Abstract
Many developmental disorders of the inner ear are manifested clinically as delayed motor development and challenges in maintaining posture and balance, indicating involvement of central vestibular circuits. How the vestibular circuitry is rewired in pediatric cases is poorly understood due to lack of a suitable animal model. Based on this, our lab designed and validated a chick embryo model to study vestibular development in congenital vestibular disorders. The developing inner ear or “otocyst” on the right side of 2-day-old chick embryos (E2) was surgically rotated 180° in the anterior–posterior axis, forming the “anterior–posterior axis rotated otocyst chick” or ARO chick. The ARO chick has a reproducible pathology of a sac with truncated or missing semicircular canals. A sac is the most common inner ear defect found in children with congenital vestibular disorders. In E13 ARO chicks, the sac contained all three cristae and maculae utriculi and sacculi, but the superior crista and macula utriculi were shortened in anterior–posterior extent. Also, the number of principal cells of the tangential vestibular nucleus, a major avian vestibular nucleus, was decreased 66 % on the rotated side. After hatching, no difference was detected between ARO and normal chicks in their righting reflex times. However, unlike normal chicks, ARO hatchlings had a constant, right head tilt, and after performing the righting reflex, ARO chicks stumbled and walked with a widened base. Identifying the structure and function of abnormally developed brain regions in ARO chicks may assist in improving treatments for patients with congenital vestibular disorder.
Keywords: otocyst rotation, chick vestibular nuclei
INTRODUCTION
All vertebrates must maintain body equilibrium for well-being and survival. This universal requirement may explain why vertebrates share a common, phylogenetically old blueprint for the vestibular system. Signals originating from vestibular sensory organs in the inner ear are processed over widespread areas, starting in the brainstem vestibular nuclei and cerebellum and then caudally in the spinal cord and rostrally in brainstem motor nuclei, thalamus, and vestibular cerebral cortex. Sensory integration starts in the vestibular nuclei, with conscious awareness of motion, spatial orientation, and navigation occurring in multiple vestibular cerebral cortical areas. Vestibular sensory organs that detect rotation are found in the semicircular canals, while gravity-detecting organs are located mainly in the utricle and saccule. In a wide spectrum of congenital vestibular disorders, children are diagnosed with inner ear pathologies, delayed motor development, and severe posture and balance problems (Abadie et al. 2000). At present, the effects of abnormal vestibular inner ear development on the vestibular nuclei are uncertain. We propose that the vestibular inner ear defects produce reorganization of the vestibular nuclei, so that they generate a feed-forward cascade of events resulting in abnormal signaling in multiple brain regions processing vestibular signals.
Identifying the neuroanatomical and functional substrates of brain dysfunction in congenital disorders is a challenge that is compromised by the lack of a satisfactory experimental animal model. Thus, our goal is to understand the outcome of inner ear pathology in an animal model with a reproducible inner ear pathology that resembles the most common inner ear defect found in children with congenital vestibular disorders. From computer tomography (CT) scans, these children most often show a sac-like inner ear with truncated or missing semicircular canals (Abadie et al. 2000; Satar et al. 2003; Morimoto et al. 2006; Sanlaville and Verloes 2007; Vesseur et al. 2016). Therefore, we designed a model that forms a sac-like inner ear in 85 % of cases (n = 44/52). A sac was formed by surgically rotating the developing inner ear or “otocyst” 180° in the anterior–posterior axis in 2-day-old chick embryos (E2), creating the “anterior–posterior axis rotated otocyst chick” or ARO chick. We selected the chick because its vestibular inner ear resembles the human inner ear and its bipedal posture resembles the human stance more than the quadruped’s. We studied ARO chicks primarily at E13, a stage two-thirds of the way through gestation because important vestibular inner ear features have already emerged, like the orthogonal position of the semicircular canals (E9–E10; Bissonnette and Fekete 1996). Since the human inner ear achieves adult size about halfway through gestation (17–19 gestation weeks) (Jeffery and Spoor 2004), we believe that characterizing a midpoint in gestation is useful to identify early-onset defects that may be corrected before their full impact materializes postnatally. As a first step in understanding the consequences of abnormal inner ear pathology on embryonic central vestibular development, inner ear structures were identified in paint-fills; the histology of cristae, macula utriculi, and macula sacculi was studied in tissue sections of ARO sacs; and a specific subset of brainstem vestibular nuclei neurons was counted in tissue sections. In addition, vestibular reflex behaviors were tested in 1–5-day-old ARO hatchling chicks.
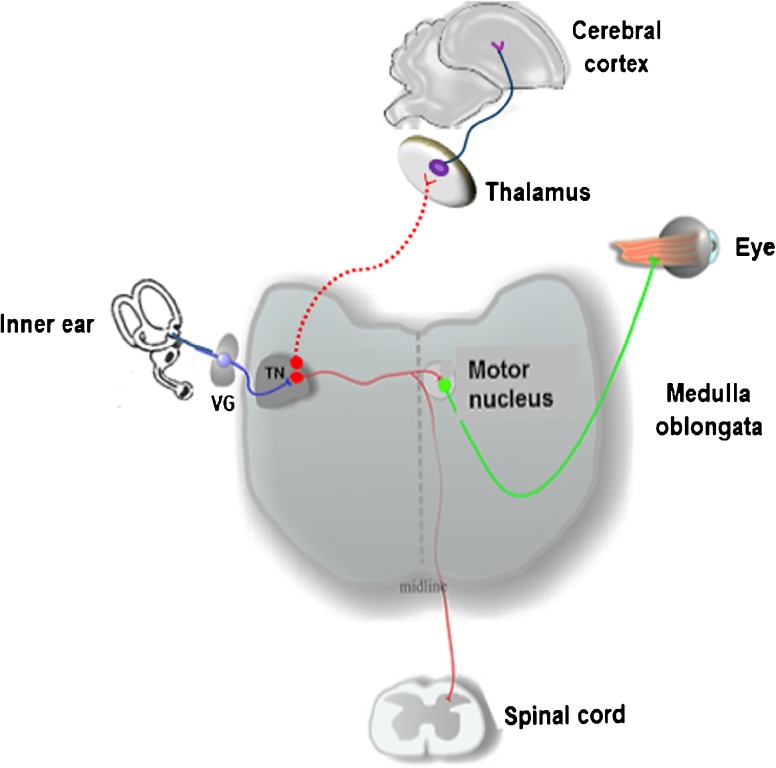
The chick tangential nucleus is a major avian vestibular nucleus with a well-defined architecture and its principal cells aligned in rows between the laterally coursing, glutamatergic primary vestibular fibers and longitudinally coursing GABAergic fibers (Popratiloff et al. 2004; Popratiloff and Peusner 2011). Principal cell axons project to the oculomotor nuclei and cervical spinal cord (Cox and Peusner 1990a; for review, see Peusner 2014) (Fig. 1). In hatchling chicks, the principal cells compose about 80 % of the tangential nucleus neurons from a total of some 300 neurons (Aldrich and Peusner 2002). Since the principal cells predominate, these studies have focused on them. The largest diameter primary vestibular fibers form large calycine endings around hair cells in central regions of the cristae and maculae (Fink and Morest 1977), while centrally, these vestibular axons form synaptic terminals on the tangential nucleus principal cells (Peusner and Morest 1977a, b). The three semicircular canals form discrete and separate projections to the tangential nucleus (Cox and Peusner 1990b). Utricular and saccular fibers do not overlap with each other, but they do share common regions with the canal fibers (Popratiloff and Peusner 2007). Given the wealth of data available, the principal cells offer an unparalleled opportunity to determine whether vestibular nuclei neurons achieve major developmental milestones at appropriate times in ARO chicks.
Fig. 1.
Diagram of the connections between the vestibular sensory organs and tangential nucleus (TN). Vestibular ganglion cells (VG; blue) are first-order neurons transmitting signals from hair cells in the vestibular sensory organs to vestibular nuclei neurons (red) in the medulla oblongata. The TN is a vestibular nucleus whose principal cells send axons to the cervical spinal cord to control neck muscles or to the oculomotor nuclei (green) to control the extrinsic eye muscles. Some vestibular nuclei neurons (not identified) project to the thalamus for relays to vestibular cerebral cortical areas for spatial orientation
METHODS
Experimental Animals
Fertilized white Leghorn chick embryo eggs (University of Connecticut Poultry Unit, Storrs, CT; Charles River, Wilmington, MA; B&E Eggs, York Springs, PA) were incubated at 100 °F with controlled humidity (60 %) and egg rotation unit.
Otocyst Rotation
The Hamburger and Hamilton (1951) staging method was used to establish the age of all chick embryos. The otic placode is the first inner ear precursor to appear at stage 9 (St. 9) from a thickened patch of ectoderm lateral to the hindbrain. By St. 10, the placode transforms into a cup, which closes by St. 16 to form an otic vesicle or “otocyst” (Brigande et al. 2000). In this study, inner ear precursors were rotated from St. 15 to St. 17, bridging the time between otic cup and otocyst, so the latter term was used here loosely to refer to inner ear precursors.
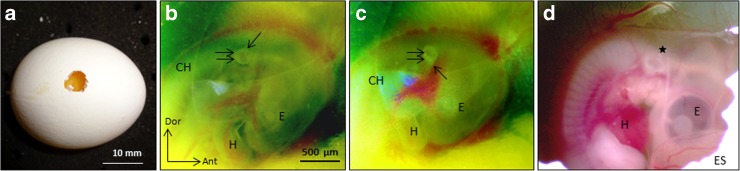
At E2 (St. 15–St. 17), the otocyst was rotated in situ. Briefly, a quarter inch oval area was sanded down on the eggshell surface (Dremel drill), and the underlying shell membranes were removed with forceps, so that the embryo was visible on top of the yolk sac using a stereo dissecting microscope (Fig. 2a). Visibility of the otocyst was improved by injecting 0.1 % fast green FCF in 0.1 M phosphate-buffered saline (PBS) beneath the embryo using a glass pipette attached to an aspirator tubing assembly (Boulland et al. 2010). The pipettes (1.5 mm OD borosilicate glass; WPI) were pulled on a Brown/Flaming horizontal pipette puller. The chorion and amnion were torn focally with fine-tipped forceps to access the otocyst (Fig. 2b). The right otocyst was rotated in ovo using a tungsten wire (125 μm diameter) with curved tip to cut the tissue surrounding the otocyst. Otocyst rotation reversed the anterior–posterior and dorsal–ventral otocyst axes, but not the medial–lateral axis (Fig. 2c) (Wu et al. 1998; Hutson et al. 1999). Accordingly, the chick model was called the anterior–posterior axis rotated otocyst chick or ARO chick. Sham operations were performed by cutting out the right otocyst without rotating it.
