Introduction
Bullous pemphigoid (BP) is a blistering autoimmune disease caused by autoantibodies that target 2 hemidesmosomal components: the transmembrane collagen XVII (BP180/BPAG2) and plakin family protein BP230 (BPAG1).1
Diagnosis of drug-induced BP is similar to that of idiopathic BP and is made when there is typical histology of subepidermal blisters with intradermal vesicles and necrotic keratinocytes.2 Prominent eosinophilic infiltrates are often present in the blister cavity and perilesional skin.3 The most sensitive test is direct immunofluorescence, which shows linear deposition of IgG at the dermoepidermal junction, while indirect immunofluorescence identifies circulating BPAG1 and BPAG2 autoantibodies.1 Because drug-induced and spontaneous BP can look identical on histology and immunofluorescence studies, a specific diagnosis is difficult.
Several different medications such as antibiotics, antihypertensives, and biologic therapies have been implicated as the cause of BP.3 The immune system has many central and peripheral checkpoints that maintain a balance between tissue growth and destruction. As a result, drugs that target these checkpoints have been linked to several immune-related adverse events.4 Pembrolizumab is an effective anti–programmed cell death 1 (PD-1) monoclonal antibody that is used in the treatment of metastatic melanoma, allowing activation of host T-cell responses against tumor cells.5 The most common immune-mediated side effects affect the skin, including lichenoid reactions, eczema, and vitiligo.6 Here we describe 2 patients who had BP after receiving treatment with pembrolizumab.
Case 1
An 86-year-old woman had metastatic melanoma diagnosed in March 2017. The first cycle of pembrolizumab, 200 mg infusion, was given in April 2017 and then every 3 weeks. In August 2018, a pruritic, erythematous, vesicular eruption developed on her bilateral upper extremities. Treatment with betamethasone, 0.05% ointment 2 times daily, and antihistamines was initiated. A 4-mm punch biopsy of a lesion on her back found a subepidermal bulla with eosinophils consistent with BP. Treatment with betamethasone 0.05% cream and pembrolizumab was continued until acute worsening 1 month later. Scattered erythematous plaques with bullae on an erythematous background developed on her chest, back, and arms. She also reported severe pruritus. Enzyme-linked immunosorbent assay testing was positive for BP180 IgG and negative for BP230 IgG. Treatment with prednisone, 60 mg with slow taper, was initiated, and pembrolizumab was discontinued. The patient responded well to treatment with resolution of bullous lesions 3 months later, while still on a weaning dose of prednisone.
Case 2
An 82-year-old man with history of non-Hodgkin lymphoma and clear cell renal carcinoma had metastatic melanoma diagnosed in July 2017. A subsequent chest computed tomography scan showed progression of disease with mediastinal and hilar nodes. He was determined a poor surgical candidate, and pembrolizumab, 200-mg infusion every 3 weeks, was initiated in September 2017.
After the fourth cycle in November 2017, an erythematous eruption developed on his forehead and face that remained stable for several months. In May 2018, pembrolizumab was held because of a contrast computed tomography scan showing increased size of axillary and pelvic lymphadenopathy with significant right pleural effusion and pleural thickening. A rebiopsy of the left axillary node showed CD19++ and CD20+++ monoclonal B cells suggestive of chronic lymphocytic leukemia Rai stage III combined with metastatic melanoma.
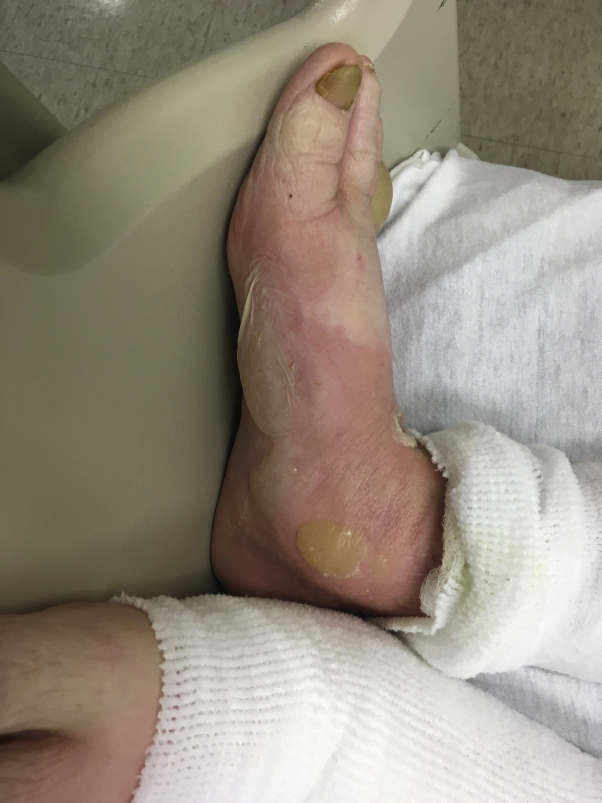
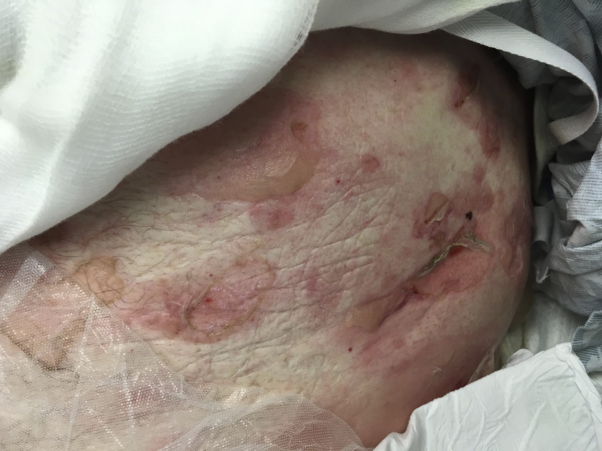
Pembrolizumab infusion therapy was restarted in June 2018 in addition to ibrutinib, 280 mg/d orally. Three days later, the patient had pruritic tense bullae on his ankles (Fig 1), bilateral upper and lower extremities, abdomen (Fig 2), back, groin, and palms. In the hospital, ibrutinib and pembrolizumab were both held while the patient received empiric treatment with intravenous acyclovir and intravenous methylprednisolone, 1 mg/kg. A punch biopsy of the skin found a subepidermal bulla and inflammatory infiltrate with eosinophils consistent with BP. The patient was then treated as an outpatient with prednisone, 60 mg. An acute exacerbation 1 month later required hospitalization during which he was treated with intravenous methylprednisolone, 1 mg/kg/d. A second punch biopsy was performed for tissue to be sent for direct immunofluorescence. The direct immunofluorescence report further supported the diagnosis of BP, as it confirmed linear staining for IgG and C3 at the dermoepidermal junction. The patient was discharged with a tapering course of prednisone, starting at 40 mg/d until re-evaluation a few weeks later. In a follow-up visit, the patient's eruption had begun to heal, but blood glucose levels were noted to be in the 200s to 300s, likely secondary to systemic steroids exacerbating his pre-existing diabetes mellitus.
