Abstract
More than 30 years after its introduction, autologous stem cell transplantation (ASCT) remains the standard of care for young patients with newly diagnosed multiple myeloma. Not only did the arrival of novel agents such as immunomodulatory drugs (IMiDs), proteasome inhibitors (PI) and monoclonal antibodies not replace ASCT, instead they solidified its central role as standard of care. Novel agent use is now inarguably essential in induction, maintenance, and possibly consolidation. In light of these new advancements, new challenges arise in deciding on optimal practice. Who is most suited to undergo ASCT? Is there an age threshold that should not be surpassed? Should transplantation be embarked on early or is it reasonable to delay it? What are the optimal induction, consolidation, and maintenance therapies? What is the role of tandem transplantation in the era of novel agents and where do patient-specific cytogenetics come into the equation when deciding on treatment? These are some of the questions addressed in this review which we will attempt to answer with the latest currently available data.
Introduction
Multiple myeloma accounts for approximately 10% of hematologic cancers and 1% of all cancers in general1. Thirty years after the work of Powles, Barlogie, and McElwain led to the initiation of the concept of high-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT)2–4, transplantation remains the standard for treating newly diagnosed multiple myeloma in young and in select, fit, elderly patients. The procedure’s superiority was initially proven by the Intergroupe Francophone du Myeloma (IFM) and later confirmed by the UK Medical Research Council5–8. Despite its confirmed significant impact on event-free survival (EFS), multiple trials failed to depict significant impact on overall survival (OS)9. Even though standard, the procedure is still challenged by inevitable relapses threatening long-term remissions, and is therefore challenged by some myeloma experts who delay ASCT till relapse or progression5,8.
The introduction of drugs such as thalidomide, lenalidomide, and bortezomib administered before and/or after HDT/ASCT gave way to the groundbreaking achievement of stringent complete response (sCR) with a normal kappa/lambda ratio (serum free light chain)10, immunophenotypic CR11, and molecular CR12, in addition to significantly increased CR and CR plus very good partial response rate (VGPR)13,14. Thus, in the era of novel agents, embarking on transplantation is further scrutinized. Current ongoing studies are investigating incorporating different agents such as daratumumab and lenalidomide before and after transplantation, respectively. The challenge is thus to evaluate the necessity of HDT/ASCT when a powerful monoclonal antibody is combined with an induction regimen incorporating an immunomodulatory drug (IMiD) and a proteasome inhibitor (PI). This review addresses ten questions around the different steps of transplantation and lays forward current evidence from ongoing trials to aid decision-making.
Which patients are candidates for ASCT?
There is no consensus regarding an age cutoff beyond which treatment with ASCT becomes questionable and as such, practice varies across institutions and countries. Generally, HDT/ASCT is reserved for patients younger than 65 years old with no severe comorbidities. Two important conditions are described below.
Patient is over 65 years of age
Most of the randomized studies have included patients younger than 65 years of age and so it becomes difficult to infer conclusions regarding this matter15. Usually, age of participants is limited to 65 years to avoid selection bias and limit toxicities and withdrawal from studies. However, this does not mean that ASCT is not feasible in older patients. On the contrary, it is in select patients16. A previous study whereby the median age of patients was 72 years old concluded that elderly multiple myeloma patients should not be excluded from transplantation displaying good results with melphalan 140 mg/m2 (ref. 16). In the very few studies that did include older patients, melphalan doses were reduced (100 mg/m2 instead of 200 mg/m2) and the transplant procedure was repeated twice5. In a single center’s experience, “young” patients (age range 30–65) who received high-dose melphalan (HDM/ASCT (200 mg/m2)) and “elderly” patients (age range 66–75) who received two cycles of HDM/ASCT (100 mg/m2) were compared17. The analysis demonstrated no significant difference in progression-free survival (PFS), OS, or treatment-related mortality between the two groups and among all subgroups17. Interestingly, PFS and OS in “elderly” patients appeared to improve after 2008, due to the increased incorporation of novel agents in the treatment, thus leading to the conclusion that the combination of ASCT and novel-based regimens were not subject to the influence of age on treatment outcome17.
Currently, in the United States, fit patients up to 75 years old, receive ASCT16. On the other hand, in Europe, autologous transplants go up to the age of 70 off-protocol18–20. The field of transplantation among elderly patients still lags behind and awaits randomized controlled trials (RCTs) to synthesize solid guidelines.
Patient has renal impairment
Renal impairment, per se, is not a contraindication to receiving HDT/ASCT. Nonetheless, it is a prompt reason to consider lower doses of therapy, as patients with renal impairment are more likely to suffer from HDM toxicities21,22. Studies, including the DAUTOS observational study of the Polish myeloma study group, demonstrated that dialysis-dependent patients were more likely to develop toxicities and complications such as mucositis and infections, but had PFS and OS comparable to matched patients with normal renal function23,24. Also, the dose of melphalan mattered, with patients achieving better outcomes with 200 mg/m2 (ref. 24). Interestingly, a proportion of patients were able to attain dialysis-independence after transplantation24. RCTs are yet to pave the way to guidelines regarding this transplantation scenario.
What is the best induction treatment prior to ASCT?
The role of induction chemotherapy prior to HDT/ASCT is to decrease tumor burden, thus deepening the response rate and increasing the likelihood of engraftment, while retaining the maximum possible tolerability and minimum possible toxicity on normal hematopoietic cells. As a result, and prior to the introduction of novel agents, alkylating agents were avoided during induction, and regimens were dexamethasone-based such as the VAD regimen (vincristine, doxorubicin and dexamethasone)1. With the advent of new drugs, multiple trials have proven the superiority of induction regimens containing one or two novel agents (thalidomide or bortezomib) over the VAD regimen in increasing CR, CR plus near-complete response (nCR), or VGPR rates pre- and post ASCT25–28. Trials that compared two-drug (TD: thalidomide–dexamethasone or VD: bortezomib–dexamethasone) to three-drug induction (VTD: bortezomib, thalidomide, dexamethasone) have proven supremacy of the latter combination13,29,30. VTD was also proven superior to bortezomib, cyclophosphamide, and dexamethasone (VCD), thus highlighting the synergistic effect of combining an IMiD with bortezomib and dexamethasone31. As such, VTD became a standard induction regimen, whereby the role of a PI such as bortezomib is irreplaceable due to its demonstrated usefulness in high-risk patients26,32,33. Furthermore, although the general practice is to use 3–4 cycles of VTD before transplant, the use of 6 cycles of VTD was associated with deeper responses. This is to be weighed against increased side effects, specifically neuropathy, upon administering 6 cycles instead of 3–4 (ref. 30).