Fig. 2.
Otocyst rotation in ovo.a Chick embryo egg with a quarter inch window made by sanding down the eggshell with a Dremel drill and removing the underlying shell membranes with forceps. b E16 chick embryo in ovo before otocyst rotation, viewed under a dissecting microscope with fiber optics illumination. Fast green was injected underneath the embryo. The chorion (CH) and amnion were torn open over the otocyst. Next, the otocyst (double arrows) was cut free from surrounding tissues and rotated 180°. The single arrow points to the endolymphatic duct. E, eye; H, heart. Scale bar in b refers to b–d. c Same E16 chick embryo in b, but after 180° otocyst rotation. Single arrow points to the endolymphatic duct, which was a useful structure to confirm the degree of otocyst rotation. Same labels as in b. d Same chick embryo seen in b and c, but 1 day after otocyst rotation. *, developing inner ear; ES, eggshell. Dor, dorsal; Ant, anterior. Same labels as in b, c
After surgery, the eggshell window was sealed and the egg returned to the incubator without daily egg rotation. The position of the otocyst in ARO chicks was checked and photographed 1 day after surgery (Zeiss Axiocam ERc 5s camera; Zen blue software; Zeiss Instruments) (Fig. 2d). ARO chicks were decapitated at E13 for paint-fills of the inner ear and Nissl-stained tissue sections of the brainstem with or without inner ear structures present. The sham-operated chicks were decapitated at E13 for paint-fills. Some ARO chicks were allowed to hatch for vestibular reflex testing. Finally, a subset of chick embryo eggs were windowed, taped closed, and reincubated without rotation until they hatched (control hatchlings). Normal chick embryos and hatchlings were prepared at the same ages as ARO chicks for comparison.
Inner Ear Imaging
Paint-Fills
Normal, ARO, and sham-operated chicks were decapitated; the lower jaw was removed; the cranium and meninges were opened dorsally; and the head was immersed in Bodian’s fixative (Martin and Swanson 1993; Bissonnette and Fekete 1996; Kiernan 2006). After 2 days, the brain was dissected out and processed as described for specimens with the chick head intact (see below). The head was cut in half to separate left and right inner ears, which were dehydrated in ethanol and cleared in methyl salicylate. With the medial surface facing upward, the half-head was pinned down to the bottom of a petri dish coated with Sylgard containing 2 % charcoal. After immersing the half-head in methyl salicylate, paint (Liquid paper; Newell Rubbermaid) dissolved in methyl salicylate was loaded into a pipette attached to a Hamilton syringe. The syringe was mounted on a micromanipulator, and under a dissecting microscope with fiber optics illumination, the pipette was advanced slowly to penetrate the inner ear and inject paint into the membranous labyrinth of the utricle (E13, 1 % paint; H5, 4 % paint). By E13, the otoliths appeared opaque white and provided good targets to position the pipette tip. Photographs of the paint-filled inner ears were taken on a camera (Zeiss Axiocam ERc 5s) attached to the dissecting microscope (Zeiss Discovery.V8) and processed using ZEN blue computer program (Zeiss Instruments).
Inner Ear Measurements
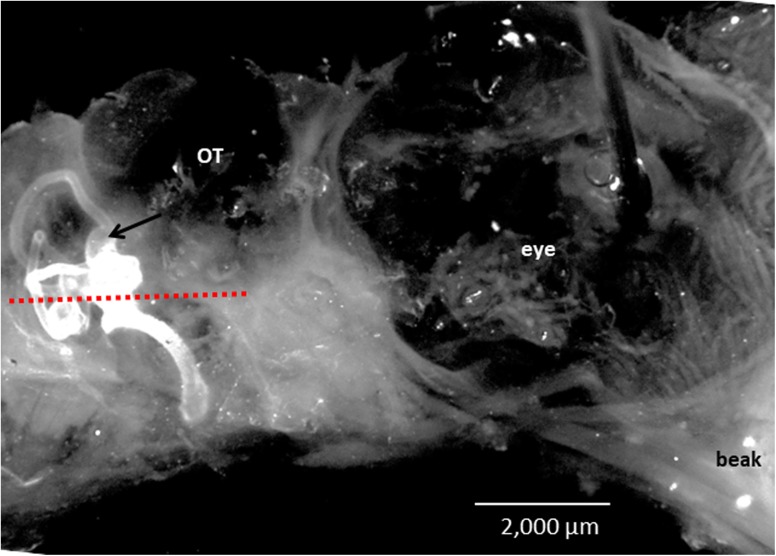
At E13, the overall dimensions of the paint-filled labyrinths were measured from photographs of the lateral surface of the inner ear using ZEN blue computer program. The background of the images was blackened using Adobe Photoshop (version CC 2017). The ear was placed with its lateral canal in the horizontal plane (Fig. 3). The anterior–posterior expanse of the superior canal was measured from its anterior to posterior tip, while its dorsal–ventral expanse was measured from its dorsal tip to the surface of the utricle. Likewise, the anterior–posterior expanse of the lateral canal, dorsal–ventral expanse of the posterior canal, and anterior–posterior and dorsal–ventral dimensions of the ampullae were measured. The length of the cochlea was measured from the apex to the base, with its width measured halfway along the length. The anterior–posterior and dorsal–ventral dimensions of the sac were measured by placing its vertical long axes in the vertical plane. The outer canal diameter of the superior and posterior canals was measured 50 μm from the ampulla, whereas the lateral canal diameter was measured near the canal center because its ampullary connection was not seen in lateral surface views. Finally, the distance was measured between the superior canal ampulla and posterior edge of the eyeball, or the anterior surface of the sac and posterior edge of the eyeball.
Fig. 3.
Right side of normal E13 chick embryo head showing the position of the right, paint-filled inner ear. A red-dotted line was drawn parallel to the lateral canal in the horizontal plane. The distance between the posterior surface of the eyeball and anterior surface of the inner ear, or sac, was similar for E13 ARO chicks forming sacs and normal E13 chicks. Arrow points to ampulla of superior canal. A pin was placed through the eyeball to hold down the head to Sylgard at the bottom of the dish. OT, site in cranium where optic tectum is seated
Imaging the Cristae and Maculae
Since the cristae and maculae were not visible in paint-filled inner ears, a series of chick embryo heads were prepared with the inner ears and brain intact from E13 ARO chicks and normal chicks and stained with the Nissl method (Luna 1968). Briefly, after immersion in Bodian’s fixative for 2 days, the heads were placed in 10 % neutral buffered formaldehyde for at least 2 days, embedded in paraffin, and sectioned transversely in 20 μm serial sections. The vestibular sensory organs were described and measured using a ×20 objective (NA 0.8) on a Zeiss Imager.A2 light microscope. Images shown in Figs. 5, 6, and 7 were virtual slides produced by stitching together adjacent fields from Nissl-stained, 20-μm-thick tissue sections using a ×10 objective (NA 0.45) on a Zeiss Cell Observer light microscope.
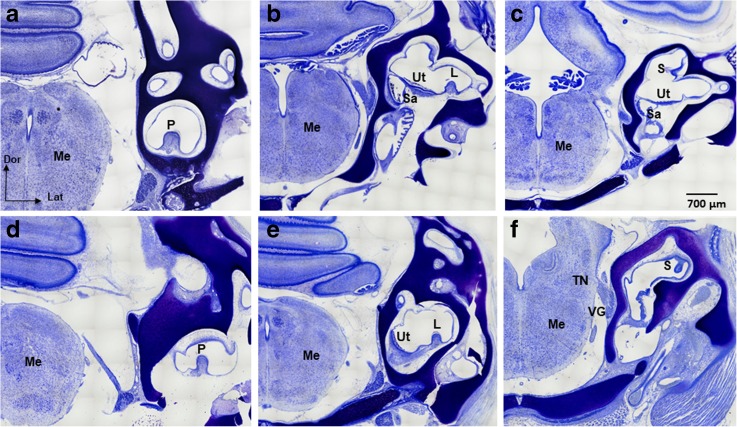
Fig. 5.
Vestibular sensory organs in Nissl-stained, transverse tissue sections of E13 normal (a–c) and ARO chicks with sacs (d–f). All sections are oriented with the inner ear on the right side of the brain for comparison. Only the superior cristae and maculae utriculi in the ARO sacs showed significantly reduced anterior–posterior extent compared to normal E13 chicks. The posterior crista (P) of normal chick embryos (a) and ARO chicks (d) faced dorsally. The lateral cristae (L) of normal chick embryos (b) and ARO chicks (e) faced dorsally. The superior cristae (S) of normal chick embryos faced medially (c), but faced ventrally in ARO chicks (f). Thus, only the superior crista was severely disoriented. Note that the utricle (Ut) is oriented horizontally but tilted more laterally in ARO chicks (e) compared to the normal (b). The saccule (Sa) is oriented vertically in normal (b, c) and in ARO chicks (not shown). VG, vestibular ganglion; TN, tangential nucleus; Me, medulla oblongata; Dor, dorsal; Lat, lateral. The scale bar in c refers to a–f
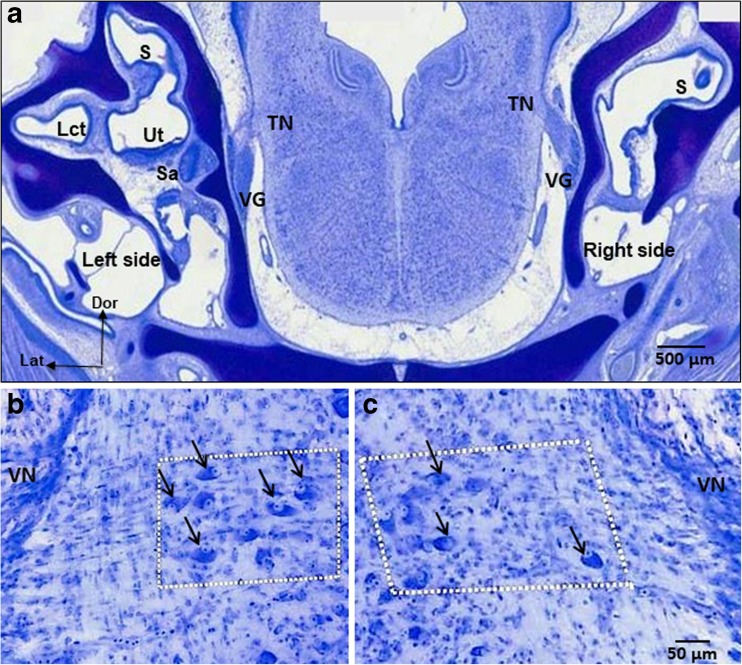
Fig. 6.
