Abstract
Oncolytic viral therapy has gained significant traction as cancer therapy over the past 2 decades. Oncolytic viruses are uniquely designed both to lyse tumor cells through their replication and to recruit immune responses against virally infected cells. Increasingly, investigators are leveraging this immune response to target the immunosuppressive tumor microenvironment and improve immune effector response against bystander tumor cells. In this article, we review the spectrum of preclinical, early-stage clinical, and potential future efforts with cytokine-secreting oncolytic viruses, with a focus on the treatment of brain tumors and solid tumors.
Keywords: interferon, IL-12, IL-15, IL-24, GM-CSF, IL-18, IL-6, tumor microenvironment, PDL-1, NK
Main Text
Oncolytic Virotherapy—Early Recognition of the Immune Response’s Role
At the advent of modern oncolytic virus (OV) design, efforts focused on improving selective viral replication in the tumor, as this was viewed as the principal mechanism governing OV efficacy. Investigators targeted intracellular antiviral mechanisms that restricted viral replication. There was also interest in suppressing the antiviral immune response to further improve viral replication, persistence, and thereby extend OV antitumor effect. In landmark studies by Andreansky et al.,1 recombinant oncolytic herpes simplex viruses (oHSVs) deleted for the neurovirulence gene γ134.5 (Δγ134.5) were constructed to express the immunosuppressive cytokines (IL-10 and IL-4) that were then tested in glioma models. These studies showed that immunosuppressive cytokine expression reduced OV antitumor activity and reduced overall survival. In contrast, immunostimulatory cytokine expression from the virus improved survival. These studies provided clear evidence that, in addition to viral replication and tumor cell lysis, the immune response was also integral to viral antitumor activity.1 Since this time, investigators have focused on engineering viruses that replicate better and improve the immunotherapeutic response. By combining virotherapy with other immunomodulatory agents, investigators now are poised to open a new chapter in cancer therapy by marshaling the immune response and redirecting it against the tumor.
Tumor Microenvironment and Associated Cytokines
The microenvironment is a complex mixture of malignant tumor cells and non-malignant immune and stromal cells that supports tumor growth, invasion, and therapeutic resistance. The immune cellular components that constitute the tumor microenvironment include both lymphoid and myeloid-derived cells, which help support the tumor. Additionally, non-cellular components such as secreted proteins and blood vessels supply the tumor. Of particular interest for oncolytic therapy are the regulatory T cells (Treg cells), and myeloid-derived suppressor cells (MDSCs). Treg cells contribute to the immunosuppressive tumor microenvironment by preventing further immune activation of effector T cells, B cells, and natural killer (NK) cells. Like Treg cells, MDSCs limit NK and T cell activation.2
Non-malignant cells also provide tumor growth factors, aid in endothelial cell growth and vascular supply, and secrete chemicals that create a hypoxic and metabolically challenging environment for many immune cells. The metabolic state of the tumor can restrict immune effectors. For example, tumor cells are well recognized to use anaerobic glycolysis and create a glucose-deficient anaerobic environment in many solid tumors. Effector T cells (Teff cells) require glycolysis for both activation and proliferation. In contrast, immunosuppressive regulatory T cells utilize oxidative phosphorylation for their proliferation and maintenance. This hypoxic and low-glucose environment favors Treg cells and further promotes tumor-associated immunosuppression. The hypoxic environment caused by rapid growth also produces reactive oxygen species. The oxidative stress and metabolic disturbances, in turn, promote a chronic inflammatory environment.3 This hypoxic and chronically inflamed environment, combined with persistent antigenic stimulation in the tumor, changes T cell activity: cells previously poised for a cytotoxic response become exhausted and convert to support cells that aid in the post-cytotoxic repair response, further benefiting tumor growth. Such inhibitory metabolic changes could also be induced by the expression of certain metabolic enzymes, such as indoleamine 2,3-dioxygenase (IDO) and arginase, which are expressed by both tumor cells and infiltrating myeloid cells and MDSCs, respectively. These enzymes locally deplete certain amino acids that are essential for anabolic functions in lymphocytes, especially T cells.
Both malignant and non-malignant cells in the tumor often express inhibitory surface markers that further restrict the immune response and limit immune surveillance.4 For instance, tumor cells or myeloid-associated cells in the tumor overexpress programmed cell death ligand 1 (PDL1), a ligand that suppresses T cell activity. Current immunotherapeutic approaches target PDL1 activity using neutralizing antibodies against PDL1 or its cognate receptor, PD1, expressed on T cells to overcome this immune resistance and improve the immune-mediated antitumor response.5 Numerous other checkpoint ligands, such as cytotoxic T-lymphocyte protein 4 (CTLA4), TIM-3 (HAVcr2), LAG-3 (CD223), TIGIT, B7-H3 (CD276), B7-H4 (VCTN1), and A2aR have each been associated with the inhibition of lymphocyte activity and may induce T lymphocyte anergy.
Tumor-Associated Cytokines and Immunosuppressive Environment
The quantity and combination of cytokines secreted in the tumor microenvironment affect how the immune system responds to tumor and influence tumor cell proliferation. Non-malignant stromal fibroblasts, endothelial cells, and infiltrating immune cells secrete cytokines (e.g., TNF-α, IL-6, TGF-β, IL-4 and IL-10) that shape the tumor microenvironment and participate in both the initiation and progression of cancer. For instance, tumor necrosis factor (TNF-α) is thought to promote carcinogenesis. Chronic low TNF-α production increases reactive oxygen species through the NADPH oxidase induction and, ultimately, produces superoxides and/or hydrogen peroxide that induces DNA damage. Thus, at low concentrations, TNF-α promotes malignant transformation in the tumor. In contrast, rapid induction of high concentrations of TNF-α contributes to an antitumor response in pre-clinical sarcoma models. IL-6 and TGF-β can also aid tumor development. Specifically, IL-6 has been implicated in multiple myeloma, and changes in TGF-β have been noticed in gastric, non-small-cell lung, colorectal, breast, bladder, and prostate cancers. However, TGF-β effects can vary from tumor suppression to tumor enhancement, depending on cell type and stage of tumorigenesis.6
A properly functioning immune response relies on a balance of pro- and anti-inflammatory effects often working in conjunction with feedback loops to attenuate the immune response. In cancer, this balance is often perturbed and benefits tumor growth. Not only does the cytokine expression level influence their activity, but also the spatiotemporal sequence of the cytokine expression influences immune activity and function. Not only does OV therapy activate acute inflammatory pathways that recruit immune cells to the site of infection, but also they have been shown to shift the cytokine balance in the tumor.
