Abstract
Background
P. aeruginosa is a pathogen frequently resistant to antibiotics and a common cause of ventilator-associated pneumonia (VAP). Non-antibiotic strategies to prevent or treat VAP are therefore of major interest. Specific polyclonal avian IgY antibodies have previously been shown to be effective against pneumonia caused by P. aeruginosa in rodents and against P. aeruginosa airway colonization in patients.
Objectives
To study the effect of specific polyclonal anti-P. aeruginosa IgY antibodies (Pa-IgY) on colonization of the airways in a porcine model.
Method
The pigs were anesthetized, mechanically ventilated, and subject to invasive hemodynamic monitoring and allocated to either receive 109 CFU nebulized P. aeruginosa (control, n = 6) or 109 CFU nebulized P. aeruginosa + 200 mg Pa-IgY antibodies (intervention, n = 6). Physiological measurement, blood samples, and tracheal cultures were then secured regularly for 27 h, after which the pigs were sacrificed and lung biopsies were cultured.
Results
After nebulization, tracheal growth of P. aeruginosa increased in both groups during the experiment, but with lower growth in the Pa-IgY-treated group during the experiment (p = 0.02). Tracheal growth was 4.6 × 103 (9.1 × 102–3.1 × 104) vs. 4.8 × 104 (7.5 × 103–1.4 × 105) CFU/mL in the intervention group vs. the control group at 1 h and 5.0 × 100 (0.0 × 100–3.8 × 102) vs. 3.3 × 104 (8.0 × 103–1.4 × 105) CFU/mL at 12 h in the same groups. During this time, growth in the intervention vs. control group was one to two orders of ten lower. After 12 h, the treatment effect disappeared and bacterial growth increased in both groups. The intervention group had lower body temperature and cardiac index and higher static compliance compared to the control group.
Conclusion
In this porcine model, Pa-IgY antibodies lessen bacterial colonization of the airways.
Electronic supplementary material
The online version of this article (10.1186/s40635-019-0246-1) contains supplementary material, which is available to authorized users.
Keywords: Pneumonia, Nosocomial, VAP, HAP
Background
Pseudomonas aeruginosa is an opportunistic pathogen in humans known to host several antibiotic resistance mechanisms [1, 2]. The most lethal infections caused by P. aeruginosa are pneumonias of which ventilator-associated pneumonia (VAP) is a common clinical phenotype [3, 4]. VAP is believed to occur as bacteria-containing fluids from the oropharynx leak into and colonize the lower airways, the natural defense mechanisms being attenuated with sedation and the use of an endotracheal tube on which biofilm formation might also contribute [5, 6]. P. aeruginosa is a common pathogen in VAP, being responsible for about one in five cases in parts of the world [1].
IgY antibodies are large (167,250 kDa) monomeric antibodies produced in birds and reptiles with a molecular weight resembling mammalian IgG. The ability to harvest IgY from eggs laid by hens inoculated with P. aeruginosa provides a cheap and effective production method of specific polyclonal anti-P. aeruginosa IgY antibodies (Pa-IgY) [7]. These antibodies bind primarily to the flagella of these bacteria and facilitate opsonization by augmenting the phagocytic activity of polymorphonuclear neutrophils [8]. Two studies have shown that Pa-IgY can increase the time to airway colonization by P. aeruginosa in patients with cystic fibrosis [9, 10]. Another study administered Pa-IgY in a murine pneumonia model and found mainly prophylactic properties but also reduced pulmonary bacterial load with post-exposure treatment [11]. No study has previously investigated the prophylactic properties of Pa-IgY in a model of VAP development.
Today, the foundation of treating VAP is the effective use of antibiotics, something that is increasingly difficult with the increasing presence of resistant bacteria. To maintain the possibility of treating VAP caused by P. aeruginosa before resistance to available treatments becomes too widespread, novel strategies have to be examined. The objective of this study was to examine if it is possible to prevent initial airway colonization by P. aeruginosa with the use of Pa-IgY in an animal model with anesthetized and mechanically ventilated piglets. We used a model with nebulized delivery of P. aeruginosa predominantly to the large airways resulting in tracheobronchial colonization [12]. Our hypothesis was that the administration of Pa-IgY decreases the colonization by P. aeruginosa of the airways. The primary endpoint of this study is the growth of P. aeruginosa in tracheal cultures over time.
