Introduction
Individuals with Down syndrome (DS) display widespread immune dysregulation, with much increased risk of diverse autoimmune and autoinflammatory skin conditions, including alopecia areata (AA).1, 2 However, the molecular basis of this autoimmune profile remains to be elucidated. Trisomy 21 (T21), the genetic cause of DS, consistently activates the interferon (IFN) response in multiple cell types, causing hypersensitivity to IFN ligands, hyperactivation of downstream Janus kinase/signal transducer activator of transcription (JAK/STAT) signaling, significant overexpression of IFN-stimulated genes,3 and changes in the circulating proteome indicative of chronic autoinflammation with many ties to increased IFN signaling.4 Here we report 2 cases of individuals with DS who were treated for AA with tofacitinib (Xeljanz, Pfizer, New York, NY), a US Food and Drug Administration–approved JAK inhibitor, to remarkable therapeutic benefit. Therefore, we hypothesize that JAK inhibition could have multidimensional benefits in DS.
Case reports
Our ongoing cohort study of people with DS, known as the Crnic Institute's Human Trisome Project (www.trisome.org, Colorado Multiple Institutional Review Board #15-2170), currently includes 2 participants with DS who take tofacitinib for AA and who have shown a remarkable recovery of hair loss. Informed consent and medical records were collected through the Human Trisome Project protocol, and additional authorization was obtained for photo release.
Case 1
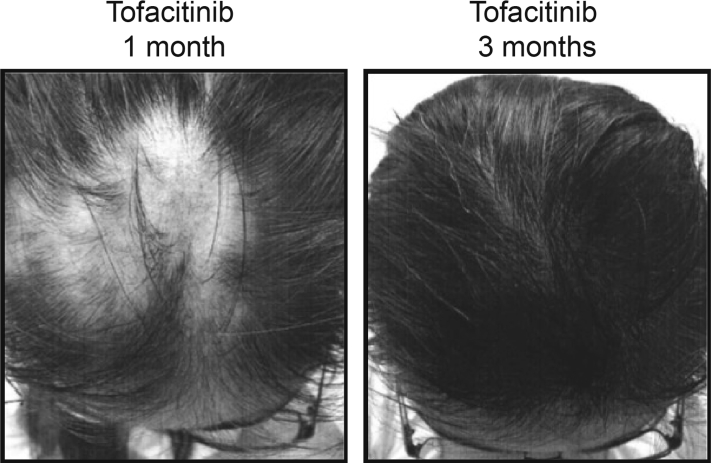
A young adult woman with DS treated with tofacitinib for AA had a stable ophiasis pattern of AA at 6 years old, which is a common pattern in individuals with DS1, 2 that affects the lower occipital scalp area and is typically very resistant to treatment.5 At 16 years, the AA began to spread to additional areas of the scalp, beginning in the occipital scalp and progressing to the parietal scalp, vertex, and frontal regions. A topical steroid (fluocinonide in FAPG cream) was not effective, and injections of corticosteroids (triamcinolone acetonide injectable suspension) were administered monthly at up to 12 scalp sites for 2 years. Although the injections did induce temporary hair growth, eventually more than 50% of the scalp was affected by hair loss. At age 20, she started taking tofacitinib, 5 mg twice daily, and within a month fine hair regrowth was evident in the vertex and frontal areas, and at 3 months hair regrowth was almost complete in these areas (Fig 1). For the first 2 years, the patient cycled on and off tofacitinib at approximate intervals of 5 mg twice daily for 8 weeks, 5 mg once daily for 4 weeks, then off drug for 10 to 12 weeks. The individual consistently relapsed with patchy hair loss approximately 8 to 10 weeks after stopping tofacitinib during each cycle. During the fourth cycle, at 22 years, she experienced an almost complete regrowth of hair in the area affected by ophiasis AA for the first time in 15 years. Most recently, the patient remained at 5 mg once daily for 7 months, then off drug for 8 weeks, followed by 5 mg twice daily for 8 weeks. No hair loss was reported at any time within the last year. Regular laboratory monitoring, including a comprehensive metabolic panel and liver function tests, have been within normal limits. This patient has not reported any undesired side effects in 3 years of tofacitinib use.
Fig 1.
Young woman with DS and AA treated with tofacitinib for nearly 3 years. Pictures show rapid hair recovery during tofacitinib treatment.