a Nissl-stained, transverse tissue section of E13 ARO chick embryo head with a sac on the right, rotated side. The section is taken at the level of the tangential nucleus (TN), which is a serial section to Fig. 5f. Labels are the same as shown in Fig. 5. Lct, connective tissue at the base of the lateral crista on the left, nonrotated side. b High power view of the tangential nucleus on the left, nonrotated side of the ARO chick in a. Principal cells (arrows in white boxed-in area) have large oval cell bodies aligned in rows between the primary vestibular fibers (not stained), so that the principal cells were readily identified and counted, as in normal E13 chick embryos. c Tangential nucleus on the right, rotated side of ARO chick in a. Principal cells (arrows in white boxed-in area) have normal cell body size and shape but were more dispersed in the nucleus than normally. The number of principal cells was reduced to 34 % of the normal number on the right, rotated side of E13 ARO chicks. Scale bar in c refers to b, c. Arrows point to some but not all of the principal cell bodies. VN, vestibular nerve on lateral brainstem surface; Dor, dorsal; Lat, lateral
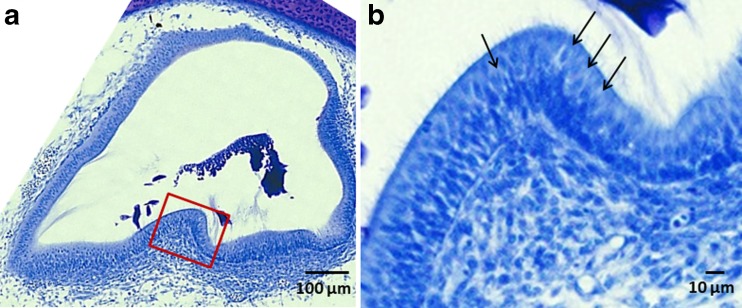
Fig. 7.
Nissl-stained, transverse tissue sections of the lateral crista on the right, rotated side of an E13 ARO chick with a sac. a The blocked in area (red line) is shown at higher magnification in b. Note the presence of large calycine endings (arrows) enclosing the type I hair cells near the central region of the crista. All cristae and maculae in E13 ARO sacs contained large calycine endings enclosing type I hair cells
Brainstem Light Microscopy
Neuron Counts
Principal cells were counted in Nissl-stained tissue sections containing the ears and brain and in additional specimens in which the brainstem was dissected out of the cranium (see above). Principal cells were counted in E13 normal and E13 ARO chick embryos when the cell’s nucleus was visible under a ×20 objective on the Zeiss Imager light microscope. No correction factors were applied because counting errors were equivalent for all groups.
Dimensions of Tangential Nucleus
In Nissl-stained tissue sections, the anterior–posterior extent of the tangential nucleus was measured by counting the number of 20 μm serial, transverse tissue sections containing the principal cells. The dorsal–ventral dimension was measured in sections of the central tangential nucleus from the dorsal- to ventral-most principal cells in the section, whereas the medial–lateral dimension was measured from the medial- to lateral-most principal cells in the section using a calibrated ocular micrometer (10 μm subdivisions) and ×20 objective. The central tangential nucleus is located halfway between the anterior–posterior extent of the nucleus, where the largest number of principal cells is usually found in one section.
Principal Cell Body Size
Principal cell body size was measured from 20 principal cells taken from the central tangential nucleus in E13 normal and E13 ARO chick embryos. A principal cell was measured when its nucleus was present in the plane of the section. The length of the principal cell was taken as its longest diameter, with the width perpendicular to the length.
Whole-Mount Immunocytochemistry
Whole mounts of E4–E5 normal and ARO chick embryos were immersion fixed in 4 % paraformaldehyde in 0.1 M PBS overnight before incubating for 1 h in blocking solution which consisted of 0.1 M PBS with 0.1 % Triton X-100 (PBS-T), 10 % normal goat serum, and 1 % Boerhinger-Mannhein blocking powder (Karpinski et al. 2014). Next, the embryos were incubated in primary antibody in blocking solution at 4 °C on a shaker for 3–4 days, followed by incubation in secondary antibody in blocking solution at 4 °C on a shaker for 1–2 days. Embryos were then dehydrated in methanol/PBS followed by clearing in 2:1 benzyl benzoate:benzyl alcohol before imaging them on a Zeiss LSM 710 confocal microscope. The primary antibodies included purified anti-tubulin β3 (TUBB3) (Biolegend, TUJ1, #801202; 1/500 or 1/1000), phalloidin conjugated to Alexa Fluor 647 (Invitrogen, A22287) (1/1000), and DAPI (Calbiochem, 268298). The secondary antibody used for tubulin was Alexa Fluor goat antimouse 488 IgG (1/1000 or 1/500) (Invitrogen, A11029). DAPI (Calbiochem, 268298) was added to the secondary antibody solution in a concentration of 0.4–1 μg/ml dissolved in water. All embryos were placed on their side in a depression slide on the confocal stage and imaged from lateral to medial.
Vestibular Reflex Testing
Some normal and ARO chicks were incubated until hatching (H; 21 days), so that vestibular reflex performance was tested from H1 to H5. Two days before hatching, the incubator temperature was reduced 1–2° (98–99 °F) for all the eggs, the humidity was kept at 60 %, and the egg rotation unit was turned off on the normal eggs (Reis et al. 1997; Yildirim and Yetisir 2004). Head tilt, eye tests, and the righting reflex test were evaluated. Head tilt was detected by comparing the position of the head to horizontal. Eye tests included identifying spontaneous eye movements (nystagmus), eye deviations at rest, and whether the eyes were open or closed. In the righting reflex test, the chick was placed on its back on a table covered with shelf lining paper with open grids, and the time it took for the chick to stand was recorded with/without a 5 × 5-in. dark cloth covering the chick’s head. Five trials/test were performed each day on each chick from 1 to 5 days after hatching. Each trial was followed by a 20–30 s rest period. The chick’s behavior between trials and at the end of the test was observed.
Statistical Analysis
Data are presented as the average ± SEM, where SEM is defined as the standard error of the mean, or the deviation of the measurements divided by the square root of the number of measurements (n). To determine significant differences between groups, a one-way ANOVA test was performed, followed by the Tukey HSD posttest (α = 0.05). Statistics were performed in R (version 3.5.1, http://www.r-project.org). Significance was set at P < 0.05. Statistical significance is reported as the statistical value and degrees of freedom of the one-way ANOVA (F(k − 1, n− k)), along with the P value from the posttest. For the reported statistics, three groups were compared: (1) normal chick embryos, including the left and right sides; (2) left side of ARO chicks; and (3) right, rotated side of ARO chicks.
RESULTS
Overview
Why Study E13 Chicks?
Although the overall shape of the semicircular canals is apparent at E6.5, their orthogonal configuration does not appear until E9–E11 (Bissonnette and Fekete 1996). Centrally, the principal cells of the tangential nucleus acquire their characteristic size and shape at E13, so that accurate identification and neuron counts are possible (Peusner 1984). Thus, ARO chicks were decapitated at E13, when both peripheral and central vestibular components have acquired major developmental features important to the study. From a group of 52 ARO chicks, 85 % formed a sac-like inner ear, with the remaining chicks forming an inner ear with three small canals (not shown). Thus, otocyst rotation in the anterior–posterior plane produced a relatively consistent and reproducible outcome of a sac-like inner ear. Below, we have focused mainly on describing the results from E13 ARO chick embryos with a sac-like inner ear phenotype.
Survival of ARO Chicks
ARO, normal, and sham-operated chicks were prepared at the same ages. When the otocyst was cut out and replaced in its normal position and orientation in sham-operated chicks, normal inner ears were produced in 8/12 cases at E13. In four cases, one canal was missing, but none of the sham-operated chicks formed a sac. Using standard laboratory incubation conditions including egg rotation, 73 % of normal chick embryo eggs hatched (n = 11/15 eggs). When an eggshell window was introduced at E2 and the chick egg was removed from egg rotation, 29 % of normal chick embryo eggs hatched (n = 2/7 eggs). Finally, 41 % of ARO chicks survived from E2 to E13 (n = 17/42), and 17 % of these hatched (n = 7/42 eggs). Thus, viability of ARO chicks decreased in a large part due to making an eggshell window, which precluded egg rotation during incubation, and in part due to the natural stresses related to hatching.
Inner Ear and Sac Dimensions
What Are the Dimensions of the Vestibular Labyrinths in E13 Normal and ARO Chicks?
From a lateral surface view, the overall expanse of the paint-filled, membranous labyrinths of E13 normal chicks was 3752 μm in the anterior–posterior axis and 4991 μm in the dorsal–ventral axis, including the cochlea (n = 10) (Fig. 3; Table 1). The superior and lateral canals extended anterior–posteriorly for 2233 and 2528 μm, respectively, while the dorsal–ventral expanses of the superior and posterior canals measured 2060 and 2119 μm, respectively (Fig. 4a). The outer diameter of the three canals was close to 200 μm. The superior ampulla measured 831 ± 33 μm in the anterior–posterior axis and 748 ± 18 μm in the dorsal–ventral axis, with the posterior and lateral ampullae of similar dimensions (not shown). The common crus outer diameter was 406 ± 29 μm (not visible). The length of the cochlea from the apex to the base was 2538 μm, and its width was 651 μm halfway along the apical–basal length.
Table 1.
