Abstract
Silver nanoparticles (AgNPs) are gaining importance in health and environment. This study synthesized AgNPs using the bark extract of a plant, Toxicodendron vernicifluum (Tv) as confirmed by a absorption peak at 420 nm corresponding to the Plasmon resonance of AgNPs. The AgNPs were spherical, oval-shaped with size range of 2–40 nm as evident by field emission transmission electron microscopy (FE-TEM) and particle size analysis (PSA). The particles formed were crystalline by the presence of (111), (220) and (200) planes, as revealed by X ray diffraction (XRD) and energy dispersive spectroscopy (EDS). The presence of amine, amide, phenolic, and alcoholic aromatics derived from Tv extract was found to be capping and or reducing agents as evident by Fourier-transform infrared spectroscopy (FTIR) spectra. The Tv-AgNPs were observed to be biocompatible to chick embryonic and NIH3T3 cells at various concentrations. Interestingly, Tv-AgNPs at the concentration of 320 µg. mL−1 induced 82.5% of cell death in human lung cancer, A549 cells and further 95% of cell death with annexin V FITC/PI based apoptosis. The Tv-AgNPs selectively targeted and damaged the cancer cells through ROS generation. The Tv-AgNPs displayed minimal inhibitory concentration (MIC) of 8.12 µg.mL−1 and 18.14 µg.mL−1 against STEC and H. pylori respectively. This multi-potent property of Tv-AgNPs was due to shape and size specific property that facilitated easy penetration into the bacterial and cancer cells for targeted therapy.
Introduction
Phytochemicals-medicated synthesis of metal nanoparticles has received due attention because of their bioactivities such as antibiotic, cytotoxic, drug cargo and photocatalytic potentials1,2. Among the metallic nanomaterials, silver nanoparticles (AgNPs) are of significance for their antibacterial effect on human pathogens3–5, wound healing6, antioxidant7, anticancer activities, and dental applications as acrylic resins, composite resins and adhesives, endodontics, periodontal materials, porcelain restoration, titanium implants, and orthodontics8. The surgical sutures, when coated with AgNPs are shown to prevent the post wound healing infections9. The potent antimicrobial properties of AgNPs has increased the demand in medical applications. AgNPs-based medical products are also available in market such as contraceptive devices, bone prostheses, biomedical devices, wound dressing, and surgical instruments10–13.
The multi-drug resistant pathogens are causing the life-threatening human diseases. In this regard, the Gram-negative Helicobacter pylori colonizes the gastric epithelium, and it causes several illnesses and chronic diseases in human14. This pathogen is known to produce urease enzyme, which converts the urea to ammonia and bicarbonate resulting in neutralization of acidic pH in stomach to create appropriate pH (4.5–7.0) for pathogenic colonization15. The eradication of H. pylori can prevent various gastrointestinal diseases including peptic ulcer, gastritis, mucosa-associated lymphoid lymphoma, and adenocarcinoma16. Recent advancement in the nanotechnology has developed several drug delivery systems to target H. pylori17–21. For the instance, the amoxillin loaded in the PLGA (poly(lactic-co-glycolic acid) functionalized with receptor UreI has enhanced targeted drug delivery towards eradication of H. pylori22. Another Gram-negative bacterium is Shiga toxin (Stx1 and Stx2) producing Escherichia coli (STEC), colonising in the human gut and causing Hemolytic uremic syndrome (HUS), hemorrhagic colitis, pneumonia, urinary infections, meningitis, and bacteremia, diarrhea23.
Environmental pollution and cigarette smoking habits have significantly increased the incidence of lung and cardiovascular diseases24,25. Chemotherapeutics, radiation, and surgical approaches for curing the diseases are expensive, often toxic to normal cells and also causing side effects1,26. In this context, AgNPs are advantageous in eliciting cancer cell death through the cell cycle arrest, mitochondrial pathways (Reactive oxygen species (ROS) generation), nucleus damage, apoptosis through up-regulation or down-regulation of apoptosis pathways related proteins and genes, necrosis, DNA damage, autophagy and oxidative stress27,28, Therefore, fabrication of biocompatible nanoparticles with no side effects can be helpful in successful treatment of the cancer cells.
Green synthesize of AgNPs using the plant extracts are potentially less in toxicity to normal cells, ecologically sustainable, economically viable and less time consuming approach29. Several reports are available on the synthesis of AgNPs from plants such as Trapa natans27, Phoenix dactylifera30, Cleome viscosa L.31, Lycium chinense32, Taxus baccata33, Clerodendrum phlomidis34 and their cytotoxicity on cancer cells. The AgNPs synthesized from Rhus coriaria under the genus Rhus (Toxicodendron) and the family Anacardiaceae are reported for cytotoxicity on human breast cancer cell line (MCF-7). Another species of the same genus and family is T. vernicifluum, reported to have antitumorigenic, antioxidant, neuroprotective, and cytotoxicity effects35–37 but not used for the synthesis of AgNPs. Hence, the present work synthesised the silver nanoparticles (Tv-AgNPs) using the aqueous extract of bark derived from T. vernicifluum and characterized using the FE-TEM-EDS, PSA, FTIR, and XRD. Antibacterial, cytotoxic and anti-proliferation activities of Tv-AgNPs were also investigated.