Virotherapy and Its Effects on the Tumor Microenvironment
Brain and solid tumors can generate an immunosuppressive environment that cloaks the tumor and restricts immune surveillance and the detection of aberrant proteins (neoantigens) produced by mutation-prone tumor cells. Upregulation of immunosuppressive factors (TGF-β, IL-10, and prostaglandin E) decreases cytotoxic immune activity to the tumor. Virotherapy reverses this suppression and induces immunostimulatory changes in the tumor. Viral infection stimulates danger signals and pro-inflammatory cytokine production and unmasks tumor-associated antigens after viral lytic damage. The cytokine and immunostimulatory changes integral to virotherapy and how they convert an immunosuppressive to an immunostimulatory environment is an area of active research.7
Innate immune cell recruitment and activation occur initially following OV treatment.8 NK and myeloid cells, like neutrophils and infiltrating macrophages, mediate this initial response directed against both OV-infected and bystander cells. NK cells specifically target cells lacking major histocompatibility complex (MHC) proteins or showing other signs of virally induced stress, and they utilize granzyme and perforin enzymes to lyse tumor cells. In addition to targeting immunoedited tumor cells, they also target virus-infected cells and may limit initial viral replication. Neutrophils use reactive oxygen species (ROS) to induce tumor cell death in the presence of OVs. This acute inflammatory response is coupled with cytokine and chemokine expression changes that promote further immune cell recruitment to the tumor.9 For example, using vesicular stomatitis virus (VSV), Breitbach et al.10 found that there was an increase in CXCL1 and CXCL5 transcripts, both neutrophil chemo-attractants, and other pro-inflammatory cytokines in a murine CT-26 colon cancer model. This study shows one way that a virus can induce an immune response against the tumor. One potential advantage of cytokine-expressing oncolytic viruses is the ability to regulate the balance between an antitumor and an anti-oncolytic virus response by the immune system by controlling the types of cells present in the tumor microenvironment. In later stages of OV antitumor response, continued inflammatory response within the tumor via activated NK cells and CD4 and CD8 T cells leads to long-term survival and can aid in the killing of remaining malignant cells.9 Development of immunologic memory cells via the adaptive immune system that contribute to long-term survival has been shown with OVs expressing granulocyte-macrophage colony-stimulating factor (GM-CSF). This response includes immunity from tumor re-challenges, as shown in B16 mouse melanoma models, indicating that cytokine-producing OVs may reduce rates of recurrence.11
Virus-Based Cytokine Expression—Preclinical Experience
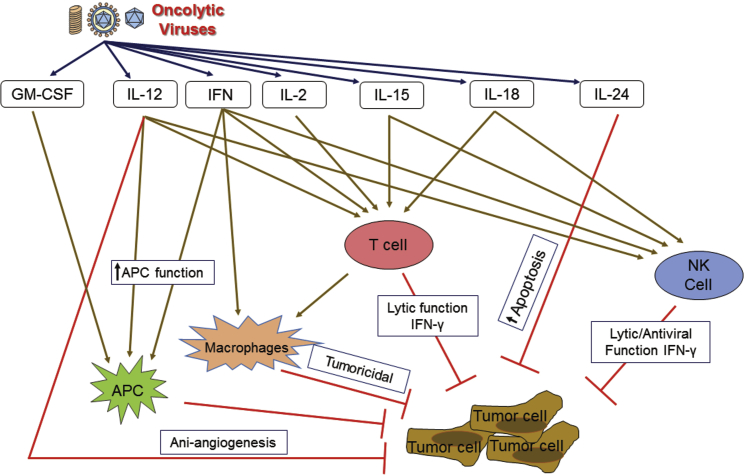
As summarized in Figure 1, investigators have engineered oncolytic viruses to express immunostimulatory cytokines in an effort to further shift the immune balance, induce tumor cytotoxicity, and improve the immune effector response. Prior studies by Andreansky et. al.1 illustrate beautifully how virus-based cytokine expression can influence the immune response and its effect on tumor growth and survival. By overexpressing a cytokine that enhances Th1 immune activity (IL-4), they showed improvement in survival. Conversely, they also showed that immunosuppressive cytokine expression in their syngeneic immunocompetent model had the opposite effect and reduced survival.
Figure 1.