Inner ear dimensions in E13 chick embryos (μm ± SEM)
| Inner ear type | Overall A/P expanse | Overall D/V expanse | Superior canal A/P expanse | Superior canal D/V expanse | Superior canal diameter | Posterior canal D/V expanse | Posterior canal diameter | Lateral canal A/P expanse | Lateral canal diameter | Cochlea length |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal inner ear n = 10 | 3752 ± 142 | 4991 ± 163 | 2233 ± 134 | 2060 ± 75 | 187 ± 10 | 2119 ± 51 | 196 ± 10 | 2528 ± 63 | 187 ± 10 | 2538 ± 64 |
| ARO chick, left inner ear n = 10 | 4102 ± 147 | 4358 ± 93 | 2178 ± 131 | 1867 ± 64 | 174 ± 5 | 2003 ± 57 | 202 ± 7 | 2180* ± 60 | 179 ± 8 | 2489 ± 84 |
| ARO chick, right inner ear (sac) n = 25 | 1700* ± 136 | 2091* ± 134 | NA | NA | NA | NA | NA | NA | NA | 1586* ± 150 |
Significant differences from normal are indicated by * (P < 0.05, one-way ANOVA followed by Tukey HSD posttest). Overall A/P expanse, ARO right: F(2, 42) = 79.09, P < 0.0001; overall D/V expanse, ARO right: F(2, 42) = 114.40, P < 0.0001; lateral canal A/P expanse, ARO left: F(1, 18) = 16.13, P = 0.00083; cochlea length, ARO right: F(2, 20) = 19.78, P < 0.0001
A/P, anterior–posterior; D/V, dorsal–ventral; NA, not applicable
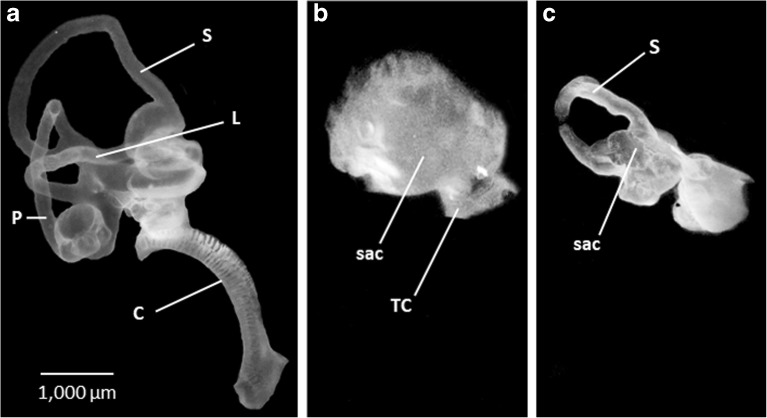
Fig. 4.
Lateral surface views of right paint-filled inner ears in normal (a) and E13 ARO chicks (b, c). In all figures, dorsal is to top and anterior is to the right. a. Normal chick embryo inner ear. S, superior canal; L, lateral canal; P, posterior canal; C, cochlea. b Sac-like inner ear with truncated canal (TC) in ARO chick. c Sac-like inner ear, forming small superior canal (S) and another truncated canal in ARO chick. The position of the truncated canals on the sacs was variable. b, c Abbreviations as in a. Scale bar in a refers to a–c
When paint was injected into the membranous labyrinths of E13 ARO chicks, the predominant inner ear pathology was a sac with truncated or absent canals (n = 25/30) (Fig. 4b, c). The cochlea was usually missing in ARO chicks. The paint-filled sacs measured 1700 μm in anterior–posterior expanse and 2091 μm in dorsal–ventral expanse (n = 25), making them < 50 % of the dimensions of the normal E13 chick inner ears (Table 1). A sac-like inner ear developed after otocyst rotation whether the otocyst wall was torn near the endolymphatic duct during rotation (n = 3) or whether the otocyst wall and neural tube were intact after otocyst rotation (n = 16). Thus, sac formation was not the result of damaging the otocyst wall or neural tube during otocyst rotation. The distance between the anterior surface of the ARO sac and posterior surface of the eyeball (5796 ± 317 μm; n = 25) did not change significantly from the distance between the normal chick inner ear and eyeball (5558 ± 176 μm; n = 10) (F(2, 40) = 0.75, P = 0.48), suggesting that the relative position of the ARO chick sac within the head was fixed and consistent with the normal position of the chick inner ear. Finally, the dimensions of the nonrotated, left inner ear of the ARO chicks were similar to those found in normal chicks, with the exception of the anterior–posterior expanse of the lateral canal (Table 1) and width of the common crus (not shown), which decreased significantly (F(1, 18) = 16.13, P = 0.00083; F(1, 18) = 6.25, P = 0.022).
Do Sensory Organs Develop in ARO Chick Sacs?
In E13 normal chick inner ears, sectioned transversely and Nissl-stained, the orientation of the cristae and maculae was similar to that described in E5 chick embryos (Battisti and Fekete 2008) (Figs. 5 and 6; Table 2). Hair cells at the top of the posterior and lateral cristae faced dorsally and the cristae measured 316 and 295 μm, respectively, in anterior–posterior extent (Fig. 5a, b; Table 2) (n = 10). In contrast, hair cells at the top of the superior cristae faced medially and the cristae measured 365 μm in anterior–posterior extent (Fig. 5c). In otolith sensory organs, hair cells in the striola region of the macula sacculi were oriented vertically and faced laterally, positioned next to the lateral brain surface, and the macula sacculi measured 423 μm in anterior–posterior extent (Fig. 5b). Hair cells in the striolar region of the macula utriculi were oriented horizontally, tilted laterally, and were positioned lateral and dorsal to the hair cells in the macula sacculi, within many of the same tissue sections. However, the macula utriculi extended more anterior and posterior than the macula sacculi due to its greater length (790 μm) (Fig. 5b).
Table 2.
Anterior–posterior extent of cristae and maculae in E13 normal and ARO chicks with sac (μm ± SEM)
| Inner ear type | Superior crista | Posterior crista | Lateral crista | Macula utriculi | Macula sacculi |
|---|---|---|---|---|---|
| Normal inner ear n = 10 |
365 ± 32 | 316 ± 28 | 295 ± 25 | 790 ± 33 | 423 ± 10 |
| ARO chick, left inner ear n = 8 |
368 ± 129 | 346 ± 90 | 290 ± 35 | 708 ± 46 | 369 ± 40 |
| ARO chick, right inner ear (sac) n = 8 |
153* ± 72 | 236 ± 105 | 238 ± 113 | 544* ± 143 | 446 ± 142 |
Significant difference from normal is indicated by * (P < 0.05, one-way ANOVA followed by Tukey HSD posttest). Superior crista, ARO right: F(2, 23) = 11.82, P = 0.00069; macula utriculi, ARO right: F(2, 23) = 12.19, P = 0.00017
In sacs of E13 ARO chicks, three cristae and utricular and saccular maculae were usually present, but the orientation and anterior–posterior extent of certain sensory organs were abnormal. The three cristae were present in all sacs (Figs. 5 and 6; Table 2), except for one case where the posterior crista was missing (n = 7/8). The orientation of the posterior crista was normal with hair cells facing dorsally and its anterior–posterior extent was not changed significantly (F(2, 23) = 2.95, P = 0.072) (Fig. 5a, d). Hair cells in the lateral crista (Fig. 5e) and macula sacculi (not shown) retained their normal orientation and anterior–posterior extent. However, hair cells in the superior crista faced ventrally, or laterally, rather than medially, and the crista underwent a significant decrease in anterior–posterior extent from 365 to 153 μm (F(2, 23) = 11.82, P = 0.00069) (Fig. 5c, f). Hair cells in the macula utriculi were positioned horizontally but were tilted more laterally than normal, while its anterior–posterior extent was reduced significantly from 790 to 544 μm (F(2, 23) = 12.19, P = 0.00017) (Fig. 5c, e). In the light microscope, hair cells in central regions of all cristae, and the striola region of the macula utriculi and macula sacculi, were surrounded by large calycine endings on the right, rotated side of ARO chicks, as in normal chicks (Fig. 7). On the left side of ARO chicks, hair cells in the cristae and maculae were normal in orientation and anterior–posterior extent, with hair cells surrounded by large calycine endings in central regions (not shown). Finally, the cochlea was not present in most sacs (n = 22/25).
Tangential Nucleus Dimensions in E13 Normal and ARO Chicks
In normal E13 chick embryos, the tangential nucleus measured 340 μm in anterior–posterior extent, 291 μm dorsoventrally, and 474 μm mediolaterally in Nissl-stained tissue sections (n = 18 tangential nuclei). Thus, by E13, the tangential nucleus has acquired about one-third of the volume found at H4–H6 when the tangential nucleus has a cuboidal shape and measures 550 μm on each axis (Aldrich and Peusner 2002). In E13 ARO chicks (n = 12), the tangential nucleus on the rotated side measured 300 μm in anterior–posterior extent, 185 μm dorsoventrally, and 248 μm mediolaterally, so that the latter two dimensions were reduced significantly compared to normal chicks (F(2, 35) = 6.89, P = 0.0033; F(2, 35) = 50.48, P < 0.0001).
Principal Cell Size, Shape, and Neuron Number in E13 ARO Chicks
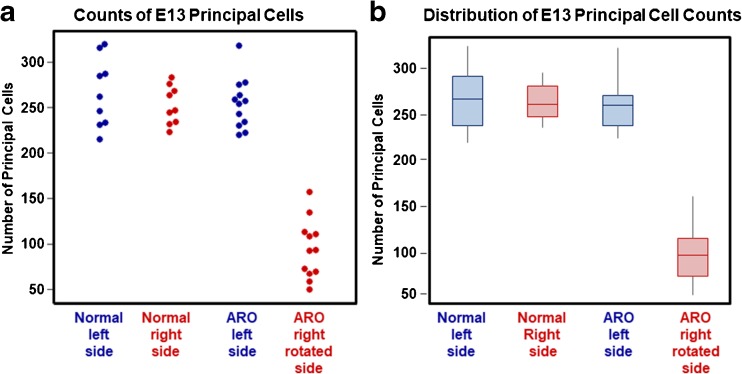
In E13 normal and ARO chicks, principal cells were distinguished by oval cell bodies that measured 26 × 15 μm, on average (normal, n = 200 neurons; ARO chicks, n = 160 neurons) (Fig. 6). Thus, up to E13, the factors controlling cell body size and shape in this class of vestibular nuclei neurons were not affected by the sac-like inner ear pathology. In ARO chicks, principal cells were found in the anterior, central, and posterior regions of the tangential nucleus, like in normal chicks, but the neurons were more dispersed on the right, rotated side due to a significantly reduced number (F(2, 39) = 123.50, P < 0.0001) (Fig. 8). At E13, the normal tangential nucleus contained 259 ± 77 principal cells (n = 18 tangential nuclei), whereas on the right, rotated side of ARO chicks, the tangential nucleus contained 94 ± 32 principal cells, a 66 % loss (n = 12 tangential nuclei). The total number of principal cells on the left side of the ARO chicks was not significantly different from normal (F(2, 39) = 123.50, P = 0.91) (compare Fig. 6b and c).
