Abstract
Objective
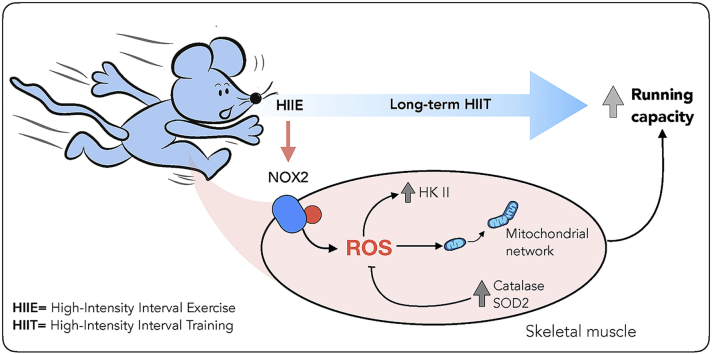
Reactive oxygen species (ROS) have been proposed as signaling molecules mediating exercise training adaptation, but the ROS source has remained unclear. This study aimed to investigate if increased NADPH oxidase (NOX)2-dependent activity during exercise is required for long-term high-intensity interval training (HIIT) in skeletal muscle using a mouse model lacking functional NOX2 complex due to absent p47phox (Ncf1) subunit expression (ncf1* mutation).
Methods
HIIT was investigated after an acute bout of exercise and after a chronic intervention (3x/week for 6 weeks) in wild-type (WT) vs. NOX2 activity-deficient (ncf1*) mice. NOX2 activation during HIIT was measured using an electroporated genetically-encoded biosensor. Immunoblotting and single-fiber microscopy was performed to measure classical exercise-training responsive endpoints in skeletal muscle.
Results
A single bout of HIIT increased NOX2 activity measured as p47-roGFP oxidation immediately after exercise but not 1 h or 4 h after exercise. After a 6-week HIIT regimen, improvements in maximal running capacity and some muscle training-markers responded less to HIIT in the ncf1* mice compared to WT, including superoxide dismutase 2, catalase, hexokinase II, pyruvate dehydrogenase and protein markers of mitochondrial oxidative phosphorylation complexes. Strikingly, HIIT-training increased mitochondrial network area and decreased fragmentation in WT mice only.
Conclusion
This study suggests that HIIT exercise increases NOX2 activity in skeletal muscle and shows that NOX2 activity is required for specific skeletal muscle adaptations to HIIT relating to antioxidant defense, glucose metabolism, and mitochondria.
Keywords: Redox, Reactive oxygen species, Exercise, High-intensity interval training
Abbreviations: HIIE, high-intensity interval exercise; HIIT, high-intensity interval training; HK II, Hexokinase II; SOD2, superoxide dismutase 2; NOX2, NADPH oxidase 2; ncf1*, B10.Q. p47phox mutated; PDH, pyruvate dehydrogenase; RER, respiratory exchange ratio; ROS, reactive oxygen species
Graphical abstract
Highlights
-
•
Acute HIIE induces transient NOX2 complex activity in vivo in muscle.
-
•
Skeletal muscle adaptations to HIIT were impaired in ncf1-deficient mice.
-
•
Functional NOX2 is necessary for HIIT-induced increased expression of antioxidants enzymes.
-
•
Ncf1-deficient mice lack HIIT-induced mitochondrial adaptations.
1. Introduction
Physical inactivity is regarded as a cause of morbidity and premature mortality worldwide [1]. Inactivity and sedentary behavior are estimated to be responsible for 6%–10% of the burden of disease from non-communicable diseases [2]. Exercise intensity is a significant variable explaining the health benefits induced by physical activity [3,4]. Indeed, structured high-intensity interval training (HIIT) has been demonstrated to improve both whole-body and skeletal muscle metabolic health in different populations [[5], [6], [7]]. Despite the proven efficacy of HIIT to promote metabolic health, the underlying mechanisms mediating the molecular adaptations in the HIIT-exercised musculature are not fully understood. Gaining a deeper understanding of the cellular mechanisms governing the acute and chronic responses to HIIT in skeletal muscle would support the development of more effective exercise training regimens and the identification of potential drug targets.
Reactive oxygen species (ROS) act as intracellular compartmentalized second messengers mediating skeletal muscle adaptations in both health and disease [8,9]. Sprint interval bicycle exercise has been shown to elicit greater post-exercise plasma hydrogen peroxide compared to moderate exercise in humans [10], suggesting that exercise-induced ROS production in skeletal muscle may be intensity-dependent. Specific ROS may be required for adaptation to chronic exercise, since ROS scavengers have been shown to disrupt some of the acute and long-term responses to exercise in skeletal muscle (reviewed in Ref. [11]). Furthermore, elevated levels of systemic oxidative stress markers were associated with greater adaptations after 6-weeks of exercise training in humans [12]. Taken together, this suggests that ROS may contribute to the intensity-dependent myocellular exercise training adaptation in skeletal muscle.