Cytokine-Expressing Oncolytic Viruses Modulate Tumor Microenvironment
IL-2-based therapies activate both innate and adaptive immunity and have produced curative effects as an anti-cancer therapy. As a cytokine, interleukin (IL)-2 induces danger-signaling increases and T cell trafficking, propagation, and activation. In addition to Andreansky’s early studies, other groups have demonstrated that virus-based IL-2 expression improves antitumor activity. Carew used an HSV-amplicon vector to deliver in trans IL-2 in tandem with G207 therapy. Combinations of G207 with the IL-2 amplicon significantly reduced SCC VII tumor growth in comparison to G207 alone and control. IL-2 is known to aid in the proliferation and cytolytic activity of effector T cells. While IL-2 demonstrated early promise, it also produced significant side effects and toxicity. Investigators using systemically delivered vaccinia-based vector expressing IL-2 and CCL5 reported reduced oncolytic activity, suboptimal immune activation, and increased toxicity related to the systemic effects of early IL-2 expression.12 This has led some to utilize IL-12 or IL-15 as an alternative method to more selectively improve the T and NK cytotoxic activity with reduced systemic toxicity.12 Recently, Liu et al.13 utilized oncolytic vaccinia virus, which has a modified IL-2 with a glycosylphosphatidylinositol anchor and rigid peptide linker in an effort to reduce its systemic toxicity. Not only did this modification diminish toxic side effects, but it also treats a variety of murine tumor models. Moreover, when combined with the PD-1 and PD-L1 blockade, this virus cured most of the mice with a high tumor burden. This increased response in the presence of an immune-inducing cytokine again shows how virus-based cytokine expression can improve oncolytic therapies.14
IL-12 is an immunostimulatory cytokine that has direct antitumor activity (anti-angiogenesis), promotes interferon (IFN)-γ production, and improves immune effector functions (T and NK cell priming and activation). Accordingly, interest in using this cytokine in anticancer therapy goes back over the past decades.15, 16, 17, 18, 19, 20, 21 The bicistronic IL-12 gene was engineered into first-generation oHSV vectors and is effective in syngeneic pre-clinical tumor models. IL-12 is known to upregulate the immune response by promoting and regulating T cells, NK cells, IFN-γ production, and Th1 response. Initial studies by Toda et al.22 showed that a defective viral vector expressing IL-12 improved antitumor therapy. They showed a significant increase in survival and decrease in tumor growth in BALB/c mice growing CT26 tumors, a murine colorectal carcinoma cell line, when mice were treated with this oncolytic virus and defective HSV vector combination.22 Next, investigators engineered selectively replication competent oncolytic viruses that express IL-12. Toda showed that defective viral IL-12 expression improved G207 oHSV therapy. Markert et al. engineered the Δγ134.5 virus M002 to express a bicistronic IL-12 transcript and that this improved oHSV activity over first-generation viruses.23, 24, 25, 26 Consistent with Liu et al.’s13 work with IL-2, Wang et al.27 was able to decrease the toxicity of IL-12 oncolytic adenovirus by cleaving the N-terminal signal peptide of IL-12 so that IL-12 was not secreted but was directly delivered to the tumor. This delivery of non-secreting IL-12 with adenovirus successfully treated pancreatic cancer in Syrian hamster models without toxicity and provided long-term immunity. Following Andreansky’s report on cytokine-mediated activity, investigators focused on ways to improve tumor-associated Th1 priming and activation. The murine IL-12 (mIL-12) virus is effective in numerous syngeneic models, which led to the clinical development of a human IL-12 (hIL-12)-expressing oHSV that is now currently in a phase I clinical trial.23 In addition to HSV-based IL-12 oncolytic vectors, combinatorial approaches using ADV-expressing IL-12 have been effective in pancreatic preclinical models. Semliki-virus-based IL-12 expression potentiated PDL1 blockade in a B16-OVA bilateral melanoma model.28 VSV vectors expressing IL-12 were also effective in murine squamous-cell-carcinoma-based studies.29, 30 Currently, multiple IL-12-based approaches are used in early-phase clinical trials and are among the more mature virus-based cytokine therapies.
IL-15 is another NK- and T cell-activating cytokine that has also proven effective in boosting antitumor response when expressed from the virus and inhibits the tumor recurrence and metastasis. Furthermore, IL-15 is less toxic than IL-2. The cytokine has been successfully used in Newcastle disease virus, influenza, adenovirus, myxoma, and VSV-based oncolytic viruses. IL-15-expressing viruses were successful in both melanoma and adenocarcinoma models.31, 32, 33, 34, 35, 36 In 2013, Gaston et al.37 successfully created an oncolytic HSV strand that produced murine IL-15 and showed successful cytotoxic effects in NK-cell cytotoxicity assays in vitro. Kowalsky et al.38 made an IL-15-expressing oncolytic vaccinia virus that showed improved survival in both colon and ovarian cancer murine models. Moreover, the authors combined this OV with anti-PD-1 therapy to enhance immune response in the colon cancer model and observed extended survival.
IL-24 is a cytokine that belongs to the IL-10 family. It can selectively induce cancer cell apoptosis and in adenovirus-based studies has also improved viral recovery.39 Qin and colleagues40 showed that the oncolytic adenovirus armed with IL-24 can inhibit the growth of breast cancer in vitro and in vivo. Lv et al.41 reported that adenovirus carrying the IL-24 gene knocked in the region of the viral thymidine kinase (TK) gene, efficiently infected and destroyed lung cancer cells via caspase-dependent apoptosis, and decreased the expression of STAT3 in a lung cancer model.
GM-CSF talimogene laherparepvec (IMLYGIC) is the first oncolytic virus to be approved and is now approved to treat melanoma.42, 43 Talimogene laherparepvec is a herpes virus modified to selectively replicate in melanoma cells and secrete GM-CSF. Secretion of GM-CSF has the theoretical benefit of improving granulocyte differentiation, monocyte migration into tissue, and macrophage and dendritic cell maturation to enhance the antigen presentation response during virotherapy. In a phase III trial, patients treated with talimogene laherparpvec had an improved durable response and median overall survival in comparison to patients treated with GM-CSF alone.44 The successful approval of this therapy shows that cytokine-secreting oncolytic viruses have a viable future as a safe and effective cancer therapy and has opened a route for future approvals for other oncolytic viruses. For example, pexastimogene devacirepvec (Pexa-Vec), a vaccinia virus expressing GM-CSF, is currently in a phase III trial for hepatocellular carcinoma and is poised to be the second OV approved by the Food and Drug Administration (FDA), pending trial results (https://clinicaltrials.gov/ct2/show/NCT02562755). Investigators are also exploring combining talimogene laherparepvec with immune checkpoint inhibitors. In a phase Ib trial for patients with advanced melanoma, Ribas et al.45 saw a high objective response rate of 62% when combining the anti-PD-1 antibody pembrolizumab with talimogene laherparepvec. Additionally, this combination treatment showed enhancement of CD8 T cell infiltration into the tumor. Similarly, patients with unresectable, advanced melanoma had a higher objective response to the combination of talimogene laherparepvex and ipilimumab, an anti-CTLA-4 monoclonal antibody compared to those treated with ipilimumab alone. Importantly, those visceral lesions that were not directly injected with the OV also showed greater response than those treated with ipilimumab alone.46 The success of these two trials points toward future OV immunotherapy combinations to improve outcomes for those patients with difficult-to-treat tumors.