Results and Discussion
Phytogenic silver nanoparticles
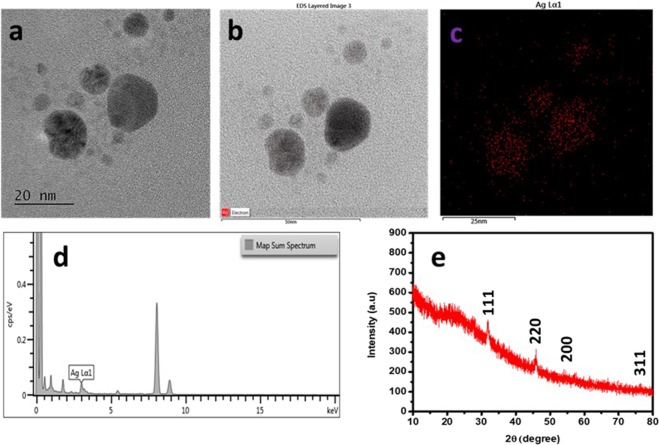
Biochemical substances including phenolics, and flavonoids from the plants act as reducing or capping agent for reduction of the silver ions, facilitating the phytogenic synthesis of AgNPs. This was confirmed through absorption plasmon resonance ranged from 400–450 nm by UV-vis spectrophotometer38, as well as through observation of color changes from pale yellow to brown colour in the reaction mixture after 12 hours of incubation as also indicated by UV-vis absorption peak at 420 nm corresponding to the AgNPs plasmon resonance (Fig. 1). Further, the FETEM analysis was made to study the morphology, size, and shape of Tv-AgNPs and the results revealed the Tv-AgNPs as anisotropic in structure, spherical and oval-shaped with size range of 2–40 nm (Fig. 2a). In addition, the FETEM-EDS based mapping and chromatographs indicated the presence of Ag in micrographs (Fig. 2b–d), in accordance with the previous reports39–41.
Figure 1.
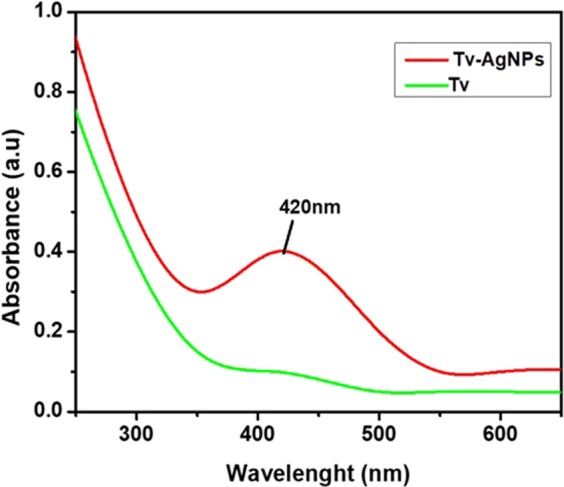
Ultraviolet-vis spectrum of Tv –AgNPs synthesized by the reaction of 3 mM AgNO3 and 5 ml of aqueous bark extract from T. vernicifluum.
Figure 2.
Field-Emission Transmission Electron Microscope (FE-TEM) observation of Tv –AgNPs synthesized by aqueous bark extract from T. vernicifluum. Anisotropic structured of Tv-AgNPs visualized by FE-TEM (a), scanning of the Ag+ in FETEM micrographs by Energy dispersive X-ray spectroscopic (EDS) (b,c) Determination of the Ag+ by FETEM-EDS spectra (d) XRD pattern of Tv-AgNPs (e).
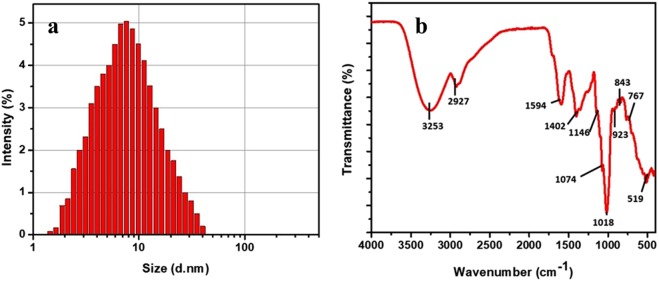
The XRD pattern as indicated in Fig. 2e confirmed the natural formation, crystallinity, and purity of Tv-AgNPs in accordance with Bragg reflection of (111), (220), (200), (311). Compared to Joint committee on powder diffraction standards (JCPDS-89–3722), the results are similar to the earlier reports of XRD patterns of silver nanoparticles42,43. The EDS and XRD results confirmed the successful synthesis of the silver nanoparticles using the bark extract of T. vernicifluum. Furthermore, the PSA analysis revealed the size range of Tv-AgNPs size from 2–40 nm with an average of 12.01 nm (Fig. 3a), which is in agreement with the FETEM results. As indicated by FETEM and PSA, different morphological structures of Tv-AgNPs generated were due to excessive capping or binding of the bark extract of T. vernicifluum. Similarly, several earlier works have reported the significant involvement of phytochemicals in the generation and properties of the AgNPs39,40,44,45.
Figure 3.
Particle size (a) and Fourier-transform infrared spectroscopy (b) analysis of the Tv-AgNPs.