Although many studies have suggested the importance of ROS molecules to exercise training adaptation, the exact myocellular source of ROS has remained unclear [13]. For many years, mitochondria were believed to be the primary source of ROS during exercise in skeletal muscle. More recently, non-mitochondrial sources have emerged as potential ROS sources during contractile activity in skeletal muscle [14,15]. Based on studies using electrically evoked contractions in isolated rodent muscle, the professional superoxide-producing enzyme complex NADPH oxidase 2 (NOX2) was strongly suggested to mediate contraction-induced ROS production [15]. Moreover, pharmacological inhibition of NOX2 has been shown to disrupt acute signaling and gene expression elicited by moderate-intensity endurance exercise in mice [14]. However, it is presently unknown if NOX2 is activated by high-intensity exercise (HIIE) and whether it is required for myocellular adaptations to HIIT.
In the present study, we investigated whether NOX2 is activated in mouse skeletal muscle by physiological acute HIIE using a previously characterized NOX2-specific ROS production biosensor, consisting of human p47phox fused to the N-terminus of redox-sensitive green fluorescent protein 2 (p47roGFP) [16]. Furthermore, we investigated if NOX2 activity was required for the long-term skeletal muscle adaptations to HIIT using a mouse model lacking functional NOX2 complex. Specifically, we used ncf1* mice, a described whole-body loss-of- function model for NOX2 activity [17] with undetectable ROS production [18]. We hypothesized that NOX2 would be activated in skeletal muscle during this HIIE modality and required for some, but not all, long-term HIIT adaptations.
2. Results
2.1. Acute HIIE increased NOX2-dependent redox changes in skeletal muscle
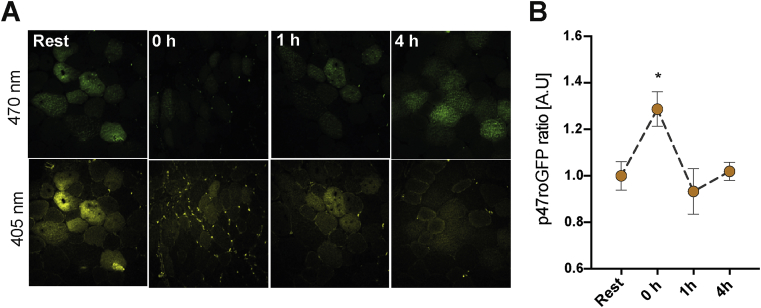
The p47roGFP reporter was expressed in tibialis anterior muscle (TA) via in vivo electroporation 1 week before exercise. Acute HIIE elicited a ratiometric increase in p47roGFP oxidation immediately after exercise (time 0) which returned to baseline by 1 h after HIIE (Fig. 1). This strongly suggests that NOX2-dependent ROS production is increased transiently by HIIE.