Materials and methods
Ethical approval
Ethical approval was received from the local animal ethics committee (application C155/14), and all animals were handled according to guidelines from the Animal Ethics Board (Uppsala, Sweden) and the European Union’s directives for animal research.
Study protocol
This study was a prospective experimental animal study. Twelve crossbred Norwegian landrace pigs, 6–8 weeks old, were used. The experiments were carried out in an animal research facility at Uppsala University with experienced staff and in an ICU-like setting. All pigs were sedated with tiletamine/zolazepam 6 mg/kg (Zoletil, Virbac, Stockholm, Sweden) and xylazine 2.2 mg/kg (Rompun, Bayer, Köpenhamn, Denmark). Anesthesia was induced with 100 mg ketamine (Ketaminol, Intervet, Stockholm, Sweden) and 20 mg morphine (Morfin Meda, Meda, Solna, Sweden) and maintained with 1 g pentobarbital (Pentobarbitalnatrium, Apoteket, Kungens Kurva, Sweden) and 32.5 mg morphine mixed in 1000 mL of 25 mg/mL glucose given at 8 mL/kg/h. Relaxation was achieved using rocuronium 10 mg/mL (Esmeron, MSD, Stockholm, Sweden) infused at 2.5 mg/kg/h. Initial fluid was given as a bolus of 20 mL/kg Ringer’s acetate (Ringer-acetat, Fresenius Kabi, Uppsala, Sweden), and the same fluid was given as maintenance at 2 mL/kg/h intravenously. All pigs were mechanically ventilated (ratio of inspiratory to expiratory time 1:2, fraction inspired oxygen (FiO2) 0.3, tidal volume 10 mL/kg, respiratory rate 25, positive end-expiratory pressure (PEEP) 5 cmH2O) through a tracheostomy and received a central venous catheter, a pulmonary artery catheter, an arterial catheter, and a suprapubic urinary catheter. After preparation, the experiment started and carried on for 27 h with regular data collection. The experiment was carried out with animals lying on their side, changing side every 6 h followed by alveolar recruitment.
Additional morphine and ketamine was administered as needed to keep the animals anesthetized. Noradrenaline 20 μg/mL (Noradrenalin, Hospira Nordic, Stockholm, Sweden) was administered as a continuous infusion starting at 5 mL/h and increased as needed to maintain mean arterial pressure (MAP) > 60 mmHg. At cardiac output < 2 L/min, a clinical decision was made to either increase the rate of noradrenaline or to give a 15 mL/kg bolus of Ringer’s acetate. Normoventilation (PaCO2 4.5–6.5 kPa) was achieved by adjusting tidal volume. Oxygenation target (PaO2 10–30 kPa) was achieved by adjusting FiO2, and for repeated hypoxemia, PEEP was incrementally increased. The animals were heated as necessary using heating pads, fluid warmers, and covers to maintain a body temperature between 35 and 42 °C. At the end of the experiment, the pigs were sacrificed using 20 mL KCl.
IgY production
The method used for the production of Pa-IgY has been previously described [13, 14]. Briefly, White Leghorn hens were injected intramuscularly with inactivated P. aeruginosa (and Freund’s incomplete adjuvant), and identical booster injections were administered at 4-week intervals. After the second booster injection, the eggs were collected and the yolks were isolated. Polyethylene glycol (PEG) was added to the yolks twice in a precipitation process to isolate the yolk proteins. Ammonium sulfate was then added to remove the PEG and further purify large yolk proteins. This achieves a Pa-IgY fraction with a purity of more than 90%. The activity of purified Pa-IgY was measured with ELISA to yield equally active batches resulting in concentrations of Pa-IgY at approximately 5 mg/mL as measured spectrophotometrically. Pa-IgY was donated by Immunsystem I. M. S. AB (Uppsala, Sweden).
Intervention
The pigs were allocated randomly to either receive Pa-IgY (intervention, n = 6) and P. aeruginosa, P. aeruginosa only (control, n = 6), Pa-IgY only (n = 3,) or anesthesia only (n = 3). The last two groups were only included as controls to detect effects of Pa-IgY and anesthesia, and their data are not included in the main analysis and can be found in (Additional file 1: Table S1A-7) together with a flowchart of the study groups (Additional file 1: Figure A1). Pa-IgY was administered immediately before the administration of P. aeruginosa. Ten milliliters of 5 mg/mL Pa-IgY was administered through a jet nebulizer at a flow of 6 L/min connected to the ventilatory circuit between the endotracheal tube and the Y-connection; this allowed for 10 mL of fluid to be nebulized during 10 min. Ten milliliters of 108 CFU/mL P. aeruginosa (PA-103, ATC 29260, CCUG31589) in log phase was then administered with the same method of nebulization; the strain is a virulent clinical isolate from sputum with a type 3 secretion system and exotoxin A production. The strain has been tested for when log phase occurs (105 min) and is resistant to pig serum. Nebulizations were administered in the supine position before laying the pigs on their side.