Similarly, the two-drug regimen, lenalidomide and dexamethasone (RD), was compared to bortezomib, lenalidomide, and dexamethasone (VRD) whereby VRD resulted in significantly increased PFS, response duration, and OS resulting in the IFM introducing VRD as induction8,34,35. In addition, the PETHEMA/GEM trial investigated induction with VRD-GEM with full dose lenalidomide from days 1 to 21, demonstrating an ORR of 85% post induction and 58% of patients achieving MRD-negativity post consolidation36.
Daratumumab (DARA), an anti-CD38+ monoclonal antibody, has been evaluated in patients with refractory disease37,38. The Cassiopeia phase III trial and the Griffin phase II trial compare DARA-VTD to VTD and DARA-VRD to VRD, respectively, demonstrating hopeful results of adding daratumumab39–41. Daratumumab plus cyclophosphamide, bortezomib, and dexamethasone (Dara-CyBorD) during induction was investigated in the phase II Lyra trial. Recent updates of the trial demonstrate activity and tolerability of Dara-CyBorD irrespective of high-risk cytogenetics with 12-month PFS and OS rates of 87% and 99%, respectively42. Finally, daratumumab is also being combined with carfilzomib, lenalidomide, and dexamethasone (KRD) in a phase Ib trial, whereby the combined regimen yielded 100% ORR, 91% ≥VGPR, and 43% ≥CR, with no negative impact on stem cell harvesting while retaining consistency of the DARA-KRD safety profile43.
MRD negativity, defined as the absence of disease within one million bone marrow cells, has been examined due to its important prognostic value at different stages of the transplantation process. The depth of response after induction and before ASCT determines patients’ prognoses after ASCT since the quality of response post induction and prior to ASCT are predictive of long-term PFS post ASCT13,33,44–49. The final analysis of the IFM2009 prospective trial demonstrated the significance of MRD negativity, whereby patients achieving MRD negativity after induction with VRD had a similar OS irrespective of whether they received an ASCT or not50. In addition, MRD negativity proved to be a more powerful predictor of outcome than cytogenetics, whereby patients with high-risk cytogenetics who achieved MRD negativity had better outcomes than patients with standard-risk cytogenetics who did not50. This could mean that MRD could potentially become essential in stratifying patients during maintenance and consolidation randomization and when deciding on maintenance duration50.
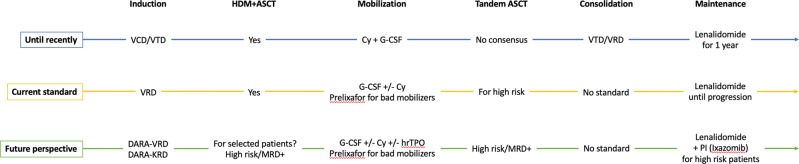
With no evidence that four-drug regimens (IMiD, PI, alkylating agent, and steroid) are superior51,52, VTD and VRD remain the most currently used pre-transplant induction regimens, awaiting the results of ongoing trials testing the efficacy of adding daratumumab or the possible substitution of bortezomib with carfilzomib, which has been found to be safe and well tolerated with exceptional response rates (Fig. 1)53,54.
Fig. 1. .
Evolution of ASCT in multiple myeloma
What is the best stem cell mobilization procedure prior to ASCT?
A critical and essential step prior to ASCT is mobilization of hematopoietic stem cells (HSC) from the bone marrow to be harvested in peripheral blood. The minimum CD34+ stem cell dose considered sufficient for successful engraftment is 2 × 106 cells CD34+/kg, but the optimal target is usually set at 5 × 106 CD34+/kg. This can be done by steady-state mobilization with granulocyte colony-stimulating factors (G-CSF)55 or chemo-mobilization by the addition of chemotherapeutic agent(s)5. Currently, two G-CSF cytokines are approved for the mobilization of autologous HSC: filgrastim (10 µg/kg/day for 4–6 consecutive days and apheresis on days 5 or 6) and lenograstim (10 µg/kg/day for 4–6 days and apheresis between days 5 and 7)5. Even though tolerable, the use of G-CSF cytokines can yield suboptimal peripheral HSC harvest56. The most commonly used agent for chemo-mobilization is high-dose cyclophosphamide (2–4 g/m2)57, followed by filgrastim or lenograstim (5 µg/kg/day 1–5 days after completion of chemotherapy till last apheresis)5. This strategy can potentially decrease tumor burden, but the time to peripheral blood stem cell (PBSC) harvest is prolonged, with increased side effects5. Since some patients fail to mobilize58–60, the addition of new mobilization agents such as plerixafor, a chemokine-receptor 4 (CXCR4) antagonist, enhanced the stem cell mobilization effect of G-CSF56,61. Even though proven to be highly effective, plerixafor is not widely available62.
Other studies have compared cyclophosphamide to mCVAD (modified cyclophosphamide, vincristine, doxorubicin and dexamethasone) and mCBAD (modified cyclophosphamide, bortezomib, doxorubicin, and dexamethasone) concluding that more intense regimens are not superior to cyclophosphamide alone in mobilization63. A randomized phase III trial compared mobilization with cytarabine (Ara-C) and G-CSF versus G-CSF alone demonstrating improved yields with the addition of Ara-C, but increased hematologic toxicities64. It is noteworthy that employing lenalidomide in induction has been found to be one of the factors compromising stem cell mobilization success65,66, mainly due to lenalidomide upregulating CXCR4 and increasing the binding of stem cells to the stroma67. A prospective randomized phase II sub-study in the Finnish Myeloma Study Group-MM02 trial compared low-dose cyclophosphamide (2 mg/m2) plus G-CSF to G-CSF alone for mobilization in patients who have received lenalidomide during induction68. The addition of cyclophosphamide to G-CSF was superior, although G-CSF could yield similar results in patients receiving no more than three cycles of VRD68. In addition, the addition of plerixafor for mobilization in patients who received lenalidomide has also been proven effective69. As such, with the availability of plerixafor, the prolonged use of lenalidomide does not hinder stem cell mobilization. Finally, combining human recombinant thrombopoietin (hrTPO) to G-CSF and cyclophosphamide improved yields compared to cyclophosphamide and G-CSF alone70.
Therefore, the most currently used regimens are either chemo-mobilization with high-dose cyclophosphamide plus G-CSF or steady-state mobilization alone, with preemptive use of plerixafor when suboptimal mobilization is predicted by a low circulating CD34 count.