Fig. 1.
High-intensity interval exercise activates NOX2 transiently in skeletal muscle. A) Representative images and b) ratio-metric quantification (405/470 nm) of p47roGFP biosensor signal at rest vs. 0, 1 and 4 h post-exercise period in tibialis anterior muscle. * denotes p < 0.05 compared to the resting condition using Holm-Sidak's multiple comparisons test. A.U. Arbitrary units. Values are mean ± SEM (n = 4 per group).
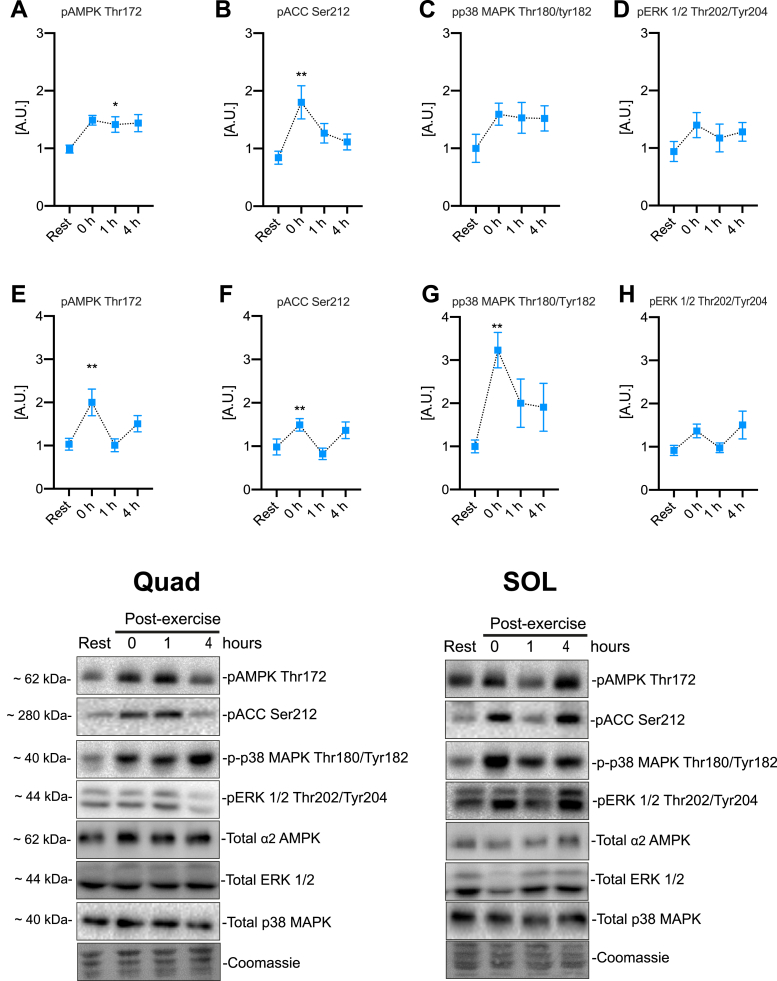
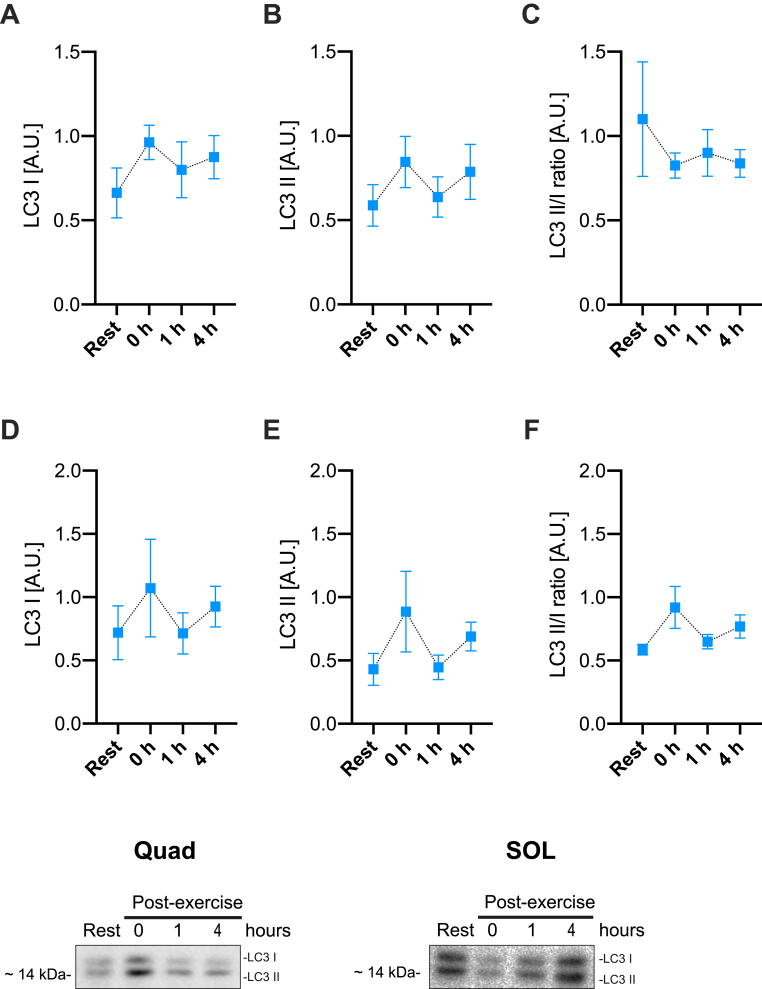
Among phosphorylations indicating activation of known exercise-responsive kinases, AMPK and its substrate ACC2 responded to exercise in both Quad and SOL muscles (Fig. 2). Phosphorylation of p38 MAPK was also significantly increased by acute HIIE in soleus muscle whereas no significant changes were observed for ERK1/2 phosphorylation (Fig. 2). To evaluate whether an acute HIIE increased the autophagy-associated LC3-I lipidation to LC3-II in skeletal muscle, we measured LC3-I and II expression in muscle lysates. Neither Quad nor SOL muscle changed their LC3 II/I ratio up to 4 h post-HIIT (Fig. 3), suggesting that HIIE does not increase autophagy during exercise or in the immediate post-exercise recovery period.
Fig. 2.
Acute cellular signaling induced by high-intensity interval exercise. Exercise-stimulated phosphorylation in quadriceps muscle for A) AMPK Thr172 B) ACC Ser212 C) p38 MAPK Thr180/Tyr182 D) ERK 1/2 Thr202/Tyr204 and in soleus muscle E) AMPK Thr172 F) ACC Ser212 G) p38 MAPK Thr180/Tyr182 H) ERK 1/2 Thr202/Tyr204. ∗/∗∗ denotes p < 0.05/0.01, respectively, compared to the resting condition using Holm-Sidak's multiple comparisons test. Representative blots are shown at the bottom. A.U. Arbitrary units. Values are mean ± SEM (n = 8 per group).
Fig. 3.
Autophagy markers are not induced by acute high-intensity interval exercise. Quantification of LC3-I, LC3-II, and LC3-II/LC3-I ratio in A-C) quadriceps and D-F) soleus muscle at rest vs. 0, 1 and 4 h post-exercise. No significant differences were observed using one-way ANOVA and Holm-Sidak's multiple comparisons test. Representative blots are shown at the bottom. A.U. Arbitrary units. Values are mean ± SEM (n = 8 per group).