Data collection
Physiological parameters were measured regularly at predefined time points. Blood samples were collected in EDTA tubes and lithium heparin tubes every 3 h at 0–12 h and 24–27 h. These tubes were analyzed at the central laboratory of the hospital for complete blood counts, plasma cytokines, and plasma and urine creatinine levels. Blood gases were analyzed in blood gas analyzers at the animal research facility (ABL835- Flex Radiometer and OSM3 Oximeter Radiometer). Urine was collected during three time intervals, 0–12 h, 12–24 h, and 24–27 h to measure urine output and for calculation of creatinine clearance. Tracheal secretions were acquired through a 10 mL tracheal lavage followed by aspiration. One hundred microliters of the lavage was then serially diluted to determine the number of P. aeruginosa colony-forming units. Each dilution was cultured on a CLED plate. Blood cultures were drawn from the arterial catheter and 100 μL of blood was cultured on CLED plates. All cultures were incubated for at least 24 h at 37 °C. The colonies were then manually counted, and the most diluted culture with a growth of 30–300 colonies was recorded as the bacterial growth for that sample. Cultures were also acquired from a lower right lobe lung biopsy measuring 1 g which was minced, diluted in 200 μL NaCl, and then cultured on CLED culture plates.
Statistical analysis
Data was tested for normality and is presented accordingly as mean ± SEM or median (IQR). Differences in baseline characteristics were tested for using an independent t test or Mann-Whitney U test according to normality. Culture results, cytokines, and arterial lactate levels were log-normally distributed and were thus log-transformed. To test differences for repeated measurements between groups and over time, mixed linear models (ANOVA III) were used. p < 0.05 was considered statistically significant. Pa-IgY and anesthesia only groups were not included in the statistical analysis.
Results
Baseline characteristics
The intervention group was similar to the control group at baseline (Table 1). Pigs in the intervention group weighed more than in the control group.
Table 1.
Characteristics at baseline
| Baseline characteristics | ||
|---|---|---|
| Intervention | Control | |
| Weight (kg)* | 31.2 ± 1.1 | 27.9 ± 0.73 |
| HR (min−1) | 98 ± 4 | 92 ± 6 |
| MAP (mmHg) | 81 ± 5 | 78 ± 5 |
| CI (L/min/m2) | 2.18 (0.72) | 2.83 (0.62) |
| Arterial lactate (mmol/L) | 2.2 ± 0.4 | 1.9 ± 0.2 |
| Hemoglobin (g/L) | 87 ± 1 | 87 ± 3 |
| WBC (109/L) | 18.0 ± 2.7 | 15.6 ± 1.8 |
| Neutrophil count (109/L) | 8.6 ± 2.1 | 7.0 ± 1.3 |
| Core temperature (°C) | 39.3 ± 0.2 | 39.5 ± 0.2 |
| IL-6 (pg/mL) | 31.0 ± 28.5 | 39.7 ± 20.2 |
| TNF-α (pg/mL) | 184.6 ± 16.9 | 166.6 ± 22.0 |
| Static compliance (mL/cmH2O) | 29.8 ± 3.9 | 29.3 ± 2.7 |
| PaO2/FiO2 ratio (mmHg) | 370 (34) | 420 (56) |
| Plasma creatinine (μmol/L) | 43 ± 2 | 50 ± 4 |
Values are mean ± SEM or median (IQR). *p < 0.05, tested according to normality with independent t test or Mann-Whitney U test. HR heart rate, MAP mean arterial pressure, CI cardiac index, aLactate arterial lactate, WBC white blood cell count, IL-6 interleukin-6, TNF-α tumor necrosis factor α
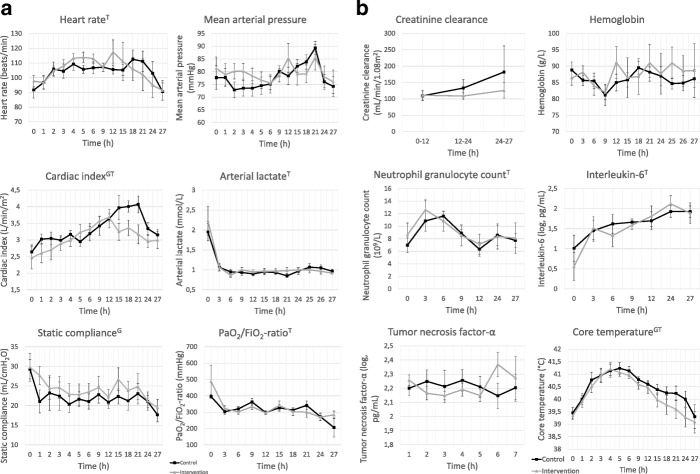
Physiological parameters and laboratory analysis (Fig. 1)
Fig. 1.