What is the optimal conditioning regimen prior to ASCT?
The current accepted standard for HDT is intravenous high-dose melphalan (200 mg/m2). Previous trials attempting to replace this with oral and intravenous busulfan have failed, due to increased toxicity and lack of superiority, respectively71,72. The effect of intravenous busulfan is being studied in a phase III trial whereby HDM is compared to busulfan-melphalan (Bu-Mel: busulfan 130 mg/m2 daily for 4 days followed by two daily doses of melphalan at 70 mg/m2)73. The trial has demonstrated increased PFS with Bu-Mel without a significant difference in response rates73. Higher doses of melphalan (>200 mg/m2), which proved useful for patients with primary refractive or relapsing disease nearly 20 years ago74, are also being investigated. A randomized study comparing conditioning with melphalan 280 to 200 mg/m2 while receiving amifostine demonstrated significantly higher ORR and nCR without an improvement in OS and PFS in patients receiving melphalan 280 mg/m2 at the cost of higher incidence of grade 2–3 mucositis and gastrointestinal toxicities75. Another study demonstrated similar results with deeper responses on melphalan 280 mg/m2 without translating into improved survival76.
Bortezomib’s effect in transplant conditioning was investigated when combined with HDM in the IFM 2014-02 phase III study77. This showed no superiority of Bortezomib-HDM over HDM alone in terms of response rate, OS or PFS77. In addition, the role of bendamustine added to melphalan as part of conditioning is being explored, highlighting improved response rates and PFS78,79.
As such, HDM remains the standard conditioning regimen prior to ASCT awaiting results of clinical trials of other conditioning regimens (if any).
What is the impact of consolidation therapy after ASCT?
The aim of short-term consolidation therapy after HDT/ASCT is to improve disease response with limited toxicity. Incorporating consolidation therapy in patients with a good response after ASCT was found to increase the CR rate and molecular remission, thus prolonging PFS12. The Italian Myeloma study group has previously investigated the effect of VTD versus TD as induction therapy before and as consolidation therapy after double ASCT, demonstrating VTD’s superior influence on CR/nCR rates and PFS13. Similarly, VRD proved superiority in consolidation35. These trials were very encouraging; nonetheless, randomized trials were needed to prove impact.
The second randomization in the EMN02/HO95 trial compared the aftermaths of receiving two cycles of VRD consolidation followed by lenalidomide versus lenalidomide maintenance alone, demonstrating the significant advantage VRD consolidation inferred in prolonging PFS80. Moreover, PFS was prolonged in most of the predefined groups in the study including ISS I and II, low-risk cytogenetics, irrespective of whether patients received VMP (bortezomib, melphalan, and prednisone) or transplantation prior to consolidation80. Nonetheless, VRD consolidation failed to improve PFS in patients with high-risk cytogenetics ((del17p and/or t(4;14) and/or t(14;16))80. This confirms the benefit of VRD consolidation followed by lenalidomide maintenance in younger, newly diagnosed multiple myeloma patients with low-risk disease80.
Along the same line of the EMN02/HO95 trial, the StaMINA phase III trial randomized patients to compare HDM/ASCT plus VRD consolidation plus lenalidomide maintenance, versus tandem HDM/ASCT plus lenalidomide maintenance, versus single HDM/ASCT plus lenalidomide maintenance81. It concluded that the addition of VRD consolidation or a tandem ASCT was not superior to standard ASCT followed by lenalidomide in upfront treatment of newly diagnosed multiple myeloma81.
With the currently available data, the role of post-transplant consolidation remains controversial.
What is the impact of maintenance therapy after ASCT?
Even though HDT/ASCT is the standard frontline treatment for newly diagnosed multiple myeloma patients, ASCT is not curative, and progressions and relapses are common even if CR is attained post-transplant6,7,82. Maintenance therapy is thus added and is expected to be gentle with the safest profile post ASCT, but unlike consolidation, it is administered long-term to deepen the response, prevent progression, and prolong OS5.
Thalidomide, having already been used in different myeloma treatment settings and being an oral agent, has been tested in several randomized trials, most of which demonstrated benefit in terms of response rates but not OS83–85. Thalidomide was repeatedly associated with peripheral neuropathy, fatigue, and other side effects, all of which resulted in patient-reported decreased quality of life despite prolonged duration of disease control85. Thus, when used in the maintenance setting, the dosage and duration should be limited to 100 mg daily and 6–12 months, respectively, as suggested by Spencer et al.’s study86.
Lenalidomide maintenance has been shown to be well tolerated and to dramatically improve PFS and OS (Table 1)87,88. A recent meta-analysis of three RCTs, CALGB, IFM, and GIMEMA, that compared lenalidomide maintenance to placebo or observation, has demonstrated clinically valuable results89. Lenalidomide significantly improved PFS in all subgroups of patients regardless of age, myeloma severity and staging, and induction regimen (52.8 versus 23.5 months), even though patients who had received lenalidomide in induction, or had achieved a deeper response post-transplant, were more likely to benefit from lenalidomide89. OS was also significantly improved in the lenalidomide arm, except in women older than 60 years with poor cytogenetics89. Overall, the addition of lenalidomide reduced the chance of death by a substantial 25%, thus increasing median survival by approximately 2.4 years89. As demonstrated in previous studies, an increased incidence of second primary malignancies, albeit modest, was associated with lenalidomide, though the time to death due to a second primary malignancy did not differ between the two groups89. Such results propose lenalidomide as a standard maintenance drug in transplant-eligible patients89. Recent updates of the Myeloma XI trial’s results were in concordance with the meta-analysis90.
Table 1.
Lenalidomide maintenance trials
| Study | Median follow-up | N | Treatment | Outcome | |
|---|---|---|---|---|---|
| Meta-analysis | 79.5 months | PFS | OS | ||
| IFM | 605 | Lenalidomide | 52.8 months | Median OS not reached | |
| CALGB | 603 | Placebo/Observation | 23.5 months | 86 months | |
| GIMEMA | (HR 0.48; 95% CI 0.41–0.55) | (HR 0.75; 95% CI 0.63–0.9) | |||
| Myeloma XI | 28.7 months | PFS | |||
| 1136 | Lenalidomide | 60.3 months | |||
| 834 | Observation | 30.1 months | |||
| (HR 0.47; 95% CI 0.39–0.57) | |||||
So far in previous trials, lenalidomide has been given in low doses until progression or adverse events develop, and this practice is currently approved by both, FDA and EMA. Given that 30% of cases with premature termination of lenalidomide were attributed to toxicities and second primary malignancies89, the question that remains is regarding the optimal duration of treatment with lenalidomide for safety and cost.