2.2. Lack of NOX2 complex activity reduced responsiveness in running capacity
Since an acute bout of HIIE seemed to activate NOX2, we next tested whether NOX2 is required for long-term HIIT adaptation. Generally, ncf1* mice were indistinguishable from WT and no cases of the previously reported spontaneous post-partum arthritis [19] were observed during >1y of breeding. Parallel results from our laboratory demonstrated the absence of in vivo treadmill exercise-stimulated ROS production in skeletal muscle of Ncf1* mice [20], in agreement with earlier in vitro studies using electrical stimulation [16].
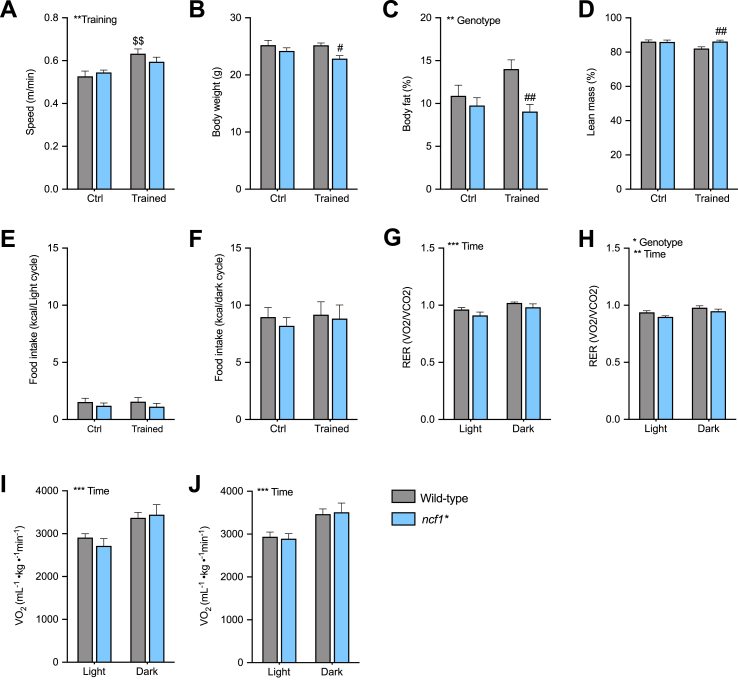
Presently, WT and ncf1* mice performed a three-times per week HIIT regimen for 6-weeks on a motorized treadmill, inspired by a published protocol [21]. The HIIT intervention increased maximal running capacity in WT but not ncf1* mice, compared to untrained mice (Fig. 4A: +23% vs. 10% in WT and ncf1* respectively). Similar body weight and composition were observed in the untrained state, but trained ncf1* mice displayed lower body weight compared to trained WT mice (Fig. 4B). Reduced body fat and increased lean mass were observed in the ncf1* HIIE group compared with the trained WT group (Fig. 4C–D). Neither training nor genotype affected energy intake (Fig. 4E and F). Overall, ncf1* mice showed a tendency towards lower RER than WT mice in the untrained state (Fig. 4G), but a significant genotype main effect was only observed in the trained state (Fig. 4H). Similar oxygen consumption was observed between genotypes in both the untrained and trained state (Fig. 4I–J). Thus, NOX2-deficient mice display impaired HIIT-induced improvements in maximal running capacity and lower body fat content after HIIT training.
Fig. 4.
Ncf1* mice show a lower responsiveness in running capacity after long-term HIIT. 6-weeks high-intensity interval training outcomes in A) maximal running speed B) Body weight C-D) Body composition E) Food intake during the light and F) dark cycle Respiratory exchange ratio in G) untrained and H) trained mice, oxygen uptake in I) Untrained and J) trained mice. A two-way ANOVA was performed to test for the overall effect of training and genotype (values shown top left in each panel */**/***p < 0.05/0.01/0.001) and Holm-Sidak's multiple comparisons test used to test for sub-group differences ($$ p < 0.01 vs. WT ctrl group, #/##p < 0.05/0.01 vs. WT trained mice). Values are mean ± SEM (n = 7–8).