Fig. 8.
a Beeswarm plot of individual data points on the number of principal cells counted in the left (blue) and right (red) tangential nuclei (TN) from E13 normal (n = 9) and E13 ARO chicks with sacs (n = 12). Significant differences were detected between the left and right side of E13 ARO chicks (F(2, 39) = 123.50, P < 0.0001. E13 ARO chicks showed a 66 % reduction in principal cell number on the right, rotated side compared to normal chicks. No significant difference was detected in principal cell number on the left, nonrotated side of ARO chicks compared to the normal chicks. b Open boxplot showing the distribution of principal cell counts in normal E13 (n = 9) and E13 ARO chicks (n = 12). Left side, blue; right side, red
Identification of the Vestibular Nerve and Vestibular Ganglion in Normal and ARO Chicks at E4–E5
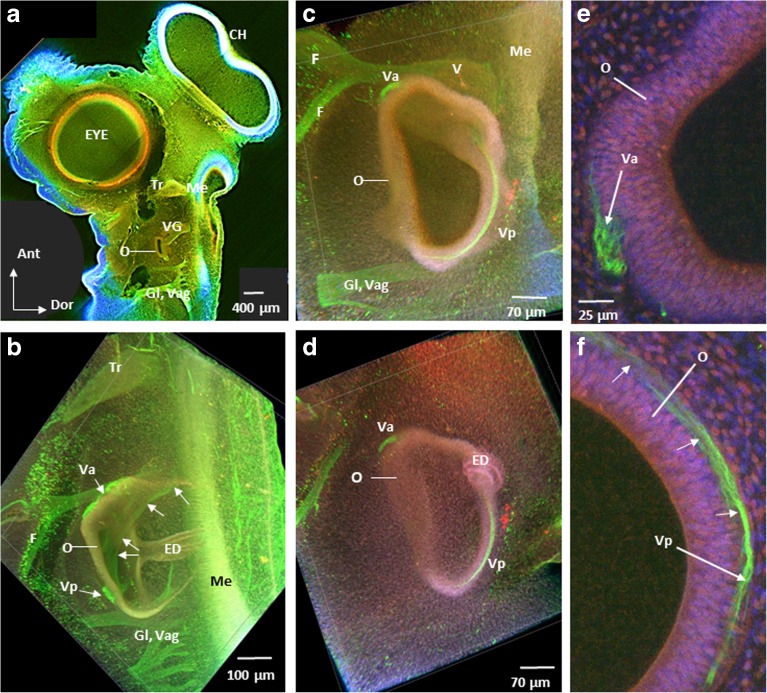
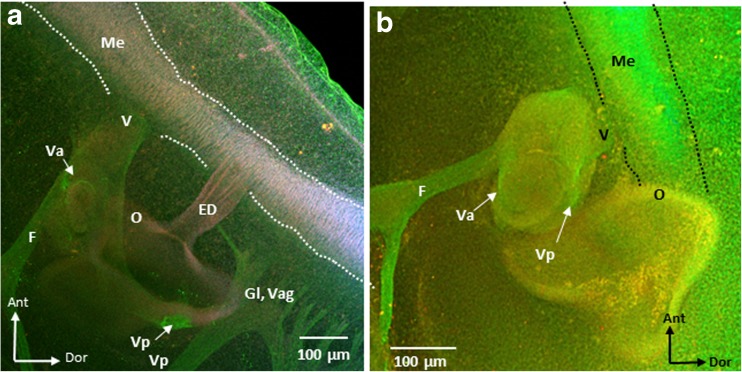
To study the presence and location of the vestibular nerve in ARO chicks, tubulin immunolabeling was performed to label microtubules (green), phalloidin to label actin filaments (red/pink/orange), and DAPI to label cell nuclei (blue) in whole mounts of E4–E5 normal (n = 9) and ARO chicks (n = 7). In lateral surface views of the ARO chick head, the presence of the vestibular ganglion and vestibular nerve was confirmed, and their location relative to the otocyst and medulla oblongata was studied in confocal images and compared to normal E4–E5 chick embryos (Figs. 9 and 10). Since only the vestibular part of the eighth nerve was imaged, auditory nerve fibers in more posterior parts were not observed (Whitehead and Morest 1985). In normal chick embryos and on the intact side of ARO chicks, the vestibular nerve ran close to the facial nerve, while the trigeminal nerve was found more anterior, and the glossopharyngeal and vagus nerves ran posterior to the otocyst (Fig. 9a, b). The same relationships were observed in ARO chicks, where the vestibular nerve formed both anterior and posterior branches (Fig. 9b). While the anterior vestibular nerve branch coursed along its normal pathway along the anterior surface of the otocyst before branching within the otocyst wall (Fig. 9c–e), the posterior vestibular nerve differed in its course in ARO chicks. Instead of traveling from anterior to posterior by curving medial and then lateral to terminate in the vestibular epithelium in the posterior parts of the otocyst wall (Fig. 9b), the posterior vestibular nerve branch in the ARO chicks ran along the dorsal otocyst wall before innervating the vestibular epithelium in posterior parts of the otocyst (Fig. 9c–f). In ARO chicks, the vestibular ganglion and vestibular nerve were present in the normal location relative to the medulla oblongata (Fig. 10a, b), and a few vestibular fibers were seen entering the medulla oblongata where they intersected longitudinal fibers running near the lateral brain surface (not shown). Longitudinal fibers run in the marginal zone of the medulla oblongata as early as E2 (Petralia and Peusner 1991). In ARO chicks, the endolymphatic duct was coiled rather than straight (Fig. 9b, d; ED).
Fig. 9.
Confocal images of E4–E5 intact side (a, b) and right, rotated side of ARO chick embryos (c–f) showing vestibular nerve immunolabeling for tubulin Alexa Fluor 488 (green channel), phalloidin Alexa Fluor 647 (red/pink/orange channel), and DAPI (blue channel). a Left, intact side of ARO chick embryo head. O, otocyst; Tr trigeminal ganglion; Me, medulla oblongata; Gl, glossopharyngeal nerve; Vag, vagus nerve, CH, cerebral hemisphere; Ant, anterior; Dor, dorsal. Labels in b–f are the same as in a, with anterior to the top and dorsal to the right. b Otocyst on the intact side. Anterior (Va) and posterior (Vp) vestibular nerve branches terminate within the otocyst wall at this age. White arrows point to the posterior branch of the vestibular nerve which travels posteriorly from the vestibular ganglion located anterior to the otocyst by curving medial and then laterally before terminating in the vestibular epithelium of posterior parts of the otocyst wall (Vp). Note that Va is located on the anterior surface of the otocyst. ED, endolymphatic duct forms a straight tube as it passed laterally above the image frame; F, facial nerve. c, d Medial and more lateral image, respectively, of the right, rotated side of ARO chick otocyst. Va followed the normal course to innervate the otocyst wall, whereas Vp ran along the dorsal surface of the otocyst wall rather than medially and then laterally before ending on the posterior surface of the otocyst wall. V, vestibular nerve near its entry into the medulla oblongata. Note the abnormal coiled structure of the endolymphatic duct (ED) in d compared to b. e, f High power views of restricted volume from c of the anterior (Va) and posterior (Vp) vestibular nerve branches on the otocyst wall. Note that the posterior branch runs along the dorsal surface of the otocyst before terminating posteriorly (white arrows). Scale bar shown in e also refers to f
Fig. 10.
Confocal images of E4–E5 ARO chick showing the vestibular nerve entering the medulla oblongata on the intact (a) and right, rotated side (b). Note that the vestibular nerve runs close to the facial nerve and anterior to the otocyst on both the intact and rotated sides, like in normal chick embryos (not shown). Panel a is a confocal image medial to Fig. 9b. Labels are the same as shown in Fig. 9
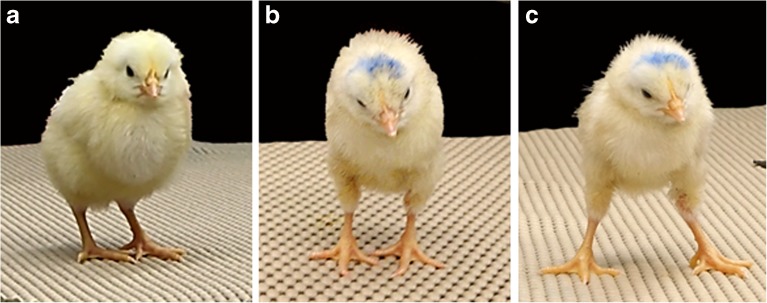
Does Vestibular Reflex Performance Change in ARO Hatchling Chicks?
Vestibular reflex activity was studied from H1 to H5 in normal (n = 13), ARO (n = 13), and control hatchlings (n = 6). In controls, a small window was made in the eggshell at E2 followed by incubating the eggs without egg rotation. After hatching, normal chicks kept their head level at all times, except for occasional brief cocking to one side, and control chick behaviors were indistinguishable from normal chicks (Fig. 11a). At all ages, ARO hatchlings had a constant head tilt toward the right side (Fig. 11b). At rest, none of the chicks showed eye deviations or nystagmus. However, ARO hatchlings tended to close one or both eyes for long intervals between the righting reflex trials (see below).
Fig. 11.