Finally, bortezomib was also tested as part of maintenance, either alone or in combination with IMiDs, demonstrating improved PFS, but not OS28. Nonetheless, bortezomib poses an obstacle due to its subcutaneous/i.v. administration. The first oral PI, ixazomib, is currently being investigated. So far, it appears to have positive effects, with a safety profile comparable to that of lenalidomide alone, and is manageable by dose reductions (Fig. 1)91. Ixazomib was also compared to placebo in the multicenter TOURMALINE-MM3 trial with a median follow-up of 31 months, whereby there was a 39% improvement in PFS and a 28% reduction in progression or death. Ixazomib also allowed for deeper responses to be achieved92.
What is the value of single versus tandem ASCT?
In the 1990s, in an attempt to improve survival and overall outcome, the concept of tandem transplant came about in an era where conventional chemotherapy was the only available drug93. Previous randomized trials had demonstrated improved outcomes with tandem transplantation in terms of PFS and OS even in patients who had not achieved a VGPR after the first transplant1,94. An alternative treatment approach, total therapy 3 (TT3), including induction, tandem ASCT, consolidation, and maintenance, has allowed one of the best results to be achieved (CR/nCR rate of 83%, 2-year PFS of 84%, and 2-year OS of 86%)95.
Long-term analysis of the GMMG-HD2 trial compared single versus tandem transplantation with conditioning with melphalan (200 mg/m2)96. The study proved the non-inferiority of single transplantation compared to tandem in the sense that OS and EFS did not significantly differ96. Nonetheless, the CR rates were significantly improved after the second transplantation96. Due to high drop-out rates, lack of use of novel therapy, and lack of subgroup analysis, the results of this study are to be cautiously interpreted96.
In the era of novel drugs, we needed trials to evaluate the impact of tandem transplantation such as the EMN02/HO95 and StaMINA trials81,97. The EMN02/HO95 trial explored the result of tandem versus single transplantation in newly diagnosed multiple myeloma patients97. Tandem transplantation was shown to improve the depth of the response by 25% with more than 50% of the patients achieving at least a CR97. PFS and OS were significantly improved after a second transplant, with approximately 30% reduction in the risk of death and progression97. Updated results of the EMN02/HO95 confirmed the improved 3-year PFS from 63 months after one ASCT to 73.1 after two ASCTs98. Importantly, the positive effect of tandem ASCT was seen in high-risk groups, in which randomization to receive double ASCT was found to be an independent predictor of PFS97,98. The analysis thus concluded that double frontline ASCT was superior to single ASCT in terms of PFS and OS in all patients, including poor prognosis subgroups, indicating that the latter were the most likely to benefit97,98.
On the other hand, the StaMINA trial failed to show superiority of tandem versus single transplant in the era of novel agents81. It is noteworthy that more than 30% of patients randomized to tandem transplant did not receive the second transplant81.
Overall, with the currently available data, a second ASCT may be beneficial in high-risk patients including patients with high-risk cytogenetics and RISS 3 category of disease.
What is the added value of HDT/ASCT in the era of triple novel agent regimens?
With the advent of novel agents, it becomes questionable whether or not HDT/ASCT has any added value at all. The previously mentioned SWOG S0777 trial compared outcomes of lenalidomide and dexamethasone alone (RD) to bortezomib, lenalidomide, and dexamethasone (VRD) without an intent to transplant34. The results confirmed the superiority of VRD in increasing PFS, response duration, and OS34. As such, it is suggested that VRD alone is not only safe but has comparable PFS/OS to HDT/ASCT. Trials were thus necessary to compare novel agents in combination to ASCT to novel agents alone.
A randomized phase III trial for the IFM2009 was conducted to compare the efficacy of combination therapy with lenalidomide, bortezomib, and dexamethasone (RVD) alone to RVD plus HDT/ASCT in newly diagnosed multiple myeloma patients younger than 65 years old8. Patients were randomized so as to receive induction therapy with three cycles of RVD, and then consolidation with either five more cycles of RVD or high-dose melphalan followed by ASCT and two cycles of RVD8. All patients received lenalidomide maintenance for 1 year8. The use of transplantation in addition to novel agents as opposed to RVD alone resulted in significant improvement in PFS (50 versus 36 months, adjusted HR 0.65), CR rate (59% versus 48%), MRD negativity (79% versus 65%), and median time to disease progression (50 versus 36 months), with no advantage regarding OS8. In the phase III EMN02/HO95 study mentioned earlier, the first randomization compared the outcomes of HDT/ASCT (single or double) versus bortezomib–melphalan–prednisone (VMP) after induction with VCD97. Even though bortezomib has been repeatedly shown to increase PFS and OS, upfront ASCT was associated with decreased risk of progression and death and improved 3-year PFS irrespective of initial prognostic factors97.
On the other hand, two studies whereby transplantation was compared to alkylating agent-based regimens and lenalidomide associated a survival benefit with first-line transplantation99,100. Nonetheless, these trials did not incorporate bortezomib in their non-transplant arm which could explain the improved OS99,100.
The extent of improved PFS in the transplant arm in both EMN02/HO95 and IFM2009 trials, likely attributed to a deeper response through increased CR and MRD rates, suggest that given more observational time, we could possibly find an improvement in OS as was the case for lenalidomide maintenance. This is especially relevant given that relapsed patients receive comparable treatment including a second ASCT and the use of newly introduced agents.
The next challenge is to evaluate the necessity of HDT/ASCT when a monoclonal antibody such as daratumumab is added to a powerful induction regimen combining an IMiD and a PI, and whether this strategy can cure a fraction of patients. As such, we conclude that ASCT remains first line even in the era of novel agents. The impending challenge remains whether or not transplantation will be later substituted by less intensive novel agent combinations.
What is the value of early versus late ASCT?
Frontline HDT/ASCT has been the standard for treating newly diagnosed multiple myeloma in young, fit patients and select elderly patients. Nonetheless, with the advent of present novel therapies, specialists have challenged the notion that HDT/ASCT should be administered early after diagnosis.