IL-18 is a pro-inflammatory cytokine also known as IFN-γ-inducing factor. It augments NK cell activity and stimulates IFN-γ production in T-helper type I cells. There has been interest in tandem expression of IL-18 with IL-12 as a way of augmenting adenovirus as well oHSVs.47, 48 OVs expressing IL-18 were successful in pre-clinical studies using syngeneic prostate and neuroblastoma models.47, 49 The adenovirus (ADV)-based studies are limited to human xenografts in immunocompromised models and are, therefore, more difficult to interpret.50, 51, 52
Type I and type II IFNs are secreted proteins that activate a signaling response that shifts transcription in the stimulated cell, leading to an antiviral and antitumor state. In immune cells, the IFN response is also integral to immune cell activation and functional changes in the immune response that improve the adaptive immune and cytotoxic response. IFN production both boosts MHC class I and class II expression on surrounding cells and increases immune-proteasome activity enhancing antigen presentation. Accordingly, investigators have armed attenuated OVs to express type I IFNs (α or β) as a means to both attenuate the virus and enhance direct antitumor activity and the immune-mediated response. The most mature approach currently involves VSV-based IFN-β expression as a method to activate these responses. Pre-clinical studies involving the VSV-IFN-β recombinant or prophylactic IFN-α combined with VSV treatment have demonstrated efficacy in diverse tumor types, including mesothelioma, plasmacytoma, hepatocellular carcinomas, and squamous cell carcinomas of the head and neck.53, 54, 55, 56 Adenovirus-based IFN expression has also been successful in treating hepatocellular and pancreatic carcinomas in pre-clinical syngeneic Syrian hamster models.57, 58, 59
IFN-γ, a type II IFN secreted by NK cells and activated T cells (CTLs and CD4 helper cells) is important in immune activity against infectious pathogens (viral, fungal, and mycobacterial infections), immune modulation, and antitumor function. Several researchers have shown that the presence of IFN-γ might be beneficial in the treatment of various cancers; for example, in human breast cancer samples, the expression of IFN-γ and its receptor were found to be increased in benign lesions in contrast to malignant lesions. Bell et al. reported that the IFN-γ-encoding oncolytic VSV demonstrated greater activation of dendritic cells and enhanced the secretion of proinflammatory cytokines compared to the parental virus. Also, they showed that the IFN-γ virus reduced tumor growth, minimized lung tumors, and prolonged survival in 4T1 mammary adenocarcinoma as well as other murine tumor models.60
IL-6 signals through the IL-6 receptor and activates signal transducer and activator of transcription (STAT) signaling in a manner similar to that of IFN. However, in contrast, it targets alternative STAT substrates and modifies proliferative and apoptotic responses in stimulated cells.61 Viral and bacterial infections generate an IL-6 response during natural infection. Investigators have investigated how IFN and IL-6 utilize a similar pathway to produce a distinct tumor cell response during virotherapy. Danzinger et al.61, 62 showed that, in JAK1-expressing prostate cancer cells, IL-6 sensitized and IFN-α suppressed viral cell death, depending upon the RNA virus used in their studies. The investigators go on to show that IL-6 and virus infection induced cytotoxicity through a STAT-3-dependent process and occurred in the absence of productive infection.61 Investigators have also utilized IL-6 in combination with oncolytic vaccinia therapy and chemotherapy to reduce drug-associated toxicities. Investigators expressed IL-6 from vaccinia and showed that when the virus was paired with mytomycin C (Mito-C) therapy, not only did it enhance the antitumor effect but also the IL-6 expression reduced the duration of chemotherapy-induced thrombocytopenia in their preclinical model.63 Going forward, investigators will likely use cytokine combinations to not only enhance the virotherapeutic antitumor activity but also improve therapeutic tolerance.
Viral Therapy Clinical Trials
Oncolytic viral therapies are currently in human clinical trials across a variety of tumor types. There are currently 86 clinical trials testing oncolytic viruses recorded in ClinicalTrials.gov (search criteria = “oncolytic,” “viral therapy,” and “virotherapy”). These trials (summarized in Table 1) included 43 recruiting or currently active trials as of June 6th, 2018. Nine of these ongoing trials are focused on glioma, glioblastoma, or another primary CNS tumor.
Table 1.
Summary of Active Clinical Trials in Brain and Solid Tumors Using Oncolytic Virus Therapy
| Trial Title | Phase | Intervention | Location(s) | NCT Number |
|---|---|---|---|---|
| Phase I Trial of DNX-2401 for Diffuse Intrinsic Pontine Glioma Newly Diagnosed in Pediatric Patients | I | DNX-2401, an adenovirus that replicates in cells with abnormal Rb pathway | Clinica Universidad de Navarra, Navarra, Spain | NCT03178032 |
| Safety and Efficacy of the ONCOlytic VIRus Armed for Local Chemotherapy, TG6002/5-FC, in Recurrent Glioblastoma Patients | I/IIa | combination of TG6002 and 5-flucytosine | Groupe Hospitalier Pitié-Salpêtrière, Paris, France | NCT03294486 |
| Neural Stem Cell Oncolytic Adenoviral Virotherapy of Newly Diagnosed Malignant Glioma | I | neural stem cells loaded with oncolytic adenovirus | Northwestern Memorial Hospital, Chicago, IL, USA | NCT03072134 |
| A Randomized Phase 2 Study of Oncolytic Polio/Rhinovirus Recombinant (PVSRIPO) Alone or in Combination With Lomustine in Recurrent WHO Grade IV Malignant Glioma Patients | II | oncolytic polio and rhinovirus recombinant (PVSRIPO) with or without lomustine | Preston Robert Tisch Brain Tumor Center at Duke University, Durham, NC, USA | NCT02986178 |
| Phase Ib Study of Oncolytic Polio/Rhinovirus Recombinant Against Recurrent Malignant Glioma in Children | Ib | polio and rhinovirus recombinant (PVSRIPO) | Duke University Medical Center, Durham, NC, USA | NCT03043391 |
| Phase I Clinical Trial of HSV G207 Alone or With a Single Radiation Dose in Children With Recurrent Supratentorial Brain Tumors | I | G207, an oncolytic herpes simplex virus-1 | Children’s of Alabama, Birmingham, AL, USA | NCT02457845 |
| A Phase 1b, Randomized, Multi-center, Open-label Study of a Conditionally Replicative Adenovirus (DNX-2401) and Interferon Gamma (IFN-γ) for Recurrent Glioblastoma or Gliosarcoma (TARGET-I) | Ib | intratumoral injection of DNX-2401, interferon-gamma | Moffitt Cancer Center, Tampa, FL, USA; The Ohio State University, Columbus, OH, USA; Baylor Charles A. Sammons Cancer Center, Dallas, TX, USA; University of Texas MD Anderson Cancer Center, Houston, TX, USA | NCT02197169 |
| A Phase 1 Study of M032 (NSC 733972), a Genetically Engineered HSV-1 Expressing IL-12, in Patients With Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma | I | M032, an oncolytic herpes simplex virus that secretes IL-12 | University of Alabama at Birmingham, Birmingham, AL, USA | NCT02062827 |