H5, normal (a) and ARO hatchling chicks (b, c). a Note the absence of head tilt and the foot position of the normal hatchling chick. b ARO hatchling exhibited a head tilt to the right, otocyst-rotated side at rest. c Same ARO chick as in b after performing the righting reflex. Note the widened base of the feet, which is more prominent in ARO, but also observed in normal chicks. After performing the right reflex, ARO chicks often stumbled on standing and closed both eyes. ARO chicks did not walk between trials or after completing the righting reflex, like normal chicks
The righting reflex usually took < 10 s (1–2 s, on average) to perform for normal, ARO, and control chicks at all ages tested, as reported for normal H4–H5 chicks (Aldrich and Peusner 2002). When a dark cloth was placed over the chick’s head, response times usually took < 10 s, although sometimes up to 1–2 min for chicks in all groups at all ages tested. Thus, righting reflex times could not be used to distinguish vestibular reflex competence in ARO and normal chicks. However, qualitative differences were apparent during the rest period between trials and at the end of the righting reflex test. Between trials, normal chicks never stumbled, but walked around with one foot in front of the other, eyes open, and pecked at the countertop. In contrast, ARO chicks often stumbled after righting, stood in place without walking around between trials, and usually closed their eyes. When they stood or walked, ARO chicks usually had their feet farther apart than normal chicks (Fig. 11c). Finally, when ARO chicks closed their eyes, a head nystagmus was observed. From paint-fills of the inner ears, all normal and control hatchlings had normal inner ears, whereas 9/13 ARO hatchlings had sac-like inner ears (n = 2 with abnormal, not sac-like, inner ears; n = 2 failed paint-fills).
DISCUSSION
Symptoms and Prevalence of Congenital Vestibular Disorders
Congenital disorders producing abnormal craniofacial development may include inner ear defects (Jongmans et al. 2006). Over 40 genes affect inner ear development (Wu and Kelley 2012). Since the vestibular system plays a central role in daily activities, significant damage to the vestibular circuitry is far-reaching. Despite some vestibular compensation over time, the symptoms never go away and are exacerbated by vestibular challenges. Pediatric cases of congenital vestibular disorders are manifested by disorientation, confusion, and fatigue, especially in children with other sensory deficits (Brown 2011). Without treatment, these children are severely disabled in eye–hand coordination, eye tracking, reading, and learning language (Blake et al. 2008).
CHARGE is an acronym for congenital abnormalities that include coloboma (eye defects), heart defects, choanal (nasal passage) atresia, retarded growth, genital hypoplasia, and ear defects. Features of CHARGE syndrome that are shared with other congenital disorders are semicircular canal hypoplasia and vestibular areflexia (Sando et al. 2001). The predominant inner ear defect in CHARGE syndrome is the formation of a sac lacking semicircular canals (Abadie et al. 2000). Although cristae and maculae are not visible in CT scans of CHARGE syndrome patients (Joshi et al. 2012), vestibular testing reveals otolith function, but no canal function (Wiener-Vacher et al. 1999). The prevalence of CHARGE syndrome is 1/10,000 (e.g., Layman et al. 2010; Green et al. 2014). Other congenital disorders with abnormal or missing semicircular canals and their incidence include Waardenburg (1/40,000; Elmaleh-Bergès et al. 2013), Noonan (1/1000–1/2500; Naficy et al. 1997), Wildervanck (1/100 in females with congenital hearing loss; Abu-Amero et al. 2014), Goldenhar (1/3500–1/25,000; Bisdas et al. 2005), and branchio-oto-renal (BOR) syndrome (1/40,000; Laclef et al. 2003). Despite the low incidence of any one congenital vestibular disorder, vestibular impairment occurs in 5.3–10 % of US children (Li et al. 2016; Rine et al. 2016).
Animal Models for Studies of Congenital Vestibular Disorders
In humans, heterozygous mutation of the chromodomain helicase DNA binding protein 7 gene (CHD7) is found in 60–80 % of CHARGE syndrome patients (e.g., Lalani et al. 2006). Mice heterozygous for Chd7 have inner ear phenotypes similar to those of CHARGE syndrome patients, along with vestibular reflex dysfunction and defects in vestibular epithelium innervation (Adams et al. 2007). For example, the lateral ampullae may have smaller widths or the posterior cristae may form flattened patches. Thus, mouse mutants have provided important new information on the role of CHD7 in inner ear development (e.g., Hurd et al. 2010; Layman et al. 2010; Micucci et al. 2014; Hale et al. 2016). However, Chd7 mutant mice are not optimum for studying brain development because of the gene’s influence on downstream genes involved in ear development (Vuorela et al. 2007). For example, bone morphogenetic protein 2 (Bmp2) is a downstream target of Chd7 (Hurd et al. 2012). If two mice have different alleles of Bmp2, different dosages of BMP2 are produced, despite having the same Chd7 gene copy. Thus, diverse inner ear phenotypes may result from mutant copies of Chd7 combined with mutation of downstream genes. Finally, Chd7 gene mutation produces defects in eye and cerebellar development, two structures that closely interact with the vestibular system (Gage et al. 2015; Feng et al. 2017). Thus, in the mouse model, the influence of an abnormal inner ear on central vestibular development cannot be distinguished from secondary effects due to abnormal eye and cerebellar development. Although genetically modified mice may accurately represent the complexity of the human disease, experiments using animal models for congenital vestibular disorders demand, first and foremost, large-scale repetition of the experiments, which combine diverse approaches to determine the consequences of inner ear pathology on central vestibular system function. Altogether, the ARO chick surmounts the complexities in the mouse model by providing a model with a vestibular inner ear pathology resembling that in humans, but isolated from abnormalities in other systems.
To produce ARO chicks, the otocyst was rotated in the anterior–posterior and dorsal–ventral axes simultaneously at St.15–St.17, modifying the concentration of at least four morphogens controlling inner ear development. Classical transplantation experiments demonstrated a role for regional polarity in the development of the labyrinths (Harrison 1935; for review, see Alsina et al. 2009). When the dorsal–ventral and medial–lateral axes of the chick otocyst were rotated together at St.15–St.17 (Hutson et al. 1999), or when one axis alone was rotated (Wu et al. 1998), sacs were not a common outcome and canal number was variable. It is well known that transplants can result in normal, abnormal, or absent structures (Yntema 1950). Thus, it is important to emphasize that the ARO chick does not form diverse inner ear structures, but almost exclusively produces a sac, the structure most commonly found in children with congenital vestibular disorders.
Sac Structure and Function
It is interesting that all the vestibular sensory organs form before the canals emerge (Wu and Oh 1996). The superior and posterior cristae develop at E3–3.5 (St. 19); the macula sacculi at St. 20; the lateral crista at E3.5–4 (St. 22, 23); the basilar papilla, lagena, and macula neglecta at E4 (St 23); and the macula utriculi at E4.5 (St. 24). Morphogenesis of the membranous labyrinths is elegantly shown in a staged series of chick embryos from E3 to E18 using the paint-fill technique (Bissonnette and Fekete 1996). At E4 (St. 23), the otocyst appears as a dorsoventrally elongated sac, with the cochlea emerging ventrally. By E5 (St. 26), the sac is elongated anteroposteriorly. By E6 (St. 29), the superior canal forms dorsally and the posterior canal ventrally, with the lateral canal emerging 6 h later. Also, at E6, ampullary swellings appear as single bulges on each canal to house the cristae ampullares. Finally, the orthogonal canal configuration appears at E9–E11 (St. 35–37). From these sequences, it appears that E2 otocyst rotation largely halted canal development, since the canals were missing or truncated in ARO chicks, but crista and macula development were variably affected. Indeed, ARO sacs routinely contained all three cristae, with the superior crista disoriented and reduced in anterior–posterior extent. The macula sacculi and macula utriculi were also present in the sacs, with only the latter disoriented and reduced in anterior–posterior extent. Why surgical rotation of the otocyst at E2 discretely affected canal outgrowth and differentiation of the superior crista and macula utriculi remains to be determined.
Part of evaluating the usefulness of the ARO chick model to study central vestibular development in congenital vestibular disorders involves determining the functionality of the pathological inner ear. During Xenopus development, the vestibular circuitry becomes functional only after the semicircular canals acquire a minimal lumen diameter, even if all other components in the circuitry are functional (Lambert et al. 2008). Due to phylogenetic conservation of the vestibular system in vertebrates, it is likely that birds and mammals also require a minimal canal lumen diameter to jump-start the central vestibular system. After otocyst rotation, hair cells in the cristae of ARO chick sacs with truncated or missing canals may not receive sufficient stimulation to generate signals on head rotation. This hypothesis is supported by clinical tests performed on CHARGE syndrome patients with sac-like inner ears who show otolith, but no canal function (Abadie et al. 2000). In future experiments, an infrared camera eye tracking system and turntable can be used to record phase and gain of the vestibuloocular reflex, which may reveal critical abnormalities in canal function in the ARO chicks (Beraneck et al. 2008).
Central Vestibular Development and Plasticity
In ARO chicks, 34 % of tangential principal cells survived at E13. Survival of embryonic principal cells has been shown to depend largely on receiving primary vestibular fiber input. For example, when the chick otocyst is ablated at E2, vestibular nerve fibers never enter the brainstem on the ablated side (Levi-Montalcini 1949; Peusner and Morest 1977c). Although the principal cells migrate and begin to differentiate up to E8–9, their cell bodies and dendrites shrivel and shrink by E10, and the principal cells disappear by E13. In normal chick embryos, the primary vestibular fibers form synapses on the principal cell bodies by E10 (Peusner and Morest 1977b), and both excitatory and inhibitory miniature spontaneous synaptic events are recorded (mEPSCs, 0.2 Hz; mIPSCs, 0.6 Hz) (Shao et al. 2006). Even at low frequencies, miniature synaptic events play an important role in the assembly of sensory circuitries (Zucker 2005). Thus, synaptic activity from the primary vestibular fibers may be essential for the principal cells to complete differentiation and survive, as reported for the frog Mauthner cell (Elliott et al. 2015a) and auditory brainstem neurons (Rubel and Fritzsch 2002).
From the finding of significant numbers of principal cell in ARO chicks, it can be concluded that these neurons receive at least some primary vestibular fiber input. Certain vestibular sensory organs were disoriented and decreased in anterior–posterior extent, likely resulting in decreased number of vestibular fibers in ARO chick brainstem. Confocal imaging of tubulin immunolabeling confirmed that the vestibular nerve on the rotated side formed separate anterior and posterior branches in the ARO chicks, with the anterior branch following a normal course, but the posterior branch deviating to reach the vestibular epithelium at E4–E5. In addition, some primary vestibular fibers entered the medulla oblongata at the normal site. Altogether, the fate of the principal cells (die or survive) on the rotated side of ARO chicks is likely determined by whether they receive appropriate synaptic connections from the primary vestibular fibers at the appropriate times.