a Graphs representing physiological parameters and laboratory analysis over time. Data points represent mean and error bars represent SEM. Black line represents the control group and gray line represents the intervention group. G, significant group difference; T, significant difference over time. No parameters had a significant group-time interaction. b Graphs representing physiological parameters and laboratory analysis over time. Data points represent mean and error bars represent SEM. Black line represents the control group and gray line represents the intervention group. G, significant group difference; T, significant difference over time. No parameters had a significant group-time interaction
HR changed over time (p = 0.024) with no difference between groups. There was no difference in MAP between groups, and there was no difference in the total administered noradrenaline between groups, 137 μg (1705) in the control group vs 0 μg (4463) in the intervention group. CI increased over time in both groups (p = 0.001) with higher levels in the intervention group (p = 0.009). aLactate decreased over time in both groups with no difference between groups. There was no difference over time or between groups in Hb or WBC. Neutrophil granulocyte count (NGC) changed over time (p = 0.040) with no difference between groups. Core temperature changed over time in both groups (p < 0.001) with higher temperatures in the control group compared to the intervention group (0.027). IL-6 increased over time with no difference between groups (p < 0.001). There was no difference over time or between groups for TNF-α. Static compliance was lower in the control group compared to the intervention group (p = 0.039) during the experiment. PaO2/FiO2 ratio decreased over time in both groups (p = 0.021) with no difference between groups. There was no difference in creatinine clearance between groups or over time. The Pa-IgY only and anesthesia only groups had numerically lower HR, CI, and core temperature (see Additional file 1 for values). MAP and Hb were numerically lower in these two groups. The anesthesia only group had numerically higher static compliance and PaO2/FiO2 ratio.
Bacterial cultures
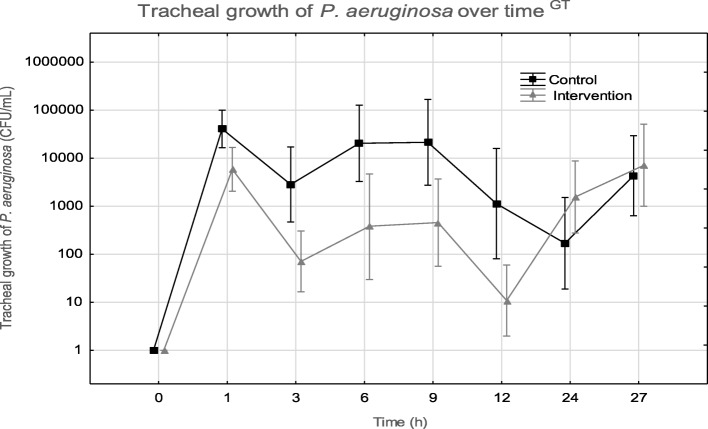
There was an increase in tracheal growth of P. aeruginosa over time (p = 0.001) in all pigs with less growth in the intervention group compared to the control group (p = 0.02) (Fig. 2, Additional file 1: Table S1B for individual data). The bacterial growth was one to two orders of magnitude lower in the intervention group until 12 h. After 12 h, growth increased in both groups and the numerical difference between groups disappeared. Three pigs in the intervention group showed cultures with no growth of P. aeruginosa at various time points 0–12 h with one pig where P. aeruginosa was eradicated 3–27 h. In the control group, one pig had one culture without growth between 0 and 12 h and two pigs at 12 h. Pulmonary biopsies showed growth in only one pig from each group, 190 CFU/mL in the control group vs. 126 CFU/mL in the intervention group. There was no growth in blood cultures. The Pa-IgY only and anesthesia only groups had no growth of P. aeruginosa in cultures. The growth of endogenous flora (Bordetella bronchiseptica) at 0 h was seen in two pigs in the intervention group; B. bronchiseptica did not grow in any subsequent cultures. The growth of E. coli occurred in one pig from the control group and one pig from the intervention group at 24 h with E. coli with continued growth at 27 h in the pig from the control group.