| A Phase I Study of the Treatment of Recurrent Malignant Glioma With rQNestin34.5v.2, a Genetically Engineered HSV-1 Virus, and Immunomodulation With Cyclophosphamide | I | rQNestin34.5v.2, a viral vector made from HSV | Dana-Farber/Brigham and Women’s Cancer Center, Boston, MA, USA | |
| Trial of C134 in Patients With Recurrent GBM (C134-HSV-1) | I | C134, a chimeric HCMV/HSV virus | University of Alabama at Birmingham, Birmingham, AL, USA | NCT03657576 |
| Phase I Dose Escalation Study of Intravenous VCN-01 With or Without Gemcitabine and Abraxane in Patients With Advanced Solid Tumors | I | VCN-01, an oncolytic adenovirus, with or without gemcitabine and abraxane | Hospital Vall d’Hebron, Institut Català d’Oncologia, Barcelona, Spain | NCT02045602 |
| Immunization Strategy with Intra-tumoral Injections of Pexa-Vec With Ipilimumab in Metastatic/Advanced Solid Tumors (ISI-JX) | I | Pexa-Vec, a recombinant vaccinia virus with GM-GCF and ipilimumab | Centre Léon Bérard. Lyon, France | NCT02977156 |
Investigators, in some instances, are augmenting OV therapy with cytokines. Preclinical studies demonstrated benefits, and this approach is now being incorporated into clinical trials. Northwestern University is running a phase I study for “Neural Stem Cell Based Virotherapy of Newly Diagnosed Malignant Glioma.” This trial utilizes an oncolytic adenovirus that has been loaded into neural stem cells (https://clinicaltrials.gov/ct2/show/NCT03072134?term=virotherapy&rank=1). Although results from this trial are not available at this time, preclinical evaluation in human-derived glioblastomas showed survival improvement when the oncolytic virus was delivered in neural stem cells compared to oncolytic virus alone.64 The results of a phase I trial in malignant glioma for the oncolytic virus G207, discussed earlier, showed that this virus was safe to administer in humans. Additionally, G207 showed some promising antitumor effects both radiographically and pathologically.65 Although G207 was studied in combination with an HSV amplicon vector delivering IL-12—with preclinical success, as described earlier—a Δγ134.5-based HSV that expresses hIL-12 (M032) is currently being examined in a phase I study in patients with recurrent or progressive glioma not eligible for surgical resection.23 Investigators are also targeting advanced solid tumors using OV therapy in combination with adjuvant immunomodulator therapy (adenovirus + gemcitabine + abraxane [ClinicalTrials.gov: NCT02045602] or Pexa-Vex + GM-CSF + ipilimumab [ClinicalTrials.gov: NCT02977156]). Overall, the growing number of clinical trials in viral therapy indicate a strong potential for new successes in difficult to treat tumors.
Potential Future Directions for Virotherapy
Future studies in virotherapy can focus on altering myeloid behavior, altering T cell response, or altering NK cell recruitment and response. Current research has focused on either directly infecting and killing a tumor cell or creating an immune response using the effects of a virus around the tumor and the tumor microenvironment. Further research should look to enhancing the body’s immune response to the tumor from outside the tumor itself—either by affecting T cells so that they concentrate on attacking the tumor or by recruiting and activating NK cells at the tumor origin. The advantage of a more systemic approach may be the reduction of small, distant metastases that may have otherwise gone unnoticed or may be an increased effectiveness against diffuse brain tumors but at the expense of potential greater systemic side effects.
One option is to focus future therapies on the recruitment and activation of NK cells. The role of NK cells fits perfectly into using viral therapy for cancer treatment. NK cells are known not only to be a key player in regulating anti-viral response but also for having a response against some cancers, like acute myeloid leukemia.66, 67 Because NK cells play a key role in anti-viral response, cytokine control of NK cells using a virus can allow us to regulate the level of immune response in order to balance efficacy with potentially harmful side effects of having too much immune response. This ability would make targets like IL-18, which activates NK cells when combined with IL-12, attractive opportunities. Likewise, IL-21, which also activates NK cells and improves their growth and persistence, has been incorporated into ADV therapy. Additionally, there is a significant overlap between many of the cytokines that regulate NK cells and CD8+ T cells. This overlap may make it difficult to specifically regulate NK cell activity.
A different route for research may be in altering T cell response with a cytokine-expressing oncolytic virus. Different populations of T cells, such as Treg and T effector cells, can be targeted by viral therapy for different purposes.8 Treg cells, which create an immune-suppressing environment, have already received significant attention in oncolytic viral therapy. For example, HSV expressing IL-12 was used in a glioma model by Cheema et al.68 in 2013. They found that the virus reduced Treg cells and improved survival.8 The success in this area certainly warrants more attention for future research directions. Cytotoxic T lymphocytes (CTLs) enhance the antitumor immune environment, and CTL increase in the tumor microenvironment correlates with improved patient survival. Oncolytic virotherapy has already been shown to improve recruitment of CTLs into the tumor microenvironment.69 Hence, further research into the ability of cytokine-expressing viruses to enhance CTL recruitment and activation would be a promising field. Combining chimeric antigen receptor (CAR) therapy with OV immunotherapy is a focus of the American Association for Cancer Research (AACR) for 2018 and is anticipated to progress rapidly.
Finally, myeloid behavior may be a good target for future therapies using viral therapy. Because myeloid-derived cells are part of the innate immune response, they activate in response to oncolytic viral therapy.8 An oncolytic virus that combines the native innate immune response of myeloid-derived cells caused by a viral infection with other cytokines and targets that further enhance myeloid response can have a compounding effect on activating the immune system. Again, care needs to be taken to balance the therapeutic effects of activating the immune system with the potential systemic side effects and viral-therapy-depleting property of a highly active immune system. Another myeloid target would be MDSCs. MDSCs are known to accumulate in the tumor microenvironment where they suppress T cell response. One way they do this is by expressing PDL1.2 New anti-PDL1 therapies have recently been introduced to the market that, therefore, could be used in combination with viral therapy targeted at myeloid response.6 A large variety of cytokines such as IL-1β, IL-6, and PgE2 activate MDSCs, reducing the immune response within the tumor microenvironment, which may provide antagonist targets for further therapy. Other targets, like NOTCH, can also be exploited to inhibit MDSC function and could provide alternative targets for use with viral therapy.