2.3. NOX2 deficiency impaired the HIIT-induced increase in specific antioxidant enzymes
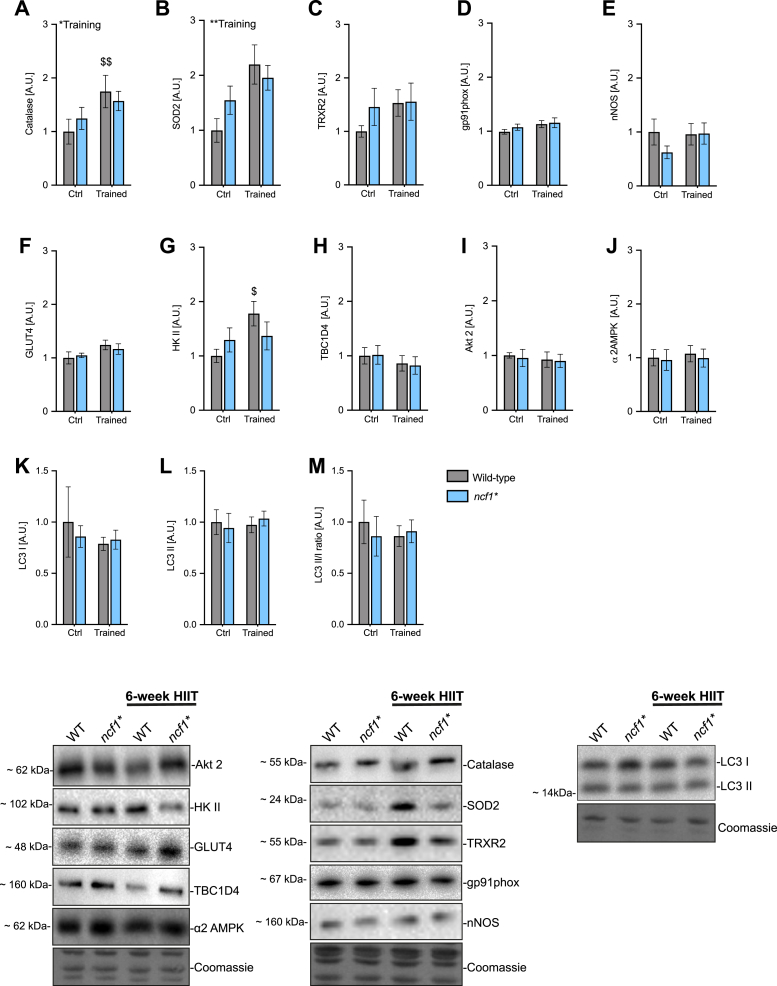
Next, we measured the expression of antioxidant and redox-signaling-related proteins. The mitochondrially localized manganese-dependent superoxide dismutase (SOD2) protein expression increased only in WT (+119%) but not in ncf1* (+26%) in response to HIIT, driven by a higher baseline SOD2 content in ncf1* vs. WT mice (Fig. 5A). A similar tendency was observed for catalase expression in WT (+74%, p = 0.06 WT trained vs. ctrl) vs. ncf1* (26%) (Fig. 5B). Neither genotype nor HIIT affected thioredoxin reductase 2 (TRX2), gp91phox, or nNOS protein expression (Fig. 5C–E). Thus, NOX2 deficiency reduces the HIIT adaptations in specific antioxidant enzymes but not globally in redox-signaling proteins.
Fig. 5.
Multiple exercise-training markers in ncf1* muscles are less responsive to 6-weeks of HIIT. A-E) High intensity interval training-induced changes in redox-related proteins in WT vs. ncf1* quadriceps muscle, F-J) Glucose handling-related proteins and K-M) autophagy-related proteins. Two-way ANOVA was performed to test for the overall effect of training and genotype (values shown top left in each panel */**p < 0.05/0.01) and Holm-Sidak's multiple comparisons test used to test for sub-group differences ($/$$ p < 0.05/0.01, respectively compared to WT ctrl). Representative blots are shown at the bottom. A.U. Arbitrary units. Values are mean ± SEM (n = 7–8).
2.4. HIIT-induced muscle HKII expression was NOX2-dependent
To evaluate the effect of exercise training on insulin sensitivity and glucose metabolism, we tested whether HIIT and/or NOX2 deficiency affected glucose handling proteins. Neither HIIT nor genotype affected glucose transport 4 (GLUT4) expression (Fig. 5F, p = 0.06 ANOVA main-effect of training). In contrast, hexokinase II, a rate-limiting glycolytic enzyme, increased +77% with HIIT in WT (p < 0.05) but did not respond to HIIT in the ncf1* mice (Fig. 5G). No effects were observed on total TBCD4, AKT2, AMPK or LC-3I and II protein abundance (Fig. 5H-M).