Case 2
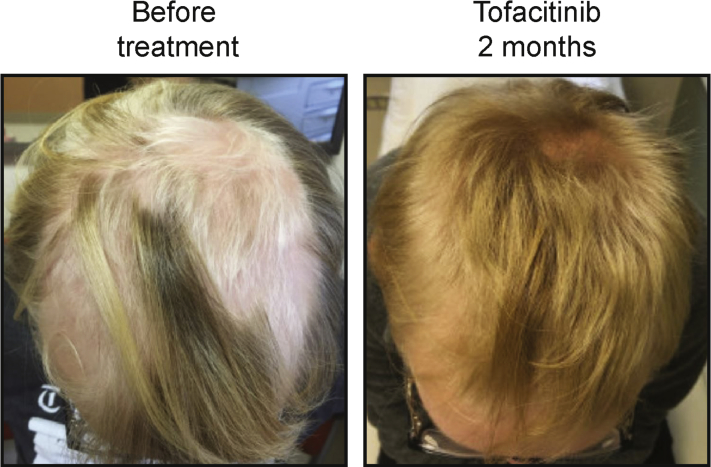
An adolescent male with DS treated with tofacitinib for patchy AA was reported to have thick hair before age 13, when AA developed with intermittent patchy hair loss over the entirety of the scalp. When the participant was 15 years old, AA significantly worsened over the course of 6 months to greater than 50% hair loss at the crown of the head, behind the ears, and the back of the head (Fig 2). Scalp itch, scalp rash, scalp scaling, scalp tenderness, or other hair loss was not noted. At this time, the patient received multiple rounds of intralesional steroid injections within a 3-month period that spurred slight and transient localized hair regrowth. The participant then began twice-daily 5-mg tofacitinib treatment and topical clobetasol external solution (0.05%) once daily, which resulted in fluffy, down-like hair regrowth after 1 week. After 2 months on tofacitinib, the participant showed complete regrowth of hair with no small patches of hair loss on the parietal, occipital, temporal, frontal or vertex areas, and subsequent growth restored the texture and color of his normal hair (Fig 2). The clobetasol solution was discontinued. A complete blood count and comprehensive metabolic panel, including liver function tests, performed after 8 weeks on tofacitinib showed no changes from baseline bloodwork. No undesirable side effects have been noted, and the individual remains on 5 mg tofacitinib twice daily.
Fig 2.
Adolescent male with DS and AA treated with tofacitinib. Note the rapid hair recovery during the initial 2 months of tofacitinib treatment.
Discussion
People with DS are highly predisposed to multiorgan autoimmunity. Several autoimmune skin diseases are more prevalent in DS including AA, atopic dermatitis, psoriasis, and vitiligo.1, 2, 6, 7 T21 causes consistent activation of the IFN response,3 concurrent with changes in the circulating proteome indicative of chronic autoinflammation, including elevated levels of histocompatibility proteins, hypocomplementemia, and increased levels of potent cytokines with established roles in autoimmunity.4 Importantly, these results could be explained by the mere fact that 4 of the 6 IFN receptors are encoded on chr21 (IFNAR1, IFNAR2, IFNGR2, and IL10RB). Given the wealth of evidence showing that IFN signaling contributes to autoimmunity in the typical population,8 we hypothesized that increased IFN signaling is a key driver of autoimmunity in DS and that pharmacologic inhibition of IFN signaling may have broad therapeutic impacts in this population. Recently, several clinical trials have tested JAK inhibitors for immune-driven skin conditions, with very encouraging results. These JAK inhibitors include tofacitinib, which inhibits primarily JAK1 and JAK3, with little impact on JAK2.9 Given that JAK1 (but not JAK3) is required for all IFN signaling, tofacitinib provides a suitable strategy to decrease IFN signaling in people with DS without the hematologic complications associated with JAK2 inhibition observed for other US Food and Drug Administration–approved JAK inhibitors.10 Tofacitinib has extensive safety data, with the most common side effects being headache and diarrhea but including infections and increased risk of some cancers and is currently approved for use in adults with rheumatic arthritis, psoriatic arthritis, and ulcerative colitis. We report these cases to show that JAK inhibitors have therapeutic benefits in DS.
Footnotes
Funding sources: University of Colorado Linda Crnic Institute for Down Syndrome, Global Down Syndrome Foundation, and the Anna and John J. Sie Foundation.
Conflicts of interest: Dr Cory Dunnick discloses that she is the principal investigator for 2 clinical trials with Pfizer using an investigational JAK inhibitor to treat alopecia areata. All clinical trial reimbursement is paid to the University of Colorado. The rest of the authors have no conflicts to disclose.
References
- 1.Madan V., Williams J., Lear J.T. Dermatological manifestations of Down's syndrome. Clin Exp Dermatol. 2006;31(5):623–629. doi: 10.1111/j.1365-2230.2006.02164.x. [DOI] [PubMed] [Google Scholar]
- 2.Sureshbabu R., Kumari R., Ranugha S., Sathyamoorthy R., Udayashankar C., Oudeacoumar P. Phenotypic and dermatological manifestations in Down Syndrome. Dermatol Online J. 2011;17(2):3. [PubMed] [Google Scholar]
- 3.Sullivan K.D., Lewis H.C., Hill A.A. Trisomy 21 consistently activates the interferon response. eLife. 2016;5 doi: 10.7554/eLife.16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan K.D., Evans D., Pandey A. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci Rep. 2017;7(1):14818. doi: 10.1038/s41598-017-13858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam N., Leung P.S., Huntley A.C., Gershwin M.E. The autoimmune basis of alopecia areata: a comprehensive review. Autoimmun Rev. 2015;14(2):81–89. doi: 10.1016/j.autrev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Schepis C., Barone C., Siragusa M., Pettinato R., Romano C. An updated survey on skin conditions in Down syndrome. Dermatology. 2002;205(3):234–238. doi: 10.1159/000065859. [DOI] [PubMed] [Google Scholar]
- 7.Marmon S., De Souza A., Strober B.E. Psoriasis and Down syndrome: a report of three cases and a potential pathophysiologic link. Dermatol Online J. 2012;18(6):13. [PubMed] [Google Scholar]
- 8.Hall J.C., Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol. 2010;6(1):40–49. doi: 10.1038/nrrheum.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shreberk-Hassidim R., Ramot Y., Zlotogorski A. Janus kinase inhibitors in dermatology: A systematic review. J Am Acad Dermatol. 2017;76(4):745–753.e719. doi: 10.1016/j.jaad.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea J.J., Kontzias A., Yamaoka K., Tanaka Y., Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]