Fig. 2.
Tracheal growth of P. aeruginosa over time. Data points represent mean and error bars represent SEM. Black line represents the control group and gray line represents the intervention group. G, significant group difference; T, significant difference over time
Discussion
In this study, we used a porcine model of large airway colonization by P. aeruginosa to investigate if a pre-exposure inhalation of Pa-IgY could reduce subsequent colonization by P. aeruginosa. Our results show that treatment with Pa-IgY reduces the growth of P. aeruginosa in tracheal cultures.
These data are in line with previous research on patients with cystic fibrosis where gargling of Pa-IgY increased time to initial colonization by P. aeruginosa [9, 10] It is also in line with studies on mice where prophylactic properties of Pa-IgY were found [11]. Yet, unlike the findings in that study, we saw a decreased effect of Pa-IgY after 12 h. Given the findings in mice, it is less likely that the loss of treatment effect after 12 h in this study is due to degradation of the IgY molecule. There is also evidence that the half-life of IgY in pig serum is closer to 48 h rather than 12 h [15]. Rather, this may be due to the fact that at the dose given there are unaffected bacteria that keep dividing, and eventually, the bacterial load is too large compared to the available Pa-IgY binding capacity for the treatment effect to persist. This could be mitigated by larger or repeated doses of Pa-IgY. Finally, theoretically emergence of bacterial resistance could also explain the decreased antibacterial effect of Pa-IgY. However, an in vivo study has shown that numbers of P. aeruginosa do not increase until 24 h after antimicrobial challenge [16]. One may speculate that given the short time to decreasing effect (12 h) and that the antibodies we use are polyclonal with several binding sites to the bacteria [8], bacterial resistance to Pa-IgY is less likely.
In our experiments, static compliance was higher in the intervention group than the control group. Although the mechanisms of this observation are not elucidated by our data, lower P. aeruginosa burden in Pa-IgY-treated animals could have contributed to better respiratory mechanics in this group. Core temperature was higher in the control group, which might imply an increased inflammatory response without Pa-IgY when the flagella of these bacteria can stimulate the immune system primarily via the TLR-5 receptors [17]. CI was also higher in the control group signifying an increased stress response. These findings in the control group are unspecific but could represent the initial signs of an infection. This is biologically plausible since colonization precedes infection and there is less colonization in the intervention group, delaying the onset of infection. The inflammatory basis of the difference in temperature and CI are contradicted by the lack of group difference in WBC, NGC, IL-6, or TNF-α. On the other hand, although the TLR-5 pathway induces several inflammatory mediators, including TNF-α and IL-6, its main effect is mediated through IL-8 which was not measured in this study [18].
This is as far as we know the first study to report the effect of Pa-IgY on lower respiratory tract colonization by P. aeruginosa. The strength of this study lies in its use of a mechanically ventilated large animal model in an ICU setting, resembling clinical VAP and allowing complex physiological measurements and repeated blood and tracheal sampling. The animals were also followed for more than 24 h, allowing the assessment of the length of Pa-IgY effect. The relatively small number of animals used is a limitation. However, these experiments are cumbersome costly and limitation of the number of animals is strongly encouraged by the ethical rules. Given the set alpha level, the number of animals impacts mainly type II error. Another limitation lies in the fact that the model only investigates P. aeruginosa colonization, not if Pa-IgY can prevent VAP by decreasing colonization, since this is not a VAP model. The nebulization of P. aeruginosa and Pa-IgY probably delivers a lower dose to the pig lung than what is nebulized due to losses in the ventilator circuit [19]. This has been accounted for by choosing larger doses of both nebulizations. The growth of P. aeruginosa and the treatment effect seen with Pa-IgY confirms effective nebulization. Also, in this study, we did not do any histopathological analysis of the lungs, since our main focus was bacterial growth. We did not take pulmonary biopsies from all lobes, as the pulmonary biopsies were taken from a standardized location in the right lower lobe to represent alveoli and bronchiole. However, we examined both lungs macroscopically, and this standardized biopsy location gave representative samples. We observed the pigs for more than a day. However, longer experiments could be of interest, especially for studying VAP, since bacterial growth may increase up to 72 h in the lungs [20]. Finally the study, although randomized, was not blinded; however, interventions allowed were performed to a preset protocol.