In 1998 before the era of novel agents, Fermand et al.101 studied the effect of autologous transplantation timing (early versus late) on OS. Patients who were randomized into the “early” arm received HDT/ASCT right away and those in the “late” arm received conventional chemotherapy until progression or relapse whereby they were supported with HDT/ASCT as well101. There was no difference in OS between the two groups101. Time without symptoms, treatment and treatment toxicity (TWiST) was also evaluated whereby the period spent without chemotherapy was longer in patients who received early HDT/ASCT, suggesting a clinical benefit of early versus late transplantation101. Several retrospective trials failed to demonstrate benefits in OS when comparing early to late HDT/ASCT, which could be attributed to selection bias regarding patients in the “late” group102.
As previously mentioned for the IFM2009 trial, comparing VRD to VRD plus transplant yielded significantly better outcome with upfront ASCT in terms of CR rate, PFS, and MRD negativity8. This highlights that, even in light of novel agents which have already been proven to drastically improve treatment outcomes, transplantation could further improve results. Nonetheless, OS was not affected by ASCT taking into account that transplantation was only done in two-third of the cases due to age, progression, and comorbidities, indicating that the benefits of upfront ASCT can be weighed against the toxicities of chemotherapy and transplantation, especially since late transplantation could secure a similar OS to early transplantation8. As such, in the absence of improvement in OS, delayed ASCT could be an option.
In the phase III EMN02/HO95 study mentioned earlier, the first randomization compared the outcomes of HDT/ASCT (single or double) versus VMP after induction with VCD97. Even though bortezomib has been repeatedly shown to increase PFS and OS, upfront ASCT was associated with a 24% reduction in risk of progression or death97. The estimated 3-year PFS was also significantly higher with upfront ASCT, regardless of the presence of poor prognostic factors97.
As such, it is safe to conclude that ASCT can improve outcomes whether performed as first line or as a rescue treatment1,101. Therefore, frontline ASCT remains the standard of treatment for fit, young and select elderly patients with newly diagnosed multiple myeloma.
What is the role of ASCT as salvage therapy?
Salvage therapy is defined as ASCT given to a patient with signs of disease progression after an earlier ASCT103. By the BSBMT/UKMF Myeloma X trial, salvage ASCT with 200 mg/m2 melphalan was superior to cyclophosphamide 400 mg/m2 weekly for 12 weeks upon relapse and re-induction with VAD104. The time to disease progression (19 versus 11 months) and OS (67 versus 52 months) were significantly in favor of salvage ASCT104. As such, ASCT can be considered for salvage in fit patients if the interval between the first ASCT and relapse is 18 months or more5,105. This awaits trials that compare salvage ASCT with novel agents including the German study by Goldschmidt et al. comparing salvage ASCT to lenalidomide/dexamethasone.
Conclusion and future prospects
Up until today, 30 years after its introduction, HDT/ASCT remains the standard of care for patients with newly diagnosed multiple myeloma. Despite the advent of novel agents, ASCT remains a very common treatment modality, especially in Europe and is included in all ongoing and proposed trials. The latter is yet to be challenged by many novel agents (including earlier use of CAR T cells) which are continuously explored. As such, and by projecting the results of the ALCYONE trial which proved superiority of DARA-VMP over VMP in transplant-ineligible patients106, we conclude that there appears to be a place for daratumumab in future induction regimens, due to its promising preliminary additive effect to triple regimens in terms of response. Multiple trials are currently looking into daratumumab in transplant-ineligible patients with recently released updates, suggesting that the addition of daratumumab allows deeper response, increased PFS, and improved MRD negativity107. Such results could be possibly extrapolated to the subset of patients who are newly diagnosed and are transplant eligible. Since melphalan, a cornerstone in conditioning prior to ASCT, is usually given at a fixed dose, patients can either reach concentrations that are above the median, thus sustaining survival but suffering increased toxicity or they can fail to reach median concentrations and suffer from untreated disease. To overcome that, pharmacokinetic (PK)-directed melphalan dosing is looked into as a potential means to determine optimal dosing in individual patients. Whether novel melphalan formulations such as FDA-approved propylene glycol-free melphalan have increased efficacy and decreased toxicity compared to melphalan is to be investigated. One study failed to demonstrate superiority of propylene glycol-free melphalan over melphalan108. Whether or not to embark on early transplantation is yet to be investigated; although it has been made clear that ASCT is synergistic to triple novel agent-based therapy, it could be delayed when toxicities outweigh benefits. When dealing with unfavorable cytogenetics and poor prognostic factors, tandem transplantation appears to be an encouraging strategy. Whereas the use of post-transplant consolidation is controversial, lenalidomide maintenance prolongs PFS and OS and can be considered as a standard of care. One important endpoint to be measured in future studies is MRD negativity in standard or high-risk disease, since it is a significant predictor of PFS, OS, and potential cure in a fraction of patients109. The objective is thus to achieve MRD negativity (<10−6), a predictor of better outcome110. To possibly achieve MRD negativity at even lower cutoff, daratumumab will likely need to be added. Finally, even though first line for multiple myeloma treatment, the use of transplantation remains limited by accessibility and availability which differs across nations and is limited in developing countries.