The FTIR analysis was performed to determine the capping of functional biomolecules in Tv-AgNPs and the results are depicted in Fig. 3b. There are several stretching vibrations as 3253 (weak O-H stretching, alcohol), 2927 (strong N-H stretching, amine), 1594 (strong C=O stretching, amide), 1402 (week C=C stretching, aromatic), 1146 (medium C-N stretching, amine), 1074 (strong C-O stretching, aromatic ester), 1018 (Strong C-F, fluoro compound), 923 (strong C=C, alkaline), 843 (C-Cl, halo compounds), and 767 (Strong C-H), 519 (C-l). This indicated that the presence of amine, amide, phenolic, alcoholic aromatics from the bark extract of T. vernicifluum involved as reducing or capping agent in the synthesis of the Tv-AgNPs and this finds support of earlier reports32,38,46–48.
Cytotoxicity and anti-proliferation assay
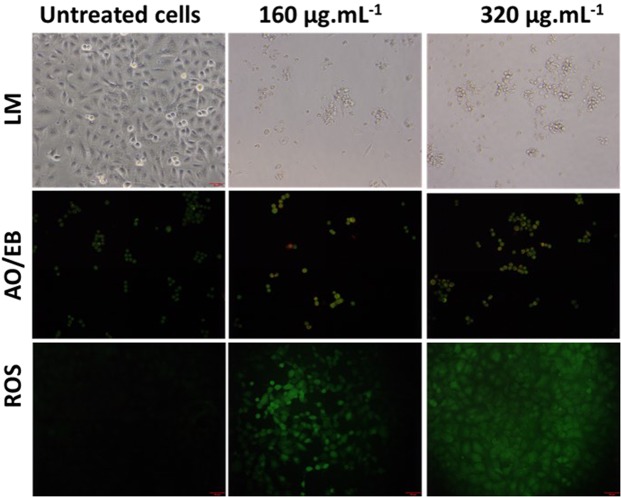
Analysis of cytotoxicity of biological materials is essential for the pharmacological trails. Hence, the present study determined the cytotoxicity of Tv-AgNPs by CAM and WST assays. CAM assay revealed that that exposure of negative control with 0.1 M NaOH induced the blood hemorrhage and coagulation, while the distilled water did not cause any irritant reaction. The Tv-AgNPs exposure did not cause any irritant reaction at 50 µg.mL−1, but slightly irritant only at 100 µg.mL−1 (S.Fig. 1). Further, in vitro cytotoxicity assay was performed on mouse embryo NIH3T3 cells and the results indicated that the NIH3T3 cells growth was not significantly reduced with the treatment of Tv-AgNPs at different concentrations (Fig. 4), In addition, AO/EB and DCFH-DA staining showed no cell death and ROS generation respectively with the treatment of Tv-AgNPs (S.Fig. 2). Further, the apoptosis analysis by flow cytometer assay indicated only negligible apoptosis (0.50%) with the treatment of Tv-AgNPs (S.Fig. 3a,b). These results of CAM assay, cell toxicity (WST assay), AO/EB, DCFH-DA staining and flow cytometric (Annexin V FITC/PI) analyses confirmed the non-toxicity of Tv-AgNPs. Similarly, the non-toxicity of phytogenic AgNPs is reported on normal cell lines of renal (MDCK)49, epithelial HBL-10043, and Human embryonic kidney HEK 29350.
Figure 4.
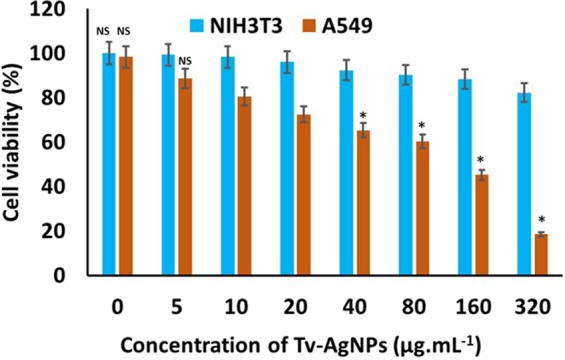
Cytotoxicity and antiproliferative effect of Tv-AgNPs on mouse embryo fibroblast cell line NIH3T3 and human lung carcinoma A549. NS- Not significant with NIH3T3 and A549. *p significantly differs.
On the other hand, the treatment of Tv-AgNPs induced the death of human lung cancer cells in A549 cell line at concentration-dependent manner (Fig. 4). About 82.5% of cells were dead in the treatment of Tv-AgNPs. Similarly, Annexin V FITC/PI based apoptosis assay also showed about 95% cell death with treatment of Tv-AgNPs at 320 µg.mL−1 (S.Fig. 3c,d). Further, AO/EB, DCFH-DA staining results showed the cell damage and ROS generation at the exposure to 320 µg.mL−1 of Tv-AgNPs (Fig. 5). This indicated the smart cancer cells sensing efficiency of Tv-AgNPs in causing cancer cell death through ROS mediated apoptosis in human lung cancer cells, induction of the oxidative stress and reduction of ATP generation required for the cellular energy51. Moreover, it is reported that AgNPs trigger the cell apoptosis in human breast cancer cell MCF-7, human lung carcinoma A549, HCT116, HepG252, colon cancer cell line HT-29, SW620 through the interactions with cell organelles including mitochondria, nucleus, proteins, and DNA53.
Figure 5.
Effect of Tv AgNPs treatments and untreated on cellular morphology changes and reactive oxygen species generation in human lung carcinoma A549.