Cytokine-expressing oncolytic viruses have been effective in pre-clinical models and are now entering early-stage clinical trials. Further work to optimize this powerful tool needs to be pursued. Both the safety and efficacy of these viruses can be improved. As clinical studies progress, we anticipate that combination therapies using the virus to drive cellular immune therapies will drive further vector development and expand the use of these experimental therapies for treatment-resistant cancers.
Acknowledgments
This work was supported by Alex's Lemonade Stand Foundation (ALSF) (to T.M.P. and K.A.C.), Cancer Free Kids (to M.G.G. and K.A.C.), and the NIH (CA222903 and CA217179 to J.M.M. and K.A.C.).
References
- 1.Andreansky S., He B., van Cott J., McGhee J., Markert J.M., Gillespie G.Y., Roizman B., Whitley R.J. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5:121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 2.Parker K.H., Beury D.W., Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv. Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan H. Novel therapeutic strategies for solid tumor based on body’s intrinsic antitumor immune system. Cell. Physiol. Biochem. 2018;47:441–457. doi: 10.1159/000489979. [DOI] [PubMed] [Google Scholar]
- 4.Andrews M.C., Reuben A., Gopalakrishnan V., Wargo J.A. Concepts collide: genomic, immune, and microbial influences on the tumor microenvironment and response to cancer therapy. Front. Immunol. 2018;9:946. doi: 10.3389/fimmu.2018.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alsaab H.O., Sau S., Alzhrani R., Tatiparti K., Bhise K., Kashaw S.K., Iyer A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitley R.J., Markert J.M. Viral therapy of glioblastoma multiforme. Proc. Natl. Acad. Sci. USA. 2013;110:11672–11673. doi: 10.1073/pnas.1310253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez-Breckenridge C.A., Choi B.D., Suryadevara C.M., Chiocca E.A. Potentiating oncolytic viral therapy through an understanding of the initial immune responses to oncolytic viral infection. Curr. Opin. Virol. 2015;13:25–32. doi: 10.1016/j.coviro.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Filley A.C., Dey M. Immune system, friend or foe of oncolytic virotherapy? Front. Oncol. 2017;7:106. doi: 10.3389/fonc.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitbach C.J., Paterson J.M., Lemay C.G., Falls T.J., McGuire A., Parato K.A., Stojdl D.F., Daneshmand M., Speth K., Kim D. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol. Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 11.Naik J.D., Twelves C.J., Selby P.J., Vile R.G., Chester J.D. Immune recruitment and therapeutic synergy: keys to optimizing oncolytic viral therapy? Clin. Cancer Res. 2011;17:4214–4224. doi: 10.1158/1078-0432.CCR-10-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H., Sampath P., Hou W., Thorne S.H. Regulating cytokine function enhances safety and activity of genetic cancer therapies. Mol. Ther. 2013;21:167–174. doi: 10.1038/mt.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Ge Y., Wang H., Ma C., Feist M., Ju S., Guo Z.S., Bartlett D.L. Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2. Nat. Commun. 2018;9:4682. doi: 10.1038/s41467-018-06954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carew J.F., Kooby D.A., Halterman M.W., Kim S.H., Federoff H.J., Fong Y. A novel approach to cancer therapy using an oncolytic herpes virus to package amplicons containing cytokine genes. Mol. Ther. 2001;4:250–256. doi: 10.1006/mthe.2001.0448. [DOI] [PubMed] [Google Scholar]
- 15.Kishima H., Shimizu K., Miyao Y., Mabuchi E., Tamura K., Tamura M., Sasaki M., Hakakawa T. Systemic interleukin 12 displays anti-tumour activity in the mouse central nervous system. Br. J. Cancer. 1998;78:446–453. doi: 10.1038/bjc.1998.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y., Jurgovsky K., Möller P., Alijagic S., Dorbic T., Georgieva J., Wittig B., Schadendorf D. Vaccination with IL-12 gene-modified autologous melanoma cells: preclinical results and a first clinical phase I study. Gene Ther. 1998;5:481–490. doi: 10.1038/sj.gt.3300619. [DOI] [PubMed] [Google Scholar]
- 17.Jean W.C., Spellman S.R., Wallenfriedman M.A., Hall W.A., Low W.C. Interleukin-12-based immunotherapy against rat 9L glioma. Neurosurgery. 1998;42:850–857. doi: 10.1097/00006123-199804000-00097. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida Y., Tasaki K., Kimurai M., Takenaga K., Yamamoto H., Yamaguchi T., Saisho H., Sakiyama S., Tagawa M. Antitumor effect of human pancreatic cancer cells transduced with cytokine genes which activate Th1 helper T cells. Anticancer Res. 1998;18(1A):333–335. [PubMed] [Google Scholar]
- 19.Coughlin C.M., Salhany K.E., Wysocka M., Aruga E., Kurzawa H., Chang A.E., Hunter C.A., Fox J.C., Trinchieri G., Lee W.M. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J. Clin. Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers J.N., Mank-Seymour A., Zitvogel L., Storkus W., Clarke M., Johnson C.S., Tahara H., Lotze M.T. Interleukin-12 gene therapy prevents establishment of SCC VII squamous cell carcinomas, inhibits tumor growth, and elicits long-term antitumor immunity in syngeneic C3H mice. Laryngoscope. 1998;108:261–268. doi: 10.1097/00005537-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Kurzawa H., Wysocka M., Aruga E., Chang A.E., Trinchieri G., Lee W.M. Recombinant interleukin 12 enhances cellular immune responses to vaccination only after a period of suppression. Cancer Res. 1998;58:491–499. [PubMed] [Google Scholar]
- 22.Toda M., Martuza R.L., Kojima H., Rabkin S.D. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J. Immunol. 1998;160:4457–4464. [PubMed] [Google Scholar]