None of the principal cells degenerate or die after vestibular ganglionectomy performed on hatchling chicks (Aldrich and Peusner 2002; Shao et al. 2009, 2012a, b). Instead, the principal cells show extensive reorganization of their synaptic inputs on the lesion and intact sides shortly after surgery. Finally, if the anterior vestibular nerve is sectioned in adult frog, the number of vestibular nuclei neurons responding to stimulation of the posterior vestibular nerve expands on the operated side 2 months after the lesion (Goto et al. 2000). Thus, the remaining vestibular nerve fibers may expand to contact additional principal cells in the ARO chicks. Vestibular fiber expansion may change the strict topographic projection pattern of the canal fibers and modify the convergence of canal and otolith fibers so that vestibular signal processing changes downstream.
Conclusions
Patients with congenital vestibular disorders likely experience asymmetric central vestibular signaling bilaterally because their two ears differ structurally (Abadie et al. 2000). This must lead to behavioral and postural abnormalities, as shown in the frog’s swimming behavior after transplanting an extra rotated otocyst on one side (Elliott et al. 2015b). In humans, asymmetric vestibular signaling may produce scoliosis (de Geus et al. 2017). However, the role of asymmetrical signaling from abnormal vestibular sensory organs onto vestibular nuclei neurons has not been fully explored (Wiener-Vacher and Mazda 1998). Thus, the ARO chick model offers this opportunity in a developing system.
Acknowledgements
We would like to acknowledge Ms. Lakshmi Kammili of GWU Pathology Core Laboratory for processing the chick specimens for paraffin embedding, tissue sectioning, and Nissl staining.
Funding information
This work was supported in part by research funds from the GWU Department of Anatomy and Cell Biology and GWU Luther Rice Undergraduate Fellowships (SJL and HES).
Compliance with Ethical Standards
Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the George Washington University. The experiments also conform to the International Guidelines for the Ethical Treatment of Animals.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sigmund J. Lilian, Email: tug89374@temple.edu
Hayley E. Seal, Email: hayleyseal@gwumail.gwu.edu
Anastas Popratiloff, Email: anastas@gwu.edu.
June C. Hirsch, Email: jvhirsch@gwu.edu
Kenna D. Peusner, Phone: 202-994-3489, Email: peusnerk@gwu.edu
References
- Abadie V, Wiener-Vacher S, Morisseau-Durand M-P, Poree C, Amiel J, Amanou L, Peigne C, Lyonet S, Manach Y. Vestibular anomalies in CHARGE syndrome: investigations on and consequences for postural development. Eur J Pediatr. 2000;159:569–574. doi: 10.1007/s004319900409. [DOI] [PubMed] [Google Scholar]
- Abu-Amero KK, Kondkar AA, Alorainy IA, Khan AO, Al-Enazy LA, Oystreck DT, Bosley TM. Xq26.3 microdeletion in a male with Wildervanck syndrome. Ophthalmic Genet. 2014;35:18–24. doi: 10.3109/13816810.2013.766218. [DOI] [PubMed] [Google Scholar]
- Adams ME, Hurd EA, Beyer LA, Martin DM. Defects in vestibular sensory epithelia and innervation in mice with loss of Chd7 function: implications for human CHARGE syndrome. J Comp Neurol. 2007;504:519–532. doi: 10.1002/cne.21460. [DOI] [PubMed] [Google Scholar]
- Aldrich EM, Peusner KD. Vestibular compensation after ganglionectomy: ultrastructural study of the tangential vestibular nucleus and behavioral study of the hatchling chick. J Neurosci Res. 2002;67:122–138. doi: 10.1002/jnr.10076. [DOI] [PubMed] [Google Scholar]
- Alsina BA, Giraldez F, Pujades C. Patterning and cell fate in ear development. Int J Dev Biol. 2009;53:1503–1513. doi: 10.1387/ijdb.072422ba. [DOI] [PubMed] [Google Scholar]
- Battisti AC, Fekete DM. Slits and Robos in the developing chicken inner ear. Dev Dyn. 2008;237:476–484. doi: 10.1002/dvdy.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100:945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisdas S, Lenarz M, Lenarz T, Becker H. Inner ear abnormalities in patients if Goldenhar syndrome. Otol Neurotol. 2005;26:398–404. doi: 10.1097/01.mao.0000169796.83695.56. [DOI] [PubMed] [Google Scholar]
- Bissonnette JP, Fekete DM. Standard atlas of the gross anatomy of the developing inner ear of the chicken. J Comp Neurol. 1996;368:620–630. doi: 10.1002/(SICI)1096-9861(19960513)368:4<620::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Blake KD, Hatshorne TS, Lawand C, Dailor AN, Thelin JW. Cranial nerve manifestations in CHARGE syndrome. Am J Med Genet A. 2008;146A:585–592. doi: 10.1002/ajmg.a.32179. [DOI] [PubMed] [Google Scholar]
- Boulland J-L, Hlasi G, Kasumacic N, Glover JC (2010) Xenotransplantation of human stem cells into the chicken embryo. J Vis Exp (41):2071 [DOI] [PMC free article] [PubMed]
- Brigande JV, Kiernan AE, Gao X, Iten LE, Fekete DM. Molecular genetics of pattern formation in the inner ear: do compartment boundaries play a role? Proc Natl Acad Sci. 2000;97:11700–11706. doi: 10.1073/pnas.97.22.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. Consequences of vestibular dysfunction. In: Hartshorne TS, Hefner MA, Davenport SLH, Thelin JW, editors. CHARGE syndrome. San Diego: Plural Publishing Inc.; 2011. [Google Scholar]
- Cox RG, Peusner KD. Horseradish peroxidase labeling of the efferent and afferent pathways of the avian tangential vestibular nucleus. J Comp Neurol. 1990;296:324–341. doi: 10.1002/cne.902960211. [DOI] [PubMed] [Google Scholar]
- Cox RG, Peusner KD. Horseradish peroxidase labeling of the central pathways in the medulla of the ampullary nerves in the chicken, Gallus gallus. J Comp Neurol. 1990;297:564–581. doi: 10.1002/cne.902970409. [DOI] [PubMed] [Google Scholar]
- de Geus CM, Free RH, Verbist BM, Sival DA, Blake KD, Meiners LC, van Ravenswaaij-Arts CMA. Guidelines in CHARGE syndrome and the missing link: cranial imaging. Am J Med Genet. 2017;175C:450–464. doi: 10.1002/ajmg.c.31593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KL, Houston DW, Fritzsch B. Ear manipulations reveal a critical period for survival and dendritic development at the single-cell level in Mauthner neurons. Dev Neurobiol. 2015;75:1339–1351. doi: 10.1002/dneu.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KL, Houston DW, Fritzsch B. Sensory afferent segregation in three-eared frogs resemble the dominance columns observed in three-eyed frogs. Sci Rep. 2015;5:8338. doi: 10.1038/srep08338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaleh-Bergès M, Baumann C, Noël-Pétroff N, Sekkal A, Couloigner V, Devriendt K, Wilson M, Marlin S, Sebag G, Pingault V. Spectrum of temporal bone abnormalities in patients with Waardenburg syndrome and SOX10 mutations. Am J Neuroradiol. 2013;34:1257–1263. doi: 10.3174/ajnr.A3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Kawauchi D, Körkel-Qu H, Deng H, Serger E, Sieber L, Lieberman JA, Jimeno-González S, Lambo S, Hanna BS, Harim Y, Jansen M, Neuerburg A, Friesen O, Zuckermann M, Rajendran V, Gronych J, Ayrault O, Korshunov A, Jones DT, Kool M, Northcott PA. Chd7 is indispensable for mammalian brain development through activation of a neuronal differentiation program. Nat Commun. 2017;8:14758. doi: 10.1038/ncomms14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink DJ, Morest DK. Formation of synaptic endings by colossal fibers in the vestibular epithelium of the chick embryo. Neuroscience. 1977;2:229–252. doi: 10.1016/0306-4522(77)90091-4. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Hurd EA, Martin DM. Mouse models for the dissection of CHD7 functions in eye development and the molecular basis for ocular defects in CHARGE syndrome. Invest Ophthalmol Vis Sci. 2015;56:7923–7930. doi: 10.1167/iovs.15-18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F, Straka H, Dieringer N. Expansion of afferent vestibular signals after the section of one of the vestibular nerve branches. J Neurophysiol. 2000;84:581–584. doi: 10.1152/jn.2000.84.1.581. [DOI] [PubMed] [Google Scholar]
- Green GE, Huq FS, Emery SB, Mukherji SK, Martin DM. CHD7 mutations and CHARGE syndrome in semicircular canal dysplasia. Otol Neurotol. 2014;35:1466–1470. doi: 10.1097/MAO.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CL, Niederriter AN, Green GE, Martin DM. Atypical phenotypes associated with pathogenic CHD7 variants and a proposal for broadening CHARGE syndrome clinical diagnostic criteria. Am J Med Genet A. 2016;170:344–354. doi: 10.1002/ajmg.a.37435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. doi: 10.1002/jmor.1050880104. [DOI] [PubMed] [Google Scholar]
- Harrison RG. Relations of symmetry in the developing ear of amblystoma punctatum. Proc Natl Acad Sci. 1935;22:238–247. doi: 10.1073/pnas.22.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd EA, Poucher HK, Cheng K, Raphael Y, Martin DM. The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear. Development. 2010;137:3139–3150. doi: 10.1242/dev.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd EA, Micucci JA, Reamer EN, Martin DM. Delayed fusion and altered gene expression contribute to semicircular canal defects in Chd7 deficient mice. Mech Dev. 2012;129:308–323. doi: 10.1016/j.mod.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MR, Lewis JE, Nguyen-Luu D, Lindberg KH, Barald KF. Expression of Pax2 and patterning of the chick inner ear. J Neurocytol. 1999;28:795–807. doi: 10.1023/A:1007057719025. [DOI] [PubMed] [Google Scholar]