Conflict of interest
M.M. discloses lectures honoraria and research support from Amgen, Celgene, Janssen, Sanofi, and Takeda whose products are discussed in this work.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Al Hamed Rama, Bazarbachi Abdul Hamid
References
- 1.Harousseau JL, Moreau P. Autologous hematopoietic stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2009;360:2645–2654. doi: 10.1056/NEJMct0805626. [DOI] [PubMed] [Google Scholar]
- 2.McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983;2:822–824. doi: 10.1016/S0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- 3.Barlogie B, Hall R, Zander A, Dicke K, Alexanian R. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood. 1986;67:1298–1301. [PubMed] [Google Scholar]
- 4.Barlogie B, et al. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood. 1987;70:869–872. [PubMed] [Google Scholar]
- 5.Mohty M, Harousseau JL. Treatment of autologous stem cell transplant-eligible multiple myeloma patients: ten questions and answers. Haematologica. 2014;99:408–416. doi: 10.3324/haematol.2013.096149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attal M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N. Engl. J. Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 7.Child JA, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 8.Attal M, et al. Lenalidomide, Bortezomib, and Dexamethasone with transplantation for myeloma. N. Engl. J. Med. 2017;376:1311–1320. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koreth J, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol. Blood Marrow Transplant. 2007;13:183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Barlogie B, van Rhee F, Shaughnessy JD, Jr, Anaissie E, Crowley J. Making progress in treating multiple myeloma with total therapies: issue of complete remission and more. Leukemia. 2008;22:1633–1636. doi: 10.1038/leu.2008.40. [DOI] [PubMed] [Google Scholar]
- 11.Paiva B, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J. Clin. Oncol. 2011;29:1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- 12.Ladetto M, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J. Clin. Oncol. 2010;28:2077–2084. doi: 10.1200/JCO.2009.23.7172. [DOI] [PubMed] [Google Scholar]
- 13.Cavo M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 14.Moreau P, Avet-Loiseau H, Harousseau JL, Attal M. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J. Clin. Oncol. 2011;29:1898–1906. doi: 10.1200/JCO.2010.32.5878. [DOI] [PubMed] [Google Scholar]
- 15.Facon T, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 16.Badros A, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br. J. Haematol. 2001;114:600–607. doi: 10.1046/j.1365-2141.2001.02976.x. [DOI] [PubMed] [Google Scholar]
- 17.Judith Neukirchen PA, et al. Favourable outcome of elderly patients with multiple myeloma treated with tandem melphalan 100 high-dose therapy, autologous stem cell transplantation and novel agents—a single center experience. Blood. 2016;128:3460. [Google Scholar]
- 18.Palumbo A, et al. Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood. 1999;94:1248–1253. [PubMed] [Google Scholar]
- 19.Gay F, et al. Bortezomib induction, reduced-intensity transplantation, and lenalidomide consolidation-maintenance for myeloma: updated results. Blood. 2013;122:1376–1383. doi: 10.1182/blood-2013-02-483073. [DOI] [PubMed] [Google Scholar]
- 20.Straka C, et al. Autotransplant with and without induction chemotherapy in older multiple myeloma patients: long-term outcome of a randomized trial. Haematologica. 2016;101:1398–1406. doi: 10.3324/haematol.2016.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badros A, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br. J. Haematol. 2001;114:822–829. doi: 10.1046/j.1365-2141.2001.03033.x. [DOI] [PubMed] [Google Scholar]
- 22.Tosi P, et al. Safety of autologous hematopoietic stem cell transplantation in patients with multiple myeloma and chronic renal failure. Leukemia. 2000;14:1310–1313. doi: 10.1038/sj.leu.2401819. [DOI] [PubMed] [Google Scholar]
- 23.Waszczuk-Gajda A, et al. Autologous peripheral blood stem cell transplantation in dialysis-dependent multiple myeloma patients—DAUTOS Study of the Polish Myeloma Study Group. Eur. J. Haematol. 2018;101:475–485. doi: 10.1111/ejh.13101. [DOI] [PubMed] [Google Scholar]
- 24.Mahindra A, et al. Autologous hematopoietic cell transplantation for multiple myeloma patients with renal insufficiency: a center for international blood and marrow transplant research analysis. Bone Marrow Transplant. 2017;52:1616–1622. doi: 10.1038/bmt.2017.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokhorst HM, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115:1113–1120. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 26.Harousseau JL, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J. Clin. Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 27.Morgan GJ, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97:442–450. doi: 10.3324/haematol.2011.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonneveld P, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J. Clin. Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 29.Moreau P, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–5758. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 30.Rosinol L, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 31.Moreau P, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569–2574. doi: 10.1182/blood-2016-01-693580. [DOI] [PubMed] [Google Scholar]
- 32.Avet-Loiseau H, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p) J. Clin. Oncol. 2010;28:4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- 33.Sonneveld P, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J. Clin. Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- 34.Durie BG, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–527. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roussel M, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J. Clin. Oncol. 2014;32:2712–2717. doi: 10.1200/JCO.2013.54.8164. [DOI] [PubMed] [Google Scholar]
- 36.Rosinol L, et al. Bortezomib, Lenalidomide and Dexamethasone (VRD-GEM) as induction therapy prior autologous stem cell transplantation (ASCT) in multiple myeloma (MM): results of a prospective phase III Pethema/GEM Trial. Blood. 2017;130(Suppl 1):2017. [Google Scholar]
- 37.Lonial S, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387:1551–1560. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 38.Costello C. An update on the role of daratumumab in the treatment of multiple myeloma. Ther. Adv. Hematol. 2017;8:28–37. doi: 10.1177/2040620716677523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genmab. Genmab announces positive topline results in Phase III CASSIOPEIA Study of Daratumumab in front line multiple myeloma. https://ir.genmab.com/news-releases/news-release-details/genmab-announces-positive-topline-results-phase-iii-cassiopeia (2018).
- 40.Voorhees, P. M. et al. Interim safety analysis of a phase 2 randomized study of Daratumumab (Dara), Lenalidomide (R), Bortezomib (V), and Dexamethasone (d; Dara-RVd) vs. RVd in patients (Pts) with newly diagnosed multiple myeloma (MM) eligible for high-dose therapy (HDT) and autologous stem cell transplantation (ASCT). Blood130(Suppl 1), 1879 (2017).
- 41.Voorhees, P. M. et al. Efficacy and updated safety analysis of a safety run-in cohort from Griffin, a phase 2 randomized study of Daratumumab (Dara), Bortezomib (V), Lenalidomide (R), and Dexamethasone (D; Dara‐Vrd) vs. Vrd in patients (Pts) with newly diagnosed (ND) multiple myeloma (MM) eligible for high‐dose therapy (HDT) and autologous stem cell transplantation (ASCT). Blood132(Suppl 1), 151 (2018).
- 42.Yimer, H. et al. Lyra: a phase 2 study of Daratumumab (Dara) plus Cyclophosphamide, Bortezomib, and Dexamethasone (Cybord) in newly diagnosed and relapsed patients (Pts) with multiple myeloma (MM). Blood, 132(Suppl 1), 152 (2018).
- 43.Andrzej J, et al. Daratumumab (DARA) in combination with Carfilzomib, Lenalidomide, and Dexamethasone (KRd) in patients (pts) with newly diagnosed multiple myeloma (MMY1001): an open-label, phase 1b study. J. Clin. Oncol. 2017;35:8000–8000. doi: 10.1200/JCO.2017.35.15_suppl.8000. [DOI] [Google Scholar]
- 44.Cavo M, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063–6073. doi: 10.1182/blood-2011-02-297325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125:3076–3084. doi: 10.1182/blood-2014-09-568915. [DOI] [PubMed] [Google Scholar]
- 46.Anderson KC, et al. Multiple myeloma, Version 2.2016: clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2015;13:1398–1435. doi: 10.6004/jnccn.2015.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gertz MA, Dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood. 2014;124:882–890. doi: 10.1182/blood-2014-03-544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreau P, et al. Achievement of VGPR to induction therapy is an important prognostic factor for longer PFS in the IFM 2005-01 trial. Blood. 2011;117:3041–3044. doi: 10.1182/blood-2010-08-300863. [DOI] [PubMed] [Google Scholar]
- 49.Binder M, et al. Predictors of early response to initial therapy in patients with newly diagnosed symptomatic multiple myeloma. Am. J. Hematol. 2015;90:888–891. doi: 10.1002/ajh.24107. [DOI] [PubMed] [Google Scholar]
- 50.Avet-Loiseau, H. L.-C. V. et al. Minimal residual disease in multiple myeloma: final analysis of the IFM2009 trial. Abstract #435. In Presented at the 2017 American Society of Hematology Annual Meeting, 10 December, 2017, Atlanta, GA (2017).