Future research in this area should explore the effect of Pa-IgY in both the prevention and post-exposure treatment of VAP. Also, studies on IgY against other pathogens than P. aeruginosa would be of interest. An earlier study has tested monoclonal antibodies for pneumonia caused by Staphylococcus aureus suggesting that antibodies are an attractive target for future human research [21]. If Pa-IgY is proven to be effective against P. aeruginosa, this can have great implications for patients even outside of VAP and should be studied accordingly. Since P. aeruginosa is an opportunistic pathogen, all immunocompromised patients could benefit from prevention and eventual treatment to spare both antibiotic use and suffering for the patient.
Conclusions
In summary, in an anesthetized and mechanically ventilated porcine model, specific polyclonal anti-P. aeruginosa IgY antibodies can be used as prophylaxis to decrease colonization in the lower respiratory tract by P. aeruginosa. These findings are an important step towards new therapies for VAP in the race against antimicrobial resistance.
Additional file
Table S1A-7. Data stratified per group for complementary experimental groups. All data is measured according to what is described in the methods section. Number are mean ± SEM. Figure S1A. Flowchart of experimental groups. A flowchart describing the different experimental groups. Table S1B. Individual tracheal growth of P. aeruginosa stratified per group. Individual data of Tracheal growth of P. aeruginosa over time per pig (colony forming unit, CFU/mL). Data presented is growth in tracheal cultures at each time point during the experiment for each animal. CFU: colony forming unit. (DOCX 28 kb)
Acknowledgements
We would like to thank the staff at the animal research facility for their help with animal handling and adherence to the experimental protocol.
Funding
This study was funded by Vinnova, grant 2016-04083 and the Uppsala University hospital research fund. Funding sources had no influence over experimental protocol, data analysis or preparation of the manuscript.
Availability of data and materials
Data is not collected from a public database. Collected data is available upon request from the corresponding author
Abbreviations
- aLactate
Arterial lactate
- CFU
Colony forming units
- CI
Cardiac index
- FiO2
Fraction of inspired oxygen
- Hb
Hemoglobin
- HR
Heart rate
- IL-6
Interleukin-6
- MAP
Mean arterial pressure
- NGC
Neutrophil granulocyte count
- Pa-IgY
Specific polyclonal anti-P. aeruginosa IgY antibodies
- PEEP
Positive end-expiratory pressure
- PEG
Polyethylene glycol
- TNF-α
Tumor necrosis factor alpha
- VAP
Ventilator-associated pneumonia
- WBC
White blood cell count
Authors’ contributions
AO contributed to the design of the experiment, animal experiments, data collection, statistical analysis, and preparation of the manuscript. KH contributed to the design of the experiment and the preparation of bacterial strain. ELL contributed to the design of the experiment, animal experiments, data collection, and preparation of the manuscript. AL contributed to the preparation of antibodies, preparation of the manuscript, and analysis of blood samples. JS contributed to the preparation of antibodies and preparation of the manuscript. ML contributed to the design of the experiment, animal experiments, data collection, statistical analysis, and preparation of the manuscript. All authors read and approved the final manuscript.
Ethics approval
Ethical approval was received from the local animal ethics committee (application C155/14) and all animals were handled according to guidelines from the Animal Ethics Board (Uppsala, Sweden) and European Union’s directives for animal research.
Consent for publication
Not applicable
Competing interests
A. Larsson and J. Stålberg are shareholders and employed by Immunsystem I. M. S. AB (Uppsala, Sweden), respectively. They had no influence on the animal experiments, data acquisition, or analysis of data.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A. Otterbeck, Email: alexander.otterbeck@surgsci.uu.se