Antibacterial activity
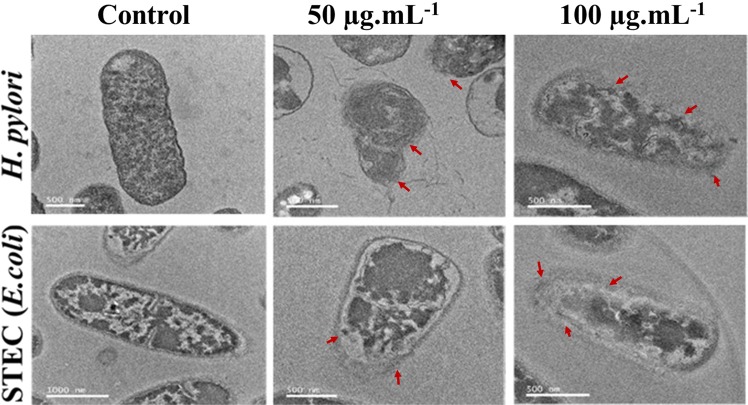
Silver nanoparticles inhibit the bacterial pathogens by penetrating through the bacterial cell wall and binding with peptidoglycan or lipopolysaccharide, subsequently damaging the bacterial membrane, forming the membrane pits, and inducing the leakage of cellular materials54–57. Similarly, the silver nanoparticles synthesized in the present study displayed potent antibacterial activity at the minimal inhibitory concentration of 8.12 µg.mL−1 for STEC and 18.14 µg.mL−1 for H. pylori. Further, the disc diffusion assay revealed that Tv-AgNPs at 100 µg.mL−1 displayed the higher zone of inhibition against H. pylori (17 mm) and STEC (22 mm) than the standard kanamycin (S.Fig. 4). The bacterial cellular damage and cell disruption due to the treatment of the Tv-AgNPs were observed under TEM, and the images clearly indicated the cell wall damage with elution of cellular inclusions by the treatment of Tv-AgNPs in comparison to untreated cells of H. pylori and STEC (Fig. 6). This potential activity is likely due to unique shape and size of Tv-AgNPs for easy penetration into the bacterial cells followed by ROS formation, DNA damage, and cellular membrane damage, growth signalling pathway and tyrosine phosphorylation42,58–60. Similarly, silver nanoparticles, synthesised from the Solanum xanthocarpum L. and Peganum harmala L are reported to significantly inhibit the growth of the H. pylori61,62.
Figure 6.
Antibacterial activity; transmission electron microscopic observation of cellular morphology changes in bacterial cells treated or untreated with Tv AgNPs, the red arrow indicates the cell damage.
Conclusion
The prevention of the microbial infections and biofilm formation by dangerous microbes such as H. pylori and STEC is essential for human health. Eco-friendly, cost-effective and green method was attempted to synthesise the Tv-AgNPs using aqueous bark extract of T. vernicifluum as reducing or capping agent. The size of Tv-AgNPs ranged from 2–40 nm with anisotropic structure, spherical and oval shape, as revealed by TEM and PSA. The Tv-AgNPs were nontoxicity as confirmed through CAM assay on Egg and cytotoxicity assay in NIH3T3. The small-sized (<40 nm) Tv-AgNPs displayed potential antibacterial, and anti-proliferative activities by inducing the ROS, oxidative stress, DNA division, nucleus damage, and apoptosis in both cancer and bacterial cells. Hence, the Tv-AgNPs deserve for the preparation of biomedical products such as would dressing cloth, and surgical devices.
Materials and Methods
Chemicals, bacterial strains, and cell culture
Gram-negative bacterial human pathogens such as Helicobacter pylori (MH179988) and Shigella toxin producing Escherichia coli (MH180008) were received from the laboratory of Professor Deog-Hwan Oh, College of Biotechnology and Bioscience, Kangwon National University, Chuncheon, Republic of Korea. The bacterial strains were preserved in 20% glycerol at −80 °C. The chemicals such as dichlorofluorescein diacetate (DCFH-DA), trypsin, acridine orange Hemi salt (AO), ethidium bromide (EB) were obtained from Sigma Aldrich, Republic of Korea. The cell viability proliferation and cytotoxicity assay kit (EZ-CYTOX water-soluble tetrazolium (WST) (EZ-CyTox)) was purchased from Daeil Lab Service, Republic of Korea. Dulbecco’s modified eagle medium (DMEM), penicillin, streptomycin, and fetal bovine serum (FBS) were procured from Thermo Fishers Scientific Seoul, the Republic of Korea. Dead Cell Apoptosis Kit with Annexin V FITC/PI - for Flow Cytometry was purchased from Invitrogen, Thermo fishers scientific, Republic of Korea. Mueller Hinton Broth was obtained from MB cell, Seoul, Republic of Korea. The cell lines such as mouse embryo fibroblast cell line NIH3T3 and human lung carcinoma A549 were received from the Korean cell line bank (Seoul, Republic of Korea). The bark sample of Toxicodendron vernicifluum was collected from Wonju-malgeun-chamott, Wonju city, Republic of Korea.