- 23.Patel D.M., Foreman P.M., Nabors L.B., Riley K.O., Gillespie G.Y., Markert J.M. Design of a phase I clinical trial to evaluate M032, a genetically engineered HSV-1 expressing IL-12, in patients with recurrent/progressive glioblastoma multiforme, anaplastic astrocytoma, or gliosarcoma. Hum. Gene Ther. Clin. Dev. 2016;27:69–78. doi: 10.1089/humc.2016.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth J.C., Cassady K.A., Cody J.J., Parker J.N., Price K.H., Coleman J.M., Peggins J.O., Noker P.E., Powers N.W., Grimes S.D. Evaluation of the safety and biodistribution of M032, an attenuated herpes simplex virus type 1 expressing hIL-12, after intracerebral administration to aotus nonhuman primates. Hum. Gene Ther. Clin. Dev. 2014;25:16–27. doi: 10.1089/humc.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker J.N., Meleth S., Hughes K.B., Gillespie G.Y., Whitley R.J., Markert J.M. Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther. 2005;12:359–368. doi: 10.1038/sj.cgt.7700784. [DOI] [PubMed] [Google Scholar]
- 26.Parker J.N., Gillespie G.Y., Love C.E., Randall S., Whitley R.J., Markert J.M. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc. Natl. Acad. Sci. USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P., Li X., Wang J., Gao D., Li Y., Li H., Chu Y., Zhang Z., Liu H., Jiang G. Re-designing interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017;8:1395. doi: 10.1038/s41467-017-01385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quetglas J.I., Labiano S., Aznar M.A., Bolaños E., Azpilikueta A., Rodriguez I., Casales E., Sánchez-Paulete A.R., Segura V., Smerdou C., Melero I. Virotherapy with a Semliki Forest virus-based vector encoding IL12 synergizes with PD-1/PD-L1 blockade. Cancer Immunol. Res. 2015;3:449–454. doi: 10.1158/2326-6066.CIR-14-0216. [DOI] [PubMed] [Google Scholar]
- 29.Sung C.K., Choi B., Wanna G., Genden E.M., Woo S.L., Shin E.J. Combined VSV oncolytic virus and chemotherapy for squamous cell carcinoma. Laryngoscope. 2008;118:237–242. doi: 10.1097/MLG.0b013e3181581977. [DOI] [PubMed] [Google Scholar]
- 30.Shin E.J., Wanna G.B., Choi B., Aguila D., 3rd, Ebert O., Genden E.M., Woo S.L. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope. 2007;117:210–214. doi: 10.1097/01.mlg.0000246194.66295.d8. [DOI] [PubMed] [Google Scholar]
- 31.Niu Z., Bai F., Sun T., Tian H., Yu D., Yin J., Li S., Li T., Cao H., Yu Q. Recombinant Newcastle Disease virus expressing IL15 demonstrates promising antitumor efficiency in melanoma model. Technol. Cancer Res. Treat. 2015;14:607–615. doi: 10.7785/tcrt.2012.500414. [DOI] [PubMed] [Google Scholar]
- 32.Yan Y., Li S., Jia T., Du X., Xu Y., Zhao Y., Li L., Liang K., Liang W., Sun H., Li R. Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity. Tumour Biol. 2015;36:4535–4543. doi: 10.1007/s13277-015-3098-7. [DOI] [PubMed] [Google Scholar]
- 33.Hock K., Laengle J., Kuznetsova I., Egorov A., Hegedus B., Dome B., Wekerle T., Sachet M., Bergmann M. Oncolytic influenza A virus expressing interleukin-15 decreases tumor growth in vivo. Surgery. 2017;161:735–746. doi: 10.1016/j.surg.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Xu X., Sun Q., Yu X., Zhao L. Rescue of nonlytic Newcastle Disease Virus (NDV) expressing IL-15 for cancer immunotherapy. Virus Res. 2017;233:35–41. doi: 10.1016/j.virusres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Stephenson K.B., Barra N.G., Davies E., Ashkar A.A., Lichty B.D. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012;19:238–246. doi: 10.1038/cgt.2011.81. [DOI] [PubMed] [Google Scholar]
- 36.Liu J., Wennier S., Reinhard M., Roy E., MacNeill A., McFadden G. Myxoma virus expressing interleukin-15 fails to cause lethal myxomatosis in European rabbits. J. Virol. 2009;83:5933–5938. doi: 10.1128/JVI.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaston D.C., Odom C.I., Li L., Markert J.M., Roth J.C., Cassady K.A., Whitley R.J., Parker J.N. Production of bioactive soluble interleukin-15 in complex with interleukin-15 receptor alpha from a conditionally-replicating oncolytic HSV-1. PLoS ONE. 2013;8:e81768. doi: 10.1371/journal.pone.0081768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kowalsky S.J., Liu Z., Feist M., Berkey S.E., Ma C., Ravindranathan R., Dai E., Roy E.J., Guo Z.S., Bartlett D.L. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 2018;26:2476–2486. doi: 10.1016/j.ymthe.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashshi A.M., El-Shemi A.G., Dmitriev I.P., Kashentseva E.A., Curiel D.T. Combinatorial strategies based on CRAd-IL24 and CRAd-ING4 virotherapy with anti-angiogenesis treatment for ovarian cancer. J. Ovarian Res. 2016;9:38. doi: 10.1186/s13048-016-0248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan S., Fang X., Xu Y., Ni A., Liu X.Y., Chu L. An oncolytic adenovirus that expresses the HAb18 and interleukin 24 genes exhibits enhanced antitumor activity in hepatocellular carcinoma cells. Oncotarget. 2016;7:60491–60502. doi: 10.18632/oncotarget.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv C., Su Q., Liang Y., Hu J., Yuan S. Oncolytic vaccine virus harbouring the IL-24 gene suppresses the growth of lung cancer by inducing apoptosis. Biochem. Biophys. Res. Commun. 2016;476:21–28. doi: 10.1016/j.bbrc.2016.05.088. [DOI] [PubMed] [Google Scholar]
- 42.Gangi A., Zager J.S. The safety of talimogene laherparepvec for the treatment of advanced melanoma. Expert Opin. Drug Saf. 2017;16:265–269. doi: 10.1080/14740338.2017.1274729. [DOI] [PubMed] [Google Scholar]