- 51.Ludwig H, et al. Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. J. Clin. Oncol. 2013;31:247–255. doi: 10.1200/JCO.2011.39.5137. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 53.Sonneveld P, et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood. 2015;125:449–456. doi: 10.1182/blood-2014-05-576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakubowiak AJ, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohty M, Ho AD. In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp. Hematol. 2011;39:723–729. doi: 10.1016/j.exphem.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Mohty M, et al. The role of plerixafor in optimizing peripheral blood stem cell mobilization for autologous stem cell transplantation. Leukemia. 2011;25:1–6. doi: 10.1038/leu.2010.224. [DOI] [PubMed] [Google Scholar]
- 57.Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–195. doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- 58.Larsen SR, Chng K, Battah F, Martiniello-Wilks R, Rasko JE. Improved granulocyte colony-stimulating factor mobilization of hemopoietic progenitors using cytokine combinations in primates. Stem Cells. 2008;26:2974–2980. doi: 10.1634/stemcells.2008-0560. [DOI] [PubMed] [Google Scholar]
- 59.Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol. Blood Marrow Transplant. 2012;18:1191–1203. doi: 10.1016/j.bbmt.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Desikan KR, et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J. Clin. Oncol. 1998;16:1547–1553. doi: 10.1200/JCO.1998.16.4.1547. [DOI] [PubMed] [Google Scholar]
- 61.Russell N, et al. Plerixafor and granulocyte colony-stimulating factor for first-line steady-state autologous peripheral blood stem cell mobilization in lymphoma and multiple myeloma: results of the prospective PREDICT trial. Haematologica. 2013;98:172–178. doi: 10.3324/haematol.2012.071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Mel S, et al. Vinorelbine-Cyclophosphamide compared to Cyclophosphamide in peripheral blood stem cell mobilization for multiple myeloma. Hematol. Oncol. Stem Cell Ther. 2018;11:225–232. doi: 10.1016/j.hemonc.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Gettys SC, et al. Modified CVAD and modified CBAD compared to high-dose cyclophosphamide for peripheral blood stem cell mobilization in patients with multiple myeloma. Eur. J. Haematol. 2017;98:388–392. doi: 10.1111/ejh.12843. [DOI] [PubMed] [Google Scholar]
- 64.Czerw Tomasz, Sadus-Wojciechowska Maria, Michalak Katarzyna, Najda Jacek, Mendrek Wlodzimierz, Sobczyk-Kruszelnicka Malgorzata, Glowala-Kosinska Magdalena, Chwieduk Agata, Mitrus Iwona, Smagur Andrzej, Holowiecki Jerzy, Giebel Sebastian. Increased Efficacy of Stem Cell Chemomobilization with Intermediate-Dose Cytarabine Plus Granulocyte Colony-Stimulating Factor (G-CSF) Compared with G-CSF Alone in Patients with Multiple Myeloma: Results of a Randomized Trial. Biology of Blood and Marrow Transplantation. 2019;25(2):248–255. doi: 10.1016/j.bbmt.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 66.Mazumder A, et al. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2008;22:1280–1281. doi: 10.1038/sj.leu.2405035. [DOI] [PubMed] [Google Scholar]
- 67.Li S, Fu J, Ma H, Mapara MY, Lentzsch S. Lenalidomide-induced upregulation of CXCR4 in CD34+ hematopoietic cells, a potential mechanism of decreased hematopoietic progenitor mobilization. Leukemia. 2013;27:1407–1411. doi: 10.1038/leu.2012.323. [DOI] [PubMed] [Google Scholar]
- 68.Silvennoinen R, et al. A randomized phase II study of stem cell mobilization with cyclophosphamide+G-CSF or G-CSF alone after lenalidomide-based induction in multiple myeloma. Bone Marrow Transplant. 2016;51:372–376. doi: 10.1038/bmt.2015.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malard F, et al. Plerixafor for autologous peripheral blood stem cell mobilization in patients previously treated with fludarabine or lenalidomide. Biol. Blood. Marrow Transplant. 2012;18:314–317. doi: 10.1016/j.bbmt.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Wang G, et al. Recombinant human thrombopoietin improves the efficacy of intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor in mobilizing peripheral blood stem cells in patients with multiple myeloma: a cohort study. Medicine (Baltimore) 2017;96:e9302. doi: 10.1097/MD.0000000000009302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lahuerta JJ, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 2010;95:1913–1920. doi: 10.3324/haematol.2010.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blanes M, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol. Blood Marrow Transplant. 2013;19:69–74. doi: 10.1016/j.bbmt.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Qazilbash, M. H. et al. A randomized phase III trial of Busulfan+Melphalan vs Melphalan alone for multiple myeloma. Blood 130(Suppl 1), 399 http://www.bloodjournal.org/content/130/Suppl_1/399. Accepted 20 Dec 2018. (2017).