Synthesis and characterization of Tv-AgNPs
The bark samples were subjected to the water extraction according to the methods reported earlier with minor modifications63,64. The bark extract was prepared by boiling the 5 g of bark samples in 100 ml of distilled H2O at 90 °C for 10 min, followed by that the extracts were cooled in room temperature then collected by the centrifugation at 10000 rpm for 20 min. Finally, the extracts were filtered through the Whatman No.1 filter paper and stored in room temperature for further use. For the synthesis of the Tv-AgNPs, 3 mM of AgNO3 was dissolved in 10 ml of bark extract at room temperature. The synthesis of Tv-AgNPs was observed by scanning the reaction mixture in a range from 200 to 700 nm using the UV spectrophotometer (Optizen 2120UV, Korea). To analysis the morphology, shape and dispersion characteristics, the Tv-AgNPs were carbon coated in copper grid and then observed under Transmission electron microscopic (TEM, JEOL-JSM 1200EX, Japan) with Energy dispersive X-ray spectroscopy (EDS), X-ray diffractometer (X’pert-pro MPD- PANalytical, Netherland) operated at 40 keV, 40 mA with Cu κα radiation in θ−2θ. The size of Tv-AgNPs was measured using particle size analyzer (PSA, Malvern Mastersizer 2000, Britain). The chemical nature and functional groups present in Tv-AgNPs were analysed by using Fourier-transform infrared spectroscopy (FTIR PerkinElmer Paragon 500, USA).
Cytotoxicity
Allergic and toxic effect of Tv-AgNPs was tested by using chick embryo chorioallantoic membrane (CAM) assay65,66. Cytotoxicity and anti-proliferation effects of Tv-AgNPs were investigated on NIH3T3 and A549 cells respectively using WST assay67. Briefly, NIH3T3 or A549 (1 × 104) cells were seeded in 96-well plates containing DMEM or RPMI 1640 medium and allowed in 5% CO2 incubator at the humidified environment for overnight to get 80–90% confluence. Then 10 µl of Tv- AgNPs at the different concentrations (0–320 µg.ml−1) were added to Tv-AgNPs; after 12 h of exposure the WST1 reagent was added and incubated for 30 min to 4 hours; and then measured the absorbance at 450 nm as per manufacturer’s instructions of WST-1 method. The experiments were conducted in three independent trials with three replicates for each trial and the cell viability was determined. Further, the effect of Tv-AgNPs treatment on NIH3T3 and A549 cells was analysed for morphological changes1,68. The DCFH-DA stain assay was used to measure the ROS generation at an excitation of 495 nm and emission of 529 nm. Apoptosis was observed by AO/EB staining assay69 and images were taken using the fluorescence microscope (Olympus, CKX53 culture microscope, Japan).
In vitro antibacterial assay
Effect of Tv-AgNPs on the eradication of H. pylori and Shigella toxin producing Escherichia coli (STEC) was analyzed using the microdilution method (Clinical and laboratory standard institute, CLSI). For the elucidation of minimal inhibitory concentration (MIC), the STEC was grown in Mueller Hinton Broth (MHB) and H. pylori in brain heart infusion (BHI) broth in a rotary shaker at 180 rpm at 37 °C for 24 h. The bacterial suspension (109 CFU.ml−1) was dispensed in 96-well (Costar) plates containing different concentrations of Tv-AgNPs (0.1–12.5 µg.ml−1). The un-inoculated MHB and untreated bacterial cells were used as negative and positive controls respectively and optical density was measured at 600 nm70. Tv-AgNPs induced bacterial cell disruption was observed using high-resolution transmission electron microscopy (HRTEM)71. For HRTEM analysis, the MIC of PDK-CE was treated to H. pylori for 24 h at 37 °C. After the treatment period, the cells were collected by centrifugation, then fixed them with 4% glutaraldehyde (v/v) for 2 h and the cells were dehydrated by acetone (70%). Finally, the cellular changes were observed using the HRTEM (JEOL-2010, Japan).
Supplementary information
Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori
Acknowledgements
This work was supported by Korea Research Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT (2017H1D3A1A01052610) and by UGC, New Delhi (K.K.).
Author Contributions
K.S. designed the experiments, performed the laboratory experiments, and drafted the manuscript. R.C. and D.H.O. performed the bactericidal experiments. K.S., D.M. and K.K. performed data analysis and revised the manuscript. M.H.W. supervised the research study and approved of the final version to be published. All the authors have read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42112-1.