- 43.Bommareddy P.K., Silk A.W., Kaufman H.L. Intratumoral approaches for the treatment of melanoma. Cancer J. 2017;23:40–47. doi: 10.1097/PPO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 44.Chesney J., Awasthi S., Curti B., Hutchins L., Linette G., Triozzi P., Tan M.C.B., Brown R.E., Nemunaitis J., Whitman E. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res. 2018;28:44–51. doi: 10.1097/CMR.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 45.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J., Hamid O., Ross M., Friedlander P., Garbe C. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ino Y., Saeki Y., Fukuhara H., Todo T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin. Cancer Res. 2006;12:643–652. doi: 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- 48.Choi I.K., Lee J.S., Zhang S.N., Park J., Sonn C.H., Lee K.M., Yun C.O. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther. 2011;18:898–909. doi: 10.1038/gt.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuhara H., Ino Y., Kuroda T., Martuza R.L., Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- 50.Zheng J.N., Pei D.S., Sun F.H., Liu X.Y., Mao L.J., Zhang B.F., Wen R.M., Xu W., Shi Z., Liu J.J., Li W. Potent antitumor efficacy of interleukin-18 delivered by conditionally replicative adenovirus vector in renal cell carcinoma-bearing nude mice via inhibition of angiogenesis. Cancer Biol. Ther. 2009;8:599–606. doi: 10.4161/cbt.8.7.7914. [DOI] [PubMed] [Google Scholar]
- 51.Zheng J.N., Pei D.S., Mao L.J., Liu X.Y., Sun F.H., Zhang B.F., Liu Y.Q., Liu J.J., Li W., Han D. Oncolytic adenovirus expressing interleukin-18 induces significant antitumor effects against melanoma in mice through inhibition of angiogenesis. Cancer Gene Ther. 2010;17:28–36. doi: 10.1038/cgt.2009.38. [DOI] [PubMed] [Google Scholar]
- 52.Yang C., Cao H., Liu N., Xu K., Ding M., Mao L.J. Oncolytic adenovirus expressing interleukin-18 improves antitumor activity of dacarbazine for malignant melanoma. Drug Des. Devel. Ther. 2016;10:3755–3761. doi: 10.2147/DDDT.S115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L., Steele M.B., Jenks N., Grell J., Suksanpaisan L., Naik S., Federspiel M.J., Lacy M.Q., Russell S.J., Peng K.W. Safety studies in tumor and non-tumor-bearing mice in support of clinical trials using oncolytic VSV-IFNβ-NIS. Hum. Gene Ther. Clin. Dev. 2016;27:111–122. doi: 10.1089/humc.2016.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel M.R., Jacobson B.A., Ji Y., Drees J., Tang S., Xiong K., Wang H., Prigge J.E., Dash A.S., Kratzke A.K. Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget. 2015;6:33165–33177. doi: 10.18632/oncotarget.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naik S., Nace R., Barber G.N., Russell S.J. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β. Cancer Gene Ther. 2012;19:443–450. doi: 10.1038/cgt.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinozaki K., Ebert O., Suriawinata A., Thung S.N., Woo S.L. Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats. J. Virol. 2005;79:13705–13713. doi: 10.1128/JVI.79.21.13705-13713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaRocca C.J., Han J., Gavrikova T., Armstrong L., Oliveira A.R., Shanley R., Vickers S.M., Yamamoto M., Davydova J. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery. 2015;157:888–898. doi: 10.1016/j.surg.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armstrong L., Arrington A., Han J., Gavrikova T., Brown E., Yamamoto M., Vickers S.M., Davydova J. Generation of a novel, cyclooxygenase-2-targeted, interferon-expressing, conditionally replicative adenovirus for pancreatic cancer therapy. Am. J. Surg. 2012;204:741–750. doi: 10.1016/j.amjsurg.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He L.F., Gu J.F., Tang W.H., Fan J.K., Wei N., Zou W.G., Zhang Y.H., Zhao L.L., Liu X.Y. Significant antitumor activity of oncolytic adenovirus expressing human interferon-beta for hepatocellular carcinoma. J. Gene Med. 2008;10:983–992. doi: 10.1002/jgm.1231. [DOI] [PubMed] [Google Scholar]
- 60.Bourgeois-Daigneault M.C., Roy D.G., Falls T., Twumasi-Boateng K., St-Germain L.E., Marguerie M., Garcia V., Selman M., Jennings V.A., Pettigrew J. Oncolytic vesicular stomatitis virus expressing interferon-γ has enhanced therapeutic activity. Mol. Ther. Oncolytics. 2016;3:16001. doi: 10.1038/mto.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danziger O., Pupko T., Bacharach E., Ehrlich M. Interleukin-6 and interferon-α signaling via JAK1-STAT differentially regulate oncolytic versus cytoprotective antiviral states. Front. Immunol. 2018;9:94. doi: 10.3389/fimmu.2018.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danziger O., Shai B., Sabo Y., Bacharach E., Ehrlich M. Combined genetic and epigenetic interferences with interferon signaling expose prostate cancer cells to viral infection. Oncotarget. 2016;7:52115–52134. doi: 10.18632/oncotarget.10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sturm J.B., Hess M., Weibel S., Chen N.G., Yu Y.A., Zhang Q., Donat U., Reiss C., Gambaryan S., Krohne G. Functional hyper-IL-6 from vaccinia virus-colonized tumors triggers platelet formation and helps to alleviate toxicity of mitomycin C enhanced virus therapy. J. Transl. Med. 2012;10:9. doi: 10.1186/1479-5876-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed A.U., Thaci B., Tobias A.L., Auffinger B., Zhang L., Cheng Y., Kim C.K., Yunis C., Han Y., Alexiades N.G. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J. Natl. Cancer Inst. 2013;105:968–977. doi: 10.1093/jnci/djt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Markert J.M., Medlock M.D., Rabkin S.D., Gillespie G.Y., Todo T., Hunter W.D., Palmer C.A., Feigenbaum F., Tornatore C., Tufaro F., Martuza R.L. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 66.Freeman B.E., Raué H.P., Hill A.B., Slifka M.K. Cytokine-mediated activation of NK cells during viral infection. J. Virol. 2015;89:7922–7931. doi: 10.1128/JVI.00199-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romee R., Rosario M., Berrien-Elliott M.M., Wagner J.A., Jewell B.A., Schappe T., Leong J.W., Abdel-Latif S., Schneider S.E., Willey S. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016;8:357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheema T.A., Wakimoto H., Fecci P.E., Ning J., Kuroda T., Jeyaretna D.S., Martuza R.L., Rabkin S.D. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc. Natl. Acad. Sci. USA. 2013;110:12006–12011. doi: 10.1073/pnas.1307935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Q., Qiu S.J., Fan J., Zhou J., Wang X.Y., Xiao Y.S., Xu Y., Li Y.W., Tang Z.Y. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]