K. Hanslin, Email: katja.hanslin@surgsci.uu.se
E. Lidberg Lantz, Email: elin.lidberg.lantz@gmail.com.
A. Larsson, Email: anders.larsson@akademiska.se
J. Stålberg, Email: johan.stalberg@immunsystem.se
M. Lipcsey, Email: miklos.lipcsey@surgsci.uu.se
References
- 1.Ding C, Yang Z, Wang J, et al. Prevalence of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa in patients with pneumonia in mainland China: a systematic review and meta-analysis. Int J Infect Dis. 2016;49:119–128. doi: 10.1016/j.ijid.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujitani S, Sun H-Y, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139:909–919. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 4.Vincent J-L, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 5.Kallet RH. The vexing problem of ventilator-associated pneumonia: observations on pathophysiology, public policy, and clinical science. Respir Care. 2015;60:1495–1508. doi: 10.4187/respcare.03774. [DOI] [PubMed] [Google Scholar]
- 6.Mietto C, Pinciroli R, Patel N, Berra L. Ventilator associated pneumonia: evolving definitions and preventive strategies. Respir Care. 2013;58:990–1007. doi: 10.4187/respcare.02380. [DOI] [PubMed] [Google Scholar]
- 7.Dias da Silva W, Tambourgi DV. IgY: a promising antibody for use in immunodiagnostic and in immunotherapy. Vet Immunol Immunopathol. 2010;135:173–180. doi: 10.1016/j.vetimm.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomsen K, Christophersen L, Jensen PØ, Bjarnsholt T, Moser C, Høiby N. Anti-Pseudomonas aeruginosa IgY antibodies promote bacterial opsonization and augment the phagocytic activity of polymorphonuclear neutrophils. Human Vaccines Immunotherapeutics. 2016;12:1690–1699. doi: 10.1080/21645515.2016.1145848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollberg H, Carlander D, Olesen H, Wejåker P-E, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol. 2003;35:433–440. doi: 10.1002/ppul.10290. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson E, Larsson A, Olesen HV, Wejåker P-E, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol. 2008;43:892–899. doi: 10.1002/ppul.20875. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen K, Christophersen L, Bjarnsholt T, Jensen PØ, Moser C, Høiby N. Anti-Pseudomonas aeruginosa IgY antibodies augment bacterial clearance in a murine pneumonia model. J Cyst Fibros. 2016;15:171–178. doi: 10.1016/j.jcf.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Li J, Leavey A, O'Neil C, Babcock HM, Biswas P. Comparative study on the size distributions, respiratory deposition, and transport of particles generated from commonly used medical nebulizers. J Aerosol Med Pulm Drug Deliv. 2017;30:132–140. doi: 10.1089/jamp.2016.1340. [DOI] [PubMed] [Google Scholar]
- 13.Pauly D, Chacana PA, Calzado EG, Brembs B, Schade R (2011) IgY technology: extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. J Vis Exp [DOI] [PMC free article] [PubMed]
- 14.Polson A, von Wechmar MB, van Regenmortel MH. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980;9:475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama H, Peralta RC, Sendo S, Ikemori Y, Kodama Y. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing. Am J Vet Res. 1993;54:867–872. [PubMed] [Google Scholar]
- 16.Feng Y, Hodiamont CJ, van Hest RM, Brul S, Schultsz C, Ter Kuile BH. Development of antibiotic resistance during simulated treatment of Pseudomonas aeruginosa in chemostats. PLoS One. 2016;11:e0149310. doi: 10.1371/journal.pone.0149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feuillet V, Medjane S, Mondor I, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett. 2005;101:117–122. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.ElHansy MHE, Boules ME, El Essawy AFM, et al. Inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during invasive mechanical ventilation. Pulm Pharmacol Ther. 2017;45:159–163. doi: 10.1016/j.pupt.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Li Bassi G, Rigol M, Marti JD, et al. A novel porcine model of ventilator-associated pneumonia caused by oropharyngeal challenge with Pseudomonas aeruginosa. Anesthesiology. 2014;120:1205–1215. doi: 10.1097/ALN.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 21.Francois B, Mercier E, Gonzalez C, et al. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med. 2018;44:1787–1796. doi: 10.1007/s00134-018-5229-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1A-7. Data stratified per group for complementary experimental groups. All data is measured according to what is described in the methods section. Number are mean ± SEM. Figure S1A. Flowchart of experimental groups. A flowchart describing the different experimental groups. Table S1B. Individual tracheal growth of P. aeruginosa stratified per group. Individual data of Tracheal growth of P. aeruginosa over time per pig (colony forming unit, CFU/mL). Data presented is growth in tracheal cultures at each time point during the experiment for each animal. CFU: colony forming unit. (DOCX 28 kb)
Data Availability Statement
Data is not collected from a public database. Collected data is available upon request from the corresponding author