- Jeffery N, Spoor F. Prenatal growth and development of the modern human labyrinth. J Anat. 2004;204:71–92. doi: 10.1111/j.1469-7580.2004.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MCJ, Admiraal RJ, van der Donk KP, Vissers LELM, Baas AF, Kapusta L, van Hagen JM, Donnai D, de Ravel TJ, Veltman JA, Geurts van Kessel A, De Vries BBA, Brunner HG, Hoefsloot LH, van Ravenswaaij CMA. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. Med Genet. 2006;43:306–314. doi: 10.1136/jmg.2005.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi VM, Shantanu KN, Kishore GR, Reddy KJ, Kumar ECV. CT and MRI imaging of the inner ear and brain with children with congenital sensorineural hearing loss. RadioGraphics. 2012;32:683–698. doi: 10.1148/rg.323115073. [DOI] [PubMed] [Google Scholar]
- Karpinski BA, Maynard TM, Fralish MS, Nuwayhid S, Zohn IE, Moody SA, LaMantia AS. Dysphagia and disrupted cranial nerve development in a mouse model of DiGeorge (22q11) deletion syndrome. Dis Model Mech. 2014;7:245–257. doi: 10.1242/dmm.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE. The paintfill method as a tool for analyzing the three-dimensional structure of the inner ear. Brain Res. 2006;1091:270–276. doi: 10.1016/j.brainres.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney, and craniofacial abnormalities in Six1 deficient mice. Mech Dev. 2003;120:669–679. doi: 10.1016/S0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Lalani SR, Hefner MA, Belmont JW, Davenport SLH. CHARGE syndrome. In: Adams MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors. GeneReviews (Internet) Seattle: University of Washington, Seattle; 2006. [Google Scholar]
- Lambert FM, Beck JC, Baker R, Straka H. Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus. J Neurosci. 2008;28:8086–8095. doi: 10.1523/JNEUROSCI.1288-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, Hurd EA, Martin DM. Chromodomain proteins in development: lessons from CHARGE syndrome. Clin Genet. 2010;78:11–20. doi: 10.1111/j.1399-0004.2010.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The development of the acoustico-vestibular centers in the chick embryo in the absence of the afferent root fibers and of descending fiber tracks. J Comp Neurol. 1949;91:209–241. doi: 10.1002/cne.900910204. [DOI] [PubMed] [Google Scholar]
- Li C-M, Hoffman HJ, Ward BK, Cohen HS, Rine RM. Epidemiology of dizziness and balance problems in children in the United States: a population–based study. J Pediatr. 2016;171:240–247. doi: 10.1016/j.jpeds.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3. New York: McGraw-Hill; 1968. [Google Scholar]
- Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodelings that control semicircular canal formation. Dev Biol. 1993;159:549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- Micucci JA, Layman WS, Hurd EA, Sperry ED, Frank SF, Durham MA, Swiderski DL, Skidmore JM, Scacheri PC, Raphael Y, Martin DM. CHD7 and retinoic acid signaling cooperate to regulate neural stem cell and inner ear development in mouse models of CHARGE syndrome. Hum Mol Genet. 2014;15:434–448. doi: 10.1093/hmg/ddt435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto AK, Wiggins IIIRH, Hudgins PA, Hedlund GL, Hamilton B, Mukherji SK, Telian SA, Harnsberger HR. Absent semicircular canals in CHARGE syndrome: radiologic spectrum of findings. Am J Neuroradiol. 2006;27:1663–1671. [PMC free article] [PubMed] [Google Scholar]
- Naficy S, Shepard NT, Telian SA. Multiple temporal bone anomalies associated with Noonan syndrome. Otolaryngol Head Neck Surg. 1997;116:265–267. doi: 10.1016/S0194-5998(97)70339-5. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Peusner KD. The earliest ultrastructural development of the tangential vestibular nucleus in the chick embryo. J Comp Neurol. 1991;310:82–93. doi: 10.1002/cne.903100108. [DOI] [PubMed] [Google Scholar]
- Peusner KD. The development of synapses and “spoon” synaptic terminal space in the tangential vestibular nucleus: a quantitative electron microscope study. J Comp Neurol. 1984;230:372–385. doi: 10.1002/cne.902300306. [DOI] [PubMed] [Google Scholar]
- Peusner K. Development of the central vestibular system. In: Romand R, Varella-Nieto I, editors. Development of auditory and vestibular systems. 4. Oxford: Elsevier; 2014. [Google Scholar]
- Peusner KD, Morest DK. The neuronal architecture and topography of the nucleus vestibularis tangentialis in the late chick embryo. Neuroscience. 1977;2:189–207. doi: 10.1016/0306-4522(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Peusner KD, Morest DK. A morphological study of neurogenesis in the nucleus vestibularis tangentialis of the check embryo. Neuroscience. 1977;2:209–227. doi: 10.1016/0306-4522(77)90090-2. [DOI] [PubMed] [Google Scholar]
- Peusner KD, Morest DK. Neurogenesis in the nucleus vestibularis tangentialis of the chick embryo in the absence of the primary afferent fibers. Neuroscience. 1977;2:253–270. doi: 10.1016/0306-4522(77)90092-6. [DOI] [PubMed] [Google Scholar]
- Popratiloff A, Peusner KD. Otolith fibers and terminals in chick vestibular nuclei. J Comp Neurol. 2007;502:19–37. doi: 10.1002/cne.21273. [DOI] [PubMed] [Google Scholar]
- Popratiloff A, Peusner KD. GABA and glycine immunolabeling in the chicken tangential nucleus. Neuroscience. 2011;175:328–343. doi: 10.1016/j.neuroscience.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Popratiloff A, Wang Y-Z, Petralia RS, Giaume C, Peusner KD. AMPA receptor subunits expression in chick vestibular nucleus neurons. J Neurosci Res. 2004;76:662–677. doi: 10.1002/jnr.20115. [DOI] [PubMed] [Google Scholar]
- Reis LH, Gama LT, Chaveriro Soares M. Effects of short storage conditions and broiler breeder age on hatchability, hatching time, and chick weights. Poult Sci. 1997;76:1459–1466. doi: 10.1093/ps/76.11.1459. [DOI] [PubMed] [Google Scholar]
- Rine RM, Dannenbaum E, Szabo J. 2015 section on pediatric knowledge translation lecture: pediatric vestibular-related impairments. Pediatr Phys Ther. 2016;28:2–6. doi: 10.1097/PEP.0000000000000226. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Sando I, Orita Y, Miura M, Balaban CD. Vestibular abnormalities in congenital disorders. Ann N Y Acad Sci. 2001;942:15–24. doi: 10.1111/j.1749-6632.2001.tb03731.x. [DOI] [PubMed] [Google Scholar]
- Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet. 2007;15:389–399. doi: 10.1038/sj.ejhg.5201778. [DOI] [PubMed] [Google Scholar]
- Satar B, Mukherji SK, Telian SA. Congenital aplasia of the semicircular canals. Otol Neurotol. 2003;24:437–446. doi: 10.1097/00129492-200305000-00014. [DOI] [PubMed] [Google Scholar]
- Shao M, Hirsch JC, Peusner KD. Emergence of action potential generation and synaptic transmission in vestibular nucleus neurons. J Neurophysiol. 2006;96:1215–1226. doi: 10.1152/jn.00180.2006. [DOI] [PubMed] [Google Scholar]
- Shao M, Popratiloff A, Yi J, Lerner A, Hirsch JC, Peusner KD. Adaptation of chicken vestibular nucleus neuron to unilateral vestibular ganglionectomy. Neuroscience. 2009;161:988–1007. doi: 10.1016/j.neuroscience.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Shao M, Hirsch JC, Peusner KD. Plasticity of spontaneous excitatory and inhibitory synaptic activity in morphologically defined vestibular nuclei neurons during early vestibular compensation. J Neurophysiol. 2012;107:29–41. doi: 10.1152/jn.00406.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Reddaway R, Hirsch JC, Peusner KD. Presynaptic GABAB receptors decrease neurotransmitter release in vestibular nuclei neurons during vestibular compensation. Neuroscience. 2012;223:333–354. doi: 10.1016/j.neuroscience.2012.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesseur AC, Verbist BM, Westerlaan HE, Kloostra FJJ, Admiraal RJC, can Ravenswaaij-Arts CMA, Free RH, Mylanus EAM. CT findings of the temporal bone in CHARGE syndrome: aspects of importance in cochlear implant surgery. Eur Arch Otorhinolaryngol. 2016;273:4225–4240. doi: 10.1007/s00405-016-4141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorela P, Ala-Mello S, Saloranta C, Penttinen M, Pöyhönen M, Huoponen K, Borozdin W, Bausch B, Botzenhart EM, Wilhelm C, Kääriäinen H, Kohlhase J. Molecular analysis of the CHD7 gene in CHARGE syndrome: identification of 22 novel mutations and evidence for a low contribution of large CHD7 deletions. Genet Med. 2007;9(10):690–694. doi: 10.1097/GIM.0b013e318156e68e. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Morest DK. The development of innervation patterns in the avian cochlea. Neuroscience. 1985;14:255–276. doi: 10.1016/0306-4522(85)90177-0. [DOI] [PubMed] [Google Scholar]
- Wiener-Vacher SR, Mazda K. Asymmetric otolith vestibulo-ocular responses in children with idiopathic scoliosis. J Pediatr. 1998;132:1028–1032. doi: 10.1016/S0022-3476(98)70403-2. [DOI] [PubMed] [Google Scholar]
- Wiener-Vacher SR, Amanou L, Denise P, Narcy P, Manach Y. Vestibular function in children with the CHARGE association. Arch Otolaryngol Head Neck Surg. 1999;125:342–347. doi: 10.1001/archotol.125.3.342. [DOI] [PubMed] [Google Scholar]
- Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4:a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DK, Oh S-H. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DK, Nunes FD, Choo D. Axial specification for sensory organs versus non-sensory structures of the chicken inner ear. Development. 1998;125:11–20. doi: 10.1242/dev.125.1.11. [DOI] [PubMed] [Google Scholar]
- Yildirim I, Yetisir R. Effects of different hatcher temperatures on hatching traits of broiler embryos during the last five days of incubation. S Afr J Anim Sci. 2004;34:211–216. [Google Scholar]
- Yntema CL. An analysis of induction of the ear from foreign ectoderm in the salamander embryo. J Exp Zool. 1950;113:211–243. doi: 10.1002/jez.1401130110. [DOI] [Google Scholar]
- Zucker RS. Minis: whence and wherefore? Neuron. 2005;45:482–484. doi: 10.1016/j.neuron.2005.02.003. [DOI] [PubMed] [Google Scholar]