Fig 1.
Tense bullae on the ankle.
Fig 2.
Tense bullae and erosions on the abdomen.
Over the next few weeks, the patient continued to experience exacerbations as prednisone was tapered off, requiring hospitalization each time in the burn unit for wound care. Serology was positive for BPAg2, consistent with BP. The patient received high-dose oral prednisone for 8 days and was transferred to a skilled nursing facility with a tapering course of prednisone. Over the course of 4 months after discontinuation of pembrolizumab, the patient continued to experience flares of BP after each steroid taper until he died in October 2018.
Discussion
There does not seem to be a difference in specific autoantibodies that cause drug-induced BP and idiopathic BP.2 This finding is supported by the enzyme-linked immunosorbent assay test that was positive for BP180 IgG in the first patient and serology positive for BPAg2 in the second patient.
Although BP is considered a humorally mediated disease, dysfunction in T-regulatory cells and inhibition of the PD-1 pathway with anti–PD-1 therapy have also been implicated in development of autoimmunity. There are several cases of pembrolizumab-induced BP described in the literature with varying times to clinical presentation. A systematic review of 10 cases showed a range of 4 to 84 weeks from initiation of pembrolizumab to cutaneous toxicity without an apparent dose-dependent relationship.7 The variable time to development of disease after starting pembrolizumab seen in the literature and in our cases presents another complicating factor in diagnosing drug-induced BP. Similar to our 2 cases, several patients also developed erythematous papules and plaques before bullae development. Most cases were successfully treated with a combination of systemic and topical steroids with steroid-sparing drugs, such as methotrexate, added for refractory disease.8, 9, 10 In certain cases, including our second patient, exacerbation of bullous lesions may be seen several months after discontinuation of anti–PD-1 therapy.
Although BP is a rare side effect of anti–PD-1 medication, it is important to understand the clinical course to develop effective therapeutic strategies and to consistently monitor cutaneous symptoms while on anti–PD-1 therapy because of the unpredictable timeline of disease progression.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Nishie W. Update on the pathogenesis of bullous pemphigoid: an autoantibody-mediated blistering disease targeting collagen XVII. J Dermatol Sci. 2014;73:179–186. doi: 10.1016/j.jdermsci.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Smith E.P., Taylor T.B., Meyer L.J., Zone J.J. Antigen identification in drug-induced bullous pemphigoid. J Am Acad Dermatol. 1993;29:879–882. doi: 10.1016/0190-9622(93)70262-r. [DOI] [PubMed] [Google Scholar]
- 3.Stavropoulos P.G., Soura E., Antonious C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133–1140. doi: 10.1111/jdv.12366. [DOI] [PubMed] [Google Scholar]
- 4.Kong Yi-Chi M., Flynn J.C. Opportunistic autoimmune disorders potentiated by immune-checkpoint inhibitory anti-CTLA-4 and anti-PD-1. Front Immunol. 2014;5:1–8. doi: 10.3389/fimmu.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquez-Rodas I., Cerezuela P., Soria A. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med. 2015;3:267. doi: 10.3978/j.issn.2305-5839.2015.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang S., Carlos G., Wakade D. Cutaneous adverse events of anti-programmed cell death 1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455–461. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Lopez A.T., Khanna T., Antonov N., Audrey-Bayan C., Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664–669. doi: 10.1111/ijd.13984. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen K., Diernaes J., Øllegaard T., Spaun E., Vestergaard C. Bullous pemphigoid as an adverse reaction to pembrolizumab: two case reports. Case Rep Dermatol. 2018;10(2):154–157. doi: 10.1159/000489661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rofe O., Bar-Sela G., Keidar Z., Sezin T., Sadik C.D., Bergman R. Severe bullous pemphigoid associated with pembrolizumab therapy for metastatic melanoma with complete regression. Clin Exp Dermatol. 2017;42:309–312. doi: 10.1111/ced.13042. [DOI] [PubMed] [Google Scholar]
- 10.Kartha V., Owen C., Callen J.P. Pembrolizumab-induced bullous pemphigoid. J Am Acad Dermatol. 2018;79:218. [Google Scholar]