2.5. Lack of NOX2 activity impaired mitochondrial adaptations to HIIT
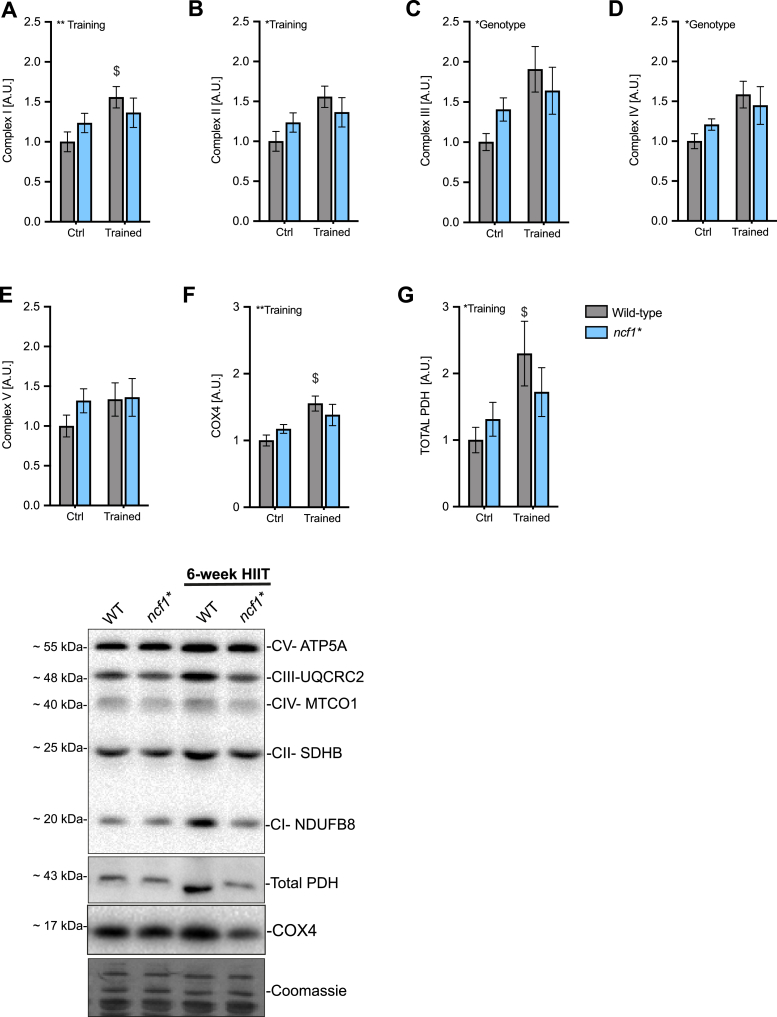
To test whether NOX2 activity is required for training-induced mitochondrial biogenesis, we first measured mitochondrial electron transport chain complex protein abundance and other mitochondrial proteins. The mitochondrial complex I marker was increased (+55%) in the trained group compared to the sedentary control (ctrl) in WT but not significantly increased in ncf1* mice (Fig. 6A). A genotype main effect was found for complex III and Complex IV markers (Fig. 6C–D). Moreover, total PDH levels increased in the WT (+129%) but not in ncf1* mice (+30%, Fig. 6G).
Fig. 6.
Mitochondria-related proteins are less responsive to HIIT in mice lacking functional NOX2 activity. Mitochondrial proteins in WT vs. ncf1* quadriceps muscle. A) Complex I B) Complex II C) Complex III D) Complex IV B) Complex V F) COX4 and G) PDH. Two-way ANOVA was performed to test for the overall effect of training and genotype (values shown top left in each panel */**p < 0.05/0.01) and Holm-Sidak's multiple comparisons test used to test for sub-group differences ($ p < 0.05, respectively compared to WT ctrl). Representative blots are shown at the bottom. A.U. Arbitrary units. Values are mean ± SEM (n = 7–8).
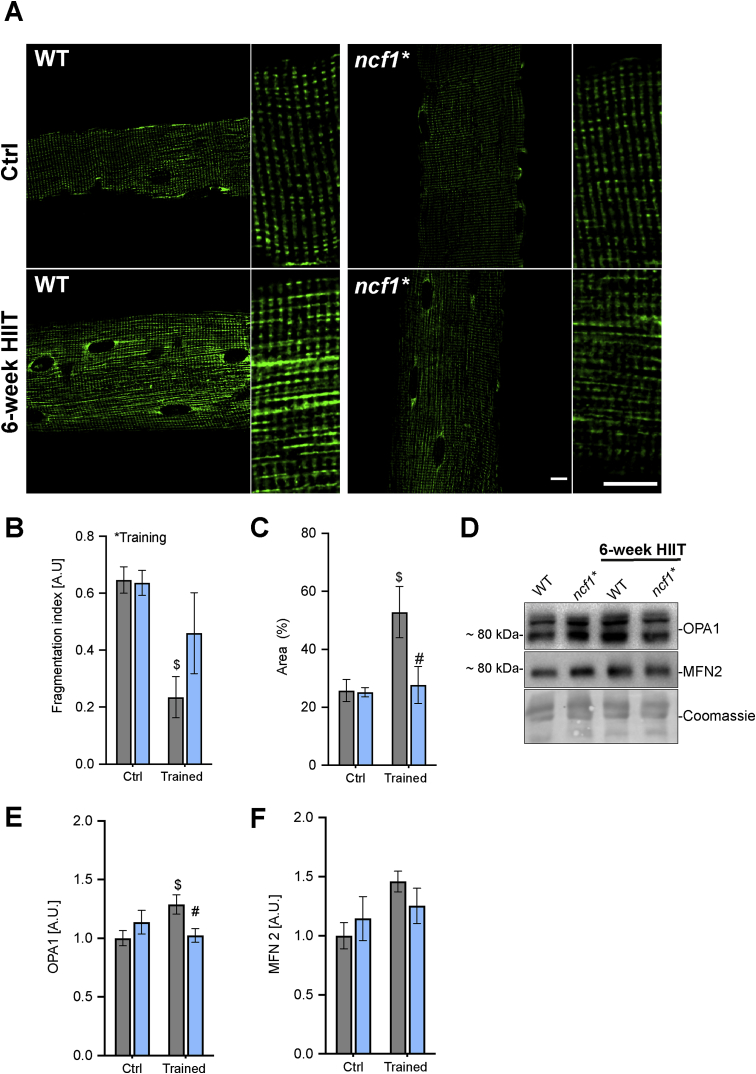
Since exercise training has been associated with both mitochondrial biogenesis and more elongated and fused mitochondria [22,23], we estimated the change in the mitochondrial network in single quadriceps muscle fibers by immunofluorescent imaging of COX4 (Fig. 7A–D). Mitochondrial fragmentation was reduced by HIIT training in WT but not ncf1* mice (Fig. 7B), and the cytosolic area occupied by mitochondria was increased by HIIT in WT but not in ncf1* mice (Fig. 7C). Consistent with a disturbed ability to fuse mitochondria in ncf1* mice, we observed an increase of the inner membrane fusion protein OPA1 by HIIT in WT but not in ncf1* mice, and a tendency towards the same for the mitochondrial outer membrane fusion protein mitofusin-2 (MFN2) (p = 0.05 vs. WT ctrl) (Fig. 7D–F). Taken together, this shows that NOX2 is required for multiple aspects of mitochondrial adaptations to HIIT, with particularly strong effects on mitochondrial network morphology.
