplasm characterized by mutant Janus kinase 2 (JAK2) signaling leading to erythrocyte overproduction.2 Hematologic abnormalities such as anemia and lymphopenia have been reported in association with sarcoidosis, but sarcoidosis has rarely been reported with PV.3 Here we present a patient with PV and multiorgan sarcoidosis who experienced resolution of cutaneous sarcoidosis lesions after systemic JAK inhibitor therapy for PV.
Report of a case
An African American woman in her 60s with JAK2 mutation–positive PV presented with a 12-month history of nontender, nonpruritic subcutaneous nodules in the bilateral triceps. She denied cough, shortness of breath, ocular symptoms, joint pain, and palpitations. Her PV was managed with daily aspirin and phlebotomy as needed.
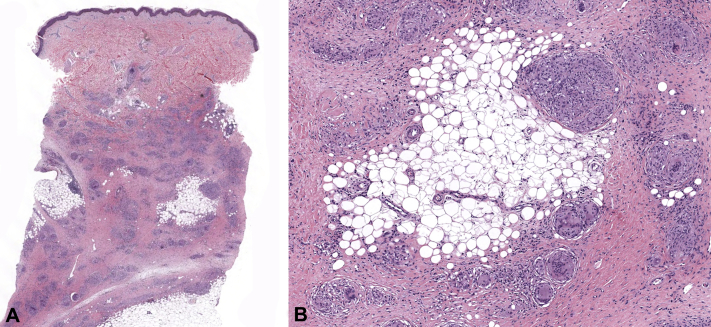
Physical examination found multiple subcutaneous nodules in the triceps bilaterally, and a punch biopsy found sarcoidal granulomatous dermatitis (Fig 1). Chest computed tomography showed extensive mediastinal and bilateral hilar lymphadenopathy and numerous pulmonary nodules. Pulmonary function testing found a mild decrease in lung function, but the patient denied experiencing respiratory symptoms. Her cutaneous lesions were treated with intralesional steroid injections, which decreased the size of the targeted lesions, although multiple nodules remained, and systemic therapy was deferred.
Fig 1.
A, Granulomatous dermatitis extending from the mid dermis to the subcutaneous tissue, with focal involvement. B, Granulomas are relatively pauci-inflammatory, overall small to medium in size, and well formed. Focally intermixed lymphocytes are seen. There is also an admixture of lymphocytes, rare neutrophils, and plasma cells within the interstitium. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×4; B, ×10.)
Eight months later, the patient had headaches and elevated blood counts refractory to phlebotomy, and she was started on the JAK1/JAK2 inhibitor ruxolitinib, 10 mg twice daily. On follow-up 5 months after initiating ruxolitinib, her PV was responding well to therapy, and her sarcoidal skin lesions had completely resolved. Repeat chest computed tomography 7 months after ruxolitinib initiation found mild interval improvement of lung nodules and mediastinal lymphadenopathy. The clinical improvement was maintained at 9-month follow-up.
Discussion
The JAK-STAT (Janus kinase–signal transducer and activator of transcription) signaling pathway plays a central role in innate and adaptive immunity as well as hematopoiesis.4 JAK inhibitors have recently emerged as a promising class of therapeutics for a growing list of inflammatory skin disorders such as psoriasis, atopic dermatitis, alopecia areata, and vitiligo.4 Additionally, a recent gene expression study found that the JAK-STAT signaling pathway is significantly differentially expressed between sarcoidosis patients and healthy controls, and a severity score developed using the gene signature identified in the study correlated with sarcoidosis disease severity.5 This evidence suggests that JAK-STAT signaling may be implicated in the pathogenesis of sarcoidosis.
In this case, we observed clinical resolution of cutaneous sarcoidosis and mild improvement of pulmonary sarcoidosis in our patient after treatment with ruxolitinib for concomitant PV. To our knowledge, there has been 1 previous report of ruxolitinib improving necrobiosis lipoidica and a report of tofacitinib improving cutaneous sarcoidosis; this is another unusual case showing the efficacy of JAK inhibitor therapy on cutaneous granulomatous disease.6, 7 Although the resolution of this patient's sarcoidosis may be secondary to successful management of her PV, a more likely explanation is the direct suppression of sarcoidosis through JAK-STAT signaling inhibition. Of note, although we observed a complete resolution of cutaneous disease in this patient, the response of the pulmonary sarcoidosis was much less striking. However, as seen in other targeted therapies for autoimmune conditions, such as ustekinumab for psoriasis and psoriatic arthritis, a discordance between the responses of cutaneous and other systemic symptoms may be observed.8 Therefore, we conclude that JAK inhibitors may be a viable treatment option for sarcoidosis, especially cutaneous sarcoidosis, and further clinical and mechanistic studies are needed.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Valeyre D., Prasse A., Nunes H., Uzunhan Y., Brillet P.Y., Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.James C., Ugo V., Le Couédic J.P. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Lower E.E., Smith J.T., Martelo 0.J., Baughman R.P. The anemia of sarcoidosis. Sarcoidosis. 1988;5:51–55. [PubMed] [Google Scholar]
- 4.Damsky W., King B.A. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–744. doi: 10.1016/j.jaad.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou T., Casanova N., Pouladi N. Identification of Jak-STAT signaling involvement in sarcoidosis severity via a novel microRNA-regulated peripheral blood mononuclear cell gene signature. Sci Rep. 2017;7(1):2–10. doi: 10.1038/s41598-017-04109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J.J., English J.C., III Improvement of ulcerative necrobiosis lipoidica after Janus kinase-inhibitor therapy. JAMA Dermatol. 2018;154(6):733–734. doi: 10.1001/jamadermatol.2018.0756. [DOI] [PubMed] [Google Scholar]
- 7.Damsky W., Thakral D., Emeagwali N., Galan A., King B. Tofacitinib treatment and molecular analysis of cutaneous sarcoidosis. N Engl J Med. 2018;379(26):2540–2546. doi: 10.1056/NEJMoa1805958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thibodaux Ross J., Triche Mallory W., Espinoza Luis R. Ustekinumab for the treatment of psoriasis and psoriatic arthritis: a drug evaluation and literature review. Exp Opin Biol Ther. 2018;18(7):821–827. doi: 10.1080/14712598.2018.1492545. [DOI] [PubMed] [Google Scholar]