References
- 1.Saravanakumar K, et al. Green synthesis and characterization of biologically active nanosilver from seed extract of Gardenia jasminoides Ellis. Journal of Photochemistry and Photobiology B: Biology. 2018;185:126–135. doi: 10.1016/j.jphotobiol.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Hemmati S, et al. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019;158:8–14. doi: 10.1016/j.poly.2018.10.049. [DOI] [Google Scholar]
- 3.Leonardo T, et al. Silver Accumulation in the Green Microalga Coccomyxa actinabiotis: Toxicity, in Situ Speciation, and Localization Investigated Using Synchrotron XAS, XRD, and TEM. Environmental Science & Technology. 2016;50:359–367. doi: 10.1021/acs.est.5b03306. [DOI] [PubMed] [Google Scholar]
- 4.Nowack B, Krug HF, Height M. 120 Years of Nanosilver History: Implications for Policy Makers. Environmental Science & Technology. 2011;45:1177–1183. doi: 10.1021/es103316q. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, et al. Silver nanoparticles: A novel antibacterial agent for control of Cronobacter sakazakii. Journal of Dairy Science. 2018;101:10775–10791. doi: 10.3168/jds.2018-15258. [DOI] [PubMed] [Google Scholar]
- 6.Parveen A, Kulkarni N, Yalagatti M, Abbaraju V, Deshpande R. In vivo efficacy of biocompatible silver nanoparticles cream for empirical wound healing. Journal of Tissue Viability. 2018;27:257–261. doi: 10.1016/j.jtv.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Keshari, A. K., Srivastava, R., Singh, P., Yadav, V. B. & Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. Journal of Ayurveda and Integrative Medicine, 10.1016/j.jaim.2017.11.003 (2018). [DOI] [PMC free article] [PubMed]
- 8.Bapat RA, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Materials Science and Engineering: C. 2018;91:881–898. doi: 10.1016/j.msec.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 9.Baygar T, Sarac N, Ugur A, Karaca IR. Antimicrobial characteristics and biocompatibility of the surgical sutures coated with biosynthesized silver nanoparticles. Bioorganic Chemistry. 2019;86:254–258. doi: 10.1016/j.bioorg.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, et al. In Vitro Analysis of a Nanocrystalline Silver-Coated Surgical Mesh. Surgical Infections. 2007;8:397–404. doi: 10.1089/sur.2006.032. [DOI] [PubMed] [Google Scholar]
- 11.Lee, H. Y., Park, H. K., Lee, Y. M., Kim, K. & Park, S. B. A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chemical Communications, 2959–2961, 10.1039/b703034g (2007). [DOI] [PubMed]
- 12.Tian J, et al. Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem. 2007;2:129–136. doi: 10.1002/cmdc.200600171. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhry Q, et al. Applications and implications of nanotechnologies for the food sector. Food Additives & Contaminants: Part A. 2008;25:241–258. doi: 10.1080/02652030701744538. [DOI] [PubMed] [Google Scholar]
- 14.Marshall B, Warren JR. Unidentified Curved Bacilli in the Stomach of Patients with Gastritis and Peptic Ulceration. The Lancet. 1984;323:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y-H, et al. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy. Biomaterials. 2009;30:3332–3342. doi: 10.1016/j.biomaterials.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 16.Sanders MK, Peura DA. Helicobacter pylori-Associated Diseases. Curr Gastroenterol Rep. 2002;4:448–454. doi: 10.1007/s11894-002-0019-x. [DOI] [PubMed] [Google Scholar]
- 17.Jing Z-W, et al. Design and evaluation of novel pH-sensitive ureido-conjugated chitosan/TPP nanoparticles targeted to Helicobacter pylori. Biomaterials. 2016;84:276–285. doi: 10.1016/j.biomaterials.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Jing Z-W, et al. Anti-Helicobacter pylori effectiveness and targeted delivery performance of amoxicillin-UCCs-2/TPP nanoparticles based on ureido-modified chitosan derivative. International Journal of Biological Macromolecules. 2018;115:367–374. doi: 10.1016/j.ijbiomac.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 19.Arif M, et al. Development of novel pH-sensitive thiolated chitosan/PMLA nanoparticles for amoxicillin delivery to treat Helicobacter pylori. Materials Science and Engineering: C. 2018;83:17–24. doi: 10.1016/j.msec.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 20.Cai J, et al. Preparation and evaluation of lipid polymer nanoparticles for eradicating H. pylori biofilm and impairing antibacterial resistance in vitro. International Journal of Pharmaceutics. 2015;495:728–737. doi: 10.1016/j.ijpharm.2015.09.055. [DOI] [PubMed] [Google Scholar]
- 21.Dey SK, et al. Floating mucoadhesive alginate beads of amoxicillin trihydrate: A facile approach for H. pylori eradication. International Journal of Biological Macromolecules. 2016;89:622–631. doi: 10.1016/j.ijbiomac.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Luo M, et al. Construction and optimization of pH-sensitive nanoparticle delivery system containing PLGA and UCCs-2 for targeted treatment of Helicobacter pylori. Colloids and Surfaces B: Biointerfaces. 2018;164:11–19. doi: 10.1016/j.colsurfb.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Hunt JM. Shiga Toxin–Producing Escherichia coli (STEC) Clinics in Laboratory Medicine. 2010;30:21–45. doi: 10.1016/j.cll.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saravanakumar K, et al. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. Journal of Photochemistry and Photobiology B: Biology. 2019;190:103–109. doi: 10.1016/j.jphotobiol.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Pope CA, 3rd, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environmental health perspectives. 2011;119:1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dadashpour M, et al. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Materials Science and Engineering: C. 2018;92:902–912. doi: 10.1016/j.msec.2018.07.053. [DOI] [PubMed] [Google Scholar]
- 27.Saber MM, Mirtajani SB, Karimzadeh K. Green synthesis of silver nanoparticles using Trapa natans extract and their anticancer activity against A431 human skin cancer cells. Journal of Drug Delivery Science and Technology. 2018;47:375–379. doi: 10.1016/j.jddst.2018.08.004. [DOI] [Google Scholar]
- 28.Lin J, et al. Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy. 2014;10:2006–2020. doi: 10.4161/auto.36293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. Journal of Nanoparticle Research. 2008;10:507–517. doi: 10.1007/s11051-007-9275-x. [DOI] [Google Scholar]
- 30.Oves M, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Materials Science and Engineering: C. 2018;89:429–443. doi: 10.1016/j.msec.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT, Murugesan K. Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: Assessment of their antibacterial and anticancer activity. Karbala International Journal of Modern Science. 2018;4:61–68. doi: 10.1016/j.kijoms.2017.10.007. [DOI] [Google Scholar]
- 32.Chokkalingam, M. et al. Facile synthesis of Au and Ag nanoparticles using fruit extract of Lycium chinense and their anticancer activity. Journal of Drug Delivery Science and Technology, 10.1016/j.jddst.2018.11.025 (2018).