Fig. 7.
HIIT-induced changes in mitochondrial network morphology and fusion/fission-related proteins are impaired in ncf1* mice. A) Representative images from mitochondrial COX4 staining in quadriceps single fibers. Scale bars are 10 μm. B) Mitochondrial fragmentation index C) Mitochondrial area D) representative images and E+F) quantification of OPA1 and MFN2 protein abundance. Two-way ANOVA was performed to test for the overall effect of training and genotype (values shown top left in each panel * p < 0.05) and Holm-Sidak's multiple comparisons test used to test for sub-group differences ($ p < 0.05 vs. WT ctrl, #p < 0.05 vs. WT trained). A.U. Arbitrary units. Values are mean ± SEM (A-C, n = 4 and E-F, n = 7–8).
3. Discussion
ROS are proposed to act as signaling molecules mediating myocellular exercise training adaptations [9]. However, the exact source of ROS involved is still debated and may differ depending on the intensity of exercise. A recent study showed that adaptive gene expression in response to acute in vivo and in vitro exercise in mice was impaired by NOX2 inhibitors [14]. Our current study supports and extends on these data by showing that the functional improvement in maximal exercise capacity, a number of exercise-responsive proteins and most strikingly, mitochondrial network adaptations were less responsive to HIIT training in mice lacking a functional NOX2 complex. The specific adaptations are briefly discussed below followed by some closing reflections on their potential connectivity.
3.1. Running performance
Some [[24], [25], [26]] but not all studies [27,28] have found that ROS scavengers impair the training-induced improvements in maximal exercise capacity in rodents and humans. The lack of consistency in previous studies has been attributed to differences in antioxidant supplementation efficacy, specificity and/or training regimens. Here, using a genetic loss-of-function model, we observed that an acute bout of HIIE was sufficient to induce p47roGFP biosensor oxidation in skeletal muscle, indicating that HIIE activated NOX2 in vivo, and that lack of NOX2 activity in skeletal muscle was associated with a blunted improvement in running capacity after a 6-week HIIT period. An immediate concern is that the decreased running capacity in ncf1* vs. WT mice after HIIT could have contributed to the decreased protein response to HIIT. However, we find this unlikely since 1) there was no difference in running capacity before training and the mean difference in maximal running speed after training was only ∼5%, arguing that these mice were exposed to a similar HIIT intensity and volume and 2) some training-responsive proteins such as GLUT4 responded equally to HIIT in both genotypes. Overall, these data suggest that NOX2 is likely a major contributor to the previously proposed ROS-mediated increase in endurance exercise capacity in rodents and humans [11].
3.2. Antioxidant capacity
Chronic adaptations to exercise training are thought to result from the cumulative stimulatory effect of repeated acute exercise bouts on gene transcription [29]. Three weeks of HIIT in humans improve antioxidant capacity in plasma of humans [30]. This is consistent with the proposal that ROS are essential mediators of the hormetic increase in endogenous antioxidant defense in response to training [31]. A previous study found that NOX2 was required for some of the acute antioxidant defense gene transcription responses to muscle activity since pharmacological blockade of NOX2 in vivo or electrically stimulated contraction in vitro blocked exercise-responsive gene transcription of SOD2 and glutathione peroxidase transcription [14]. This was suggested to be regulated by a NOX2-dependent NF-κB pathway [32]. Whether NF-kB was a contributing factor for the lack of HIIT response in current study was not investigated.