- 74.Moreau P, et al. Melphalan 220 mg/m2 followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone Marrow Transplant. 1999;23:1003–1006. doi: 10.1038/sj.bmt.1701763. [DOI] [PubMed] [Google Scholar]
- 75.Bensinger WI, et al. A randomized study of melphalan 200 mg/m(2) vs 280 mg/m(2) as a preparative regimen for patients with multiple myeloma undergoing auto-SCT. Bone Marrow Transplant. 2016;51:67–71. doi: 10.1038/bmt.2015.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hari P, et al. Final outcomes of escalated melphalan 280 mg/m(2) with amifostine cytoprotection followed autologous hematopoietic stem cell transplantation for multiple myeloma: high CR and VGPR rates do not translate into improved survival. Bone Marrow Transplant. 2019;54:293–299. doi: 10.1038/s41409-018-0261-y. [DOI] [PubMed] [Google Scholar]
- 77.Roussel M, et al. Bortezomib and high-dose Melphalan vs. high-dose melphalan as conditioning regimen before autologous stem cell transplantation in de novo multiple myeloma patients: a phase 3 study of the Intergroupe Francophone Du Myelome (IFM 2014-02) Blood. 2017;130(Suppl 1):398. doi: 10.1182/blood-2009-06-229658. [DOI] [PubMed] [Google Scholar]
- 78.Farag S, et al. Dose-intensified bendamustine and melphalan (BenMel) conditioning before second autologous transplantation in myeloma patients. Hematol. Oncol. 2018;36:671–678. doi: 10.1002/hon.2546. [DOI] [PubMed] [Google Scholar]
- 79.Martino M, et al. A phase II, single-arm, prospective study of bendamustine plus melphalan conditioning for second autologous stem cell transplantation in de novo multiple myeloma patients through a tandem transplant strategy. Bone Marrow Transplant. 2016;51:1197–1203. doi: 10.1038/bmt.2016.94. [DOI] [PubMed] [Google Scholar]
- 80.Sonneveld, P. et al. Consolidation Followed by Maintenance vs Maintenance Alone in Newly Diagnosed, Transplant Eligible Multiple Myeloma: A Randomized Phase 3 Study of Tte European Myeloma Network (Emn02/Ho95 Mm Trial). 214488 (EHA Learning Center Sonneveld P, 2018).
- 81.Stadtmauer EA, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), Bortezomib, Lenalidomide (Len) and Dexamethasone (RVD) consolidation with Len maintenance (ACM), tandem Autohct with Len maintenance (TAM) and Autohct with Len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702-StaMINA Trial) Blood. 2016;128:LBA-1. [Google Scholar]
- 82.Paiva B, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119:687–691. doi: 10.1182/blood-2011-07-370460. [DOI] [PubMed] [Google Scholar]
- 83.Morgan GJ, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119:7–15. doi: 10.1182/blood-2011-06-357038. [DOI] [PubMed] [Google Scholar]
- 84.Ludwig H, et al. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119:3003–3015. doi: 10.1182/blood-2011-11-374249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stewart AK, et al. A randomized phase 3 trial of thalidomide and prednisone as maintenance therapy after ASCT in patients with MM with a quality-of-life assessment: the National Cancer Institute of Canada Clinicals Trials Group Myeloma 10 Trial. Blood. 2013;121:1517–1523. doi: 10.1182/blood-2012-09-451872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spencer A, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J. Clin. Oncol. 2009;27:1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 87.Attal M, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 88.McCarthy PL, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCarthy PL, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J. Clin. Oncol. 2017;35:3279–3289. doi: 10.1200/JCO.2017.72.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson G, et al. Lenalidomide maintenance significantly improves outcomes compared to observation irrespective of cytogenetic risk: results of the Myeloma XI Trial. Blood. 2017;130(Suppl 1):436. [Google Scholar]
- 91.Patel KK, et al. Update on a phase II study of Ixazomib with Lenalidomide as maintenance therapy following autologous stem cell transplant in patients with multiple myeloma. Blood. 2017;130(Suppl 1):437. [Google Scholar]
- 92.Dimopoulos, M. A. et al. Maintenance therapy with the oral proteasome inhibitor (PI) Ixazomib significantly prolongs progression-free survival (PFS) following autologous stem cell transplantation (ASCT) in patients with newly diagnosed multiple myeloma (NDMM): Phase 3 Tourmaline-MM3 Trial. Blood, 132(Suppl 1), 301 (2018).
- 93.Barlogie B, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 94.Attal M, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 95.Barlogie B, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br. J. Haematol. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 96.Mai EK, et al. Single versus tandem high-dose melphalan followed by autologous blood stem cell transplantation in multiple myeloma: long-term results from the phase III GMMG-HD2 trial. Br. J. Haematol. 2016;173:731–741. doi: 10.1111/bjh.13994. [DOI] [PubMed] [Google Scholar]
- 97.Cavo M, et al. Upfront single versus double autologous stem cell transplantation for newly diagnosed multiple myeloma: an intergroup, multicenter, phase III study of the European Myeloma Network (EMN02/HO95 MM Trial) Blood. 2016;128:991. [Google Scholar]
- 98.Cavo, M. et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/H095 study. Blood, 130(Suppl 1), 401 (2017).
- 99.Palumbo A, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N. Engl. J. Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 100.Gay F, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–1629. doi: 10.1016/S1470-2045(15)00389-7. [DOI] [PubMed] [Google Scholar]
- 101.Fermand JP, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 102.Richardson PG, Laubach JP, Munshi NC, Anderson KC. Early or delayed transplantation for multiple myeloma in the era of novel therapy: does one size fit all? Hematol Am. Soc. Hematol. Educ. Program. 2014;2014:255–261. doi: 10.1182/asheducation-2014.1.255. [DOI] [PubMed] [Google Scholar]
- 103.Giralt S, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol. Blood. Marrow Transplant. 2015;21:2039–2051. doi: 10.1016/j.bbmt.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cook G, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3:e340–e351. doi: 10.1016/S2352-3026(16)30049-7. [DOI] [PubMed] [Google Scholar]
- 105.Atanackovic D, Schilling G. Second autologous transplant as salvage therapy in multiple myeloma. Br. J. Haematol. 2013;163:565–572. doi: 10.1111/bjh.12579. [DOI] [PubMed] [Google Scholar]
- 106.Mateos MV, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for untreated myeloma. N. Engl. J. Med. 2018;378:518–528. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 107.Thierry Facon, S. K. K. et al. Phase 3 randomized study of Daratumumab plus Lenalidomide and Dexamethasone (D-Rd) versus Lenalidomide and Dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA). In ASH Annual Meeting Abstracts, Late-Breaking Abstracts Session, LBA-2 (2018).
- 108.Miller KC, et al. Comparable outcomes using propylene glycol-free melphalan for autologous stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2019;54:587–594. doi: 10.1038/s41409-018-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paiva, B. P. N. et al. Impact of next-generation flow (NGF) minimal residual disease (MRD) monitoring in multiple myeloma (MM): results from the Pethema/GEM2012 Trial. Blood, ASH Annual Meeting Abstracts, Vol. 130, Suppl.1, 905 (2017).
- 110.Perrot A, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132:2456–2464. doi: 10.1182/blood-2018-06-858613. [DOI] [PMC free article] [PubMed] [Google Scholar]