- 33.Kajani AA, et al. Anticancer effects of silver nanoparticles encapsulated by Taxus baccata extracts. Journal of Molecular Liquids. 2016;223:549–556. doi: 10.1016/j.molliq.2016.08.064. [DOI] [Google Scholar]
- 34.Sriranjani R, et al. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. Journal of Molecular Liquids. 2016;220:926–930. doi: 10.1016/j.molliq.2016.05.042. [DOI] [Google Scholar]
- 35.Nam TG, et al. Rhus verniciflua Stokes Extract and Its Flavonoids Protect PC-12 Cells against H2O2-Induced Cytotoxicity. J Microbiol Biotechnol. 2017;27:1090–1097. doi: 10.4014/jmb.1612.12018. [DOI] [PubMed] [Google Scholar]
- 36.Jung CH, et al. Rhus verniciflua stokes extract: radical scavenging activities and protective effects on H2O2-induced cytotoxicity in macrophage RAW 264.7 cell lines. Biol Pharm Bull. 2006;29:1603–1607. doi: 10.1248/bpb.29.1603. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, et al. Antiplatelet effects of Rhus verniciflua stokes heartwood and its active constituents–fisetin, butein, and sulfuretin–in rats. J Med Food. 2015;18:21–30. doi: 10.1089/jmf.2013.3116. [DOI] [PubMed] [Google Scholar]
- 38.Patil MP, et al. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microbial Pathogenesis. 2018;121:184–189. doi: 10.1016/j.micpath.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 39.Patil MP, et al. Sasa borealis leaf extract-mediated green synthesis of silver–silver chloride nanoparticles and their antibacterial and anticancer activities. New Journal of Chemistry. 2017;41:1363–1371. doi: 10.1039/c6nj03454c. [DOI] [Google Scholar]
- 40.Patil MP, Seo YB, Kim G-D. Morphological changes of bacterial cells upon exposure of silver-silver chloride nanoparticles synthesized using Agrimonia pilosa. Microbial Pathogenesis. 2018;116:84–90. doi: 10.1016/j.micpath.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Saravanakumar K, Wang M-H. Trichoderma based synthesis of anti-pathogenic silver nanoparticles and their characterization, antioxidant and cytotoxicity properties. Microbial Pathogenesis. 2018;114:269–273. doi: 10.1016/j.micpath.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Ravichandran A, et al. Phyto-mediated synthesis of silver nanoparticles using fucoidan isolated from Spatoglossum asperum and assessment of antibacterial activities. Journal of Photochemistry and Photobiology B: Biology. 2018;185:117–125. doi: 10.1016/j.jphotobiol.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 43.Sukirtha R, et al. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochemistry. 2012;47:273–279. doi: 10.1016/j.procbio.2011.11.003. [DOI] [Google Scholar]
- 44.Liu Wj, Hou Xq, Chen H, Liang Jy, Sun Jb. Chemical constituents from Agrimonia pilosa Ledeb. and their chemotaxonomic significance. Natural Product Research. 2016;30:2495–2499. doi: 10.1080/14786419.2016.1198351. [DOI] [PubMed] [Google Scholar]
- 45.Alishah H, Pourseyedi S, Mahani SE, Ebrahimipour SY. Extract-mediated synthesis of Ag@AgCl nanoparticles using Conium maculatum seeds: characterization, antibacterial activity and cytotoxicity effect against MCF-7 cell line. RSC Advances. 2016;6:73197–73202. doi: 10.1039/c6ra16127h. [DOI] [Google Scholar]
- 46.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chemistry. 2011;13:2638–2650. doi: 10.1039/c1gc15386b. [DOI] [Google Scholar]
- 47.Devi TB, Ahmaruzzaman M, Begum S. A rapid, facile and green synthesis of Ag@AgCl nanoparticles for the effective reduction of 2,4-dinitrophenyl hydrazine. New Journal of Chemistry. 2016;40:1497–1506. doi: 10.1039/c5nj02367j. [DOI] [Google Scholar]
- 48.Yang N, Li W-H. Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Industrial Crops and Products. 2013;48:81–88. doi: 10.1016/j.indcrop.2013.04.001. [DOI] [Google Scholar]
- 49.Sreekanth TVM, et al. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. Journal of Photochemistry and Photobiology B: Biology. 2018;188:6–11. doi: 10.1016/j.jphotobiol.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Arya G, et al. Catalytic, antibacterial and antibiofilm efficacy of biosynthesised silver nanoparticles using Prosopis juliflora leaf extract along with their wound healing potential. Journal of Photochemistry and Photobiology B: Biology. 2019;190:50–58. doi: 10.1016/j.jphotobiol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 51.R JR, Vaali-Mohammed M-A, Al-Lohedan HA, Appaturi JN. Synthesis and bio-physical characterization of Silver nanoparticle and Ag-mesoporous MnO2 nanocomposite for anti-microbial and anti-cancer activity. Journal of Molecular Liquids. 2017;243:348–357. doi: 10.1016/j.molliq.2017.08.037. [DOI] [Google Scholar]
- 52.Padinjarathil H, et al. Galactomannan endowed biogenic silver nanoparticles exposed enhanced cancer cytotoxicity with excellent biocompatibility. International Journal of Biological Macromolecules. 2018;118:1174–1182. doi: 10.1016/j.ijbiomac.2018.06.194. [DOI] [PubMed] [Google Scholar]