3.3. Glucose handling enzymes
Both GLUT4 and HKII are known to respond to HIIT in humans [33]. A key finding in our study was that HKII, arguably one of the most exercise training-responsive proteins in man, did not increase after training in the NOX2-deficient ncf1* mice. A ROS-dependence is consistent with previous studies showing that antioxidants blunt electrically stimulated contraction-induced HKII mRNA and activity in muscle cells [34,35] and training-induced HKII mRNA in mice [36]. In contrast, the increase in GLUT4 expression with HIIT did not appear to depend on NOX2 activity.
3.4. Mitochondrial protein expression
Endurance exercise training and HIIT are known to increase the protein content, volume, and function of skeletal muscle mitochondria [37]. Previous studies reported that the antioxidants Vitamin C and E reduced exercise training-induced mitochondrial biogenesis markers such as COX4 and CS, likely by downregulation of PCG1-alpha and TFAM in human and murine skeletal muscle [24,27,38,39]. Interestingly, exercise-induced TFAM and citrate synthase mRNA levels in mouse skeletal muscle were also blocked by pharmacological inhibition of NOX2 [14]. Presently, we observed that the well-established mitochondrial content marker COX4 [40], as well as the mitochondrial metabolic capacity-markers PDH and mitochondrial complex I, increased after HIIT in WT but not ncf1* mice. We speculate that the general tendency towards increased base-line expression of mitochondria-related proteins in ncf1* vs. WT mice might increase resting fat oxidation, consistent with the observed lower RER and leaner phenotype in trained ncf1* vs. WT mice.
3.5. Mitochondrial network morphology
Endurance exercise training is associated with an elongated mitochondrial network [22] and decreased mitochondrial fragmentation [23] in the trained musculature in rodents and humans [41]. HIIT has been reported to increase the respiratory capacity of mitochondria [42,43] but the HIIT-associated changes in mitochondrial morphology have, as far as we are aware, not been investigated in any species. Our data show for the first time that these responses also occur with HIIT. Furthermore, the HIIT-induced mitochondrial network remodeling exhibited the strongest quantitative impairment of any endpoint in mice lacking NOX2 activity. The genotype difference in mitochondrial network was accompanied by a blunted HIIT-induced increases of the mitochondrial fusion related proteins OPA1 and MFN2 in ncf1* vs WT muscle. We cannot dismiss the changes in fusion-related proteins are independent of the changes in mitochondrial content. This might provide clues to the underlying mechanism but requires further investigation.
3.6. Are the different molecular findings connected?
Many of the proteins that differed significantly between WT and ncf1* mice displayed a similar expression pattern with an increased relative expression in untrained ncf1* mice and a lower relative responsiveness to HIIT in ncf1* mice. Importantly, the changes were confined to subsets of proteins and not observed for e.g. GLUT4, TBC1D4 or Akt2 expression. Based on the literature many of these changes could be mechanistically connected. Thus, the mitochondrial adaptations to HIIT were impaired both in terms of network morphology and mitochondrial protein expression. Interestingly, NOX2 may not only affect mitochondria indirectly via biogenesis and turnover but has also been proposed to exhibit bidirectional redox-crosstalk with mitochondria [44]. For instance, NOX2 is seemingly required for palmitate-induced mitochondrial abnormalities in murine cardiomyocytes [45]. Conversely, increased mitochondrial ROS production using mitochondrial inhibitors in human leukocytes or Angiotensin-II in murine endothelial cells can activate NOX2 [46]. Whether redox-crosstalk between NOX2 and mitochondria occurs in skeletal muscle is presently unknown, but our data could be interpreted as suggestive of a connection. Regardless of how NOX2 connects to mitochondria, the baseline upregulation of several antioxidant enzymes, including SOD2 and catalase could suggest increased mitochondrial ROS production. Mitochondrial ROS production has previously been associated with mitochondrial fragmentation and higher resting fat oxidation [[47], [48], [49]], but their contribution here is unclear since genotype differences in these parameters were only observed in the trained state. Worth noting, however, we did observe a significant decrease in baseline RER in ncf1* vs. WT mice [20], indicating increased resting fat oxidation in ncf1* mice. We speculate that the increased preference for fat as an energy substrate explains the lower body fat in trained ncf1* mice compared to WT (Fig. 4C). Hexokinase II is known to shuttle to and from mitochondria [50]. Interestingly, acute disruption of HKII binding to mitochondria, using a peptide-inhibitor in a perfused mouse heart ischemia-reperfusion injury model, markedly reduced cardiac recovery and increased ROS during ischemia and reperfusion [51]. Similar links have been established in other tissues, including skeletal muscle [52]. Thus, we speculate that HKII may somehow be connected to both the increased base-line fat oxidation and changes in mitochondrial morphology/function. The mechanistic connections between the observed molecular changes in ncf1* vs WT muscle should be measured in the future.
3.7. Conclusion
This study provided evidence suggesting that NOX2 is activated by HIIE and showing that the presence of functional NOX2 is required for long-term training adaptation, including increased muscle protein expression of antioxidant defense enzymes, mitochondrial enzymes and hexokinase II, and increased mitochondrial network volume and