- 53.Manshian BB, Himmelreich U, Soenen SJ. Standard Cellular Testing Conditions Generate an Exaggerated Nanoparticle Cytotoxicity Profile. Chemical Research in Toxicology. 2017;30:595–603. doi: 10.1021/acs.chemrestox.6b00340. [DOI] [PubMed] [Google Scholar]
- 54.Bello BA, et al. Anticancer, antibacterial and pollutant degradation potential of silver nanoparticles from Hyphaene thebaica. Biochemical and Biophysical Research Communications. 2017;490:889–894. doi: 10.1016/j.bbrc.2017.06.136. [DOI] [PubMed] [Google Scholar]
- 55.Saravanakumar K, et al. Fungal enzyme-mediated synthesis of chitosan nanoparticles and its biocompatibility, antioxidant and bactericidal properties. International Journal of Biological Macromolecules. 2018;118:1542–1549. doi: 10.1016/j.ijbiomac.2018.06.198. [DOI] [PubMed] [Google Scholar]
- 56.Mirzajani F, Ghassempour A, Aliahmadi A, Esmaeili MA. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Research in Microbiology. 2011;162:542–549. doi: 10.1016/j.resmic.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Li W-R, et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Applied Microbiology and Biotechnology. 2010;85:1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- 58.Patra JK, Baek K-H. Green synthesis of silver chloride nanoparticles using Prunus persica L. outer peel extract and investigation of antibacterial, anticandidal, antioxidant potential. Green Chemistry Letters and Reviews. 2016;9:132–142. doi: 10.1080/17518253.2016.1192692. [DOI] [Google Scholar]
- 59.Manivasagan P, Venkatesan J, Sivakumar K, Kim S-K. Actinobacteria mediated synthesis of nanoparticles and their biological properties: A review. Critical Reviews in Microbiology. 2016;42:209–221. doi: 10.3109/1040841x.2014.917069. [DOI] [PubMed] [Google Scholar]
- 60.Saravanan M, Arokiyaraj S, Lakshmi T, Pugazhendhi A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microbial Pathogenesis. 2018;117:68–72. doi: 10.1016/j.micpath.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Amin M, et al. Green Synthesis of Silver Nanoparticles: Structural Features and In Vivo and In Vitro Therapeutic Effects against Helicobacter pylori Induced Gastritis. Bioinorganic chemistry and applications. 2014;2014:135824–135824. doi: 10.1155/2014/135824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amin M, Anwar F, Janjua MRSA, Iqbal MA, Rashid U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. International Journal of Molecular Sciences. 2012;13:9923–9941. doi: 10.3390/ijms13089923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bar H, et al. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009;348:212–216. doi: 10.1016/j.colsurfa.2009.07.021. [DOI] [Google Scholar]
- 64.Wang L, Wu Y, Xie J, Wu S, Wu Z. Characterization, antioxidant and antimicrobial activities of green synthesized silver nanoparticles from Psidium guajava L. leaf aqueous extracts. Materials Science and Engineering: C. 2018;86:1–8. doi: 10.1016/j.msec.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Saravanakumar K, et al. Novel metabolites from Trichoderma atroviride against human prostate cancer cells and their inhibitory effect on Helicobacter pylori and Shigella toxin producing Escherichia coli. Microbial Pathogenesis. 2019;126:19–26. doi: 10.1016/j.micpath.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 66.Rajendran I, Dhandapani H, Anantanarayanan R, Rajaram R. Apigenin mediated gold nanoparticle synthesis and their anti-cancer effect on human epidermoid carcinoma (A431) cells. RSC Advances. 2015;5:51055–51066. doi: 10.1039/c5ra04303d. [DOI] [Google Scholar]
- 67.Lee JH, Park TH, Rhee WJ. Inhibition of apoptosis in HeLa cell by silkworm storage protein 1, SP1. Biotechnology and Bioprocess Engineering. 2015;20:807–813. doi: 10.1007/s12257-015-0152-7. [DOI] [Google Scholar]
- 68.Varukattu, N. B. et al. Nanostructured pH-responsive biocompatible chitosan coated copper oxide nanoparticles: A polymeric smart intracellular delivery system for doxorubicin in breast cancer cells. Arabian Journal of Chemistry, 10.1016/j.arabjc.2018.04.012 (2018).
- 69.Kummara S, Patil MB, Uriah T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles – A comparative study. Biomedicine & Pharmacotherapy. 2016;84:10–21. doi: 10.1016/j.biopha.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Gopinath V, et al. In vitro toxicity, apoptosis and antimicrobial effects of phyto-mediated copper oxide nanoparticles. RSC Advances. 2016;6:110986–110995. doi: 10.1039/c6ra13871c. [DOI] [Google Scholar]
- 71.Hickey WJ, Shetty AR, Massey RJ, Toso DB, Austin J. Three‐dimensional bright‐field scanning transmission electron microscopy elucidate novel nanostructure in microbial biofilms. Journal of Microscopy. 2017;265:3–10. doi: 10.1111/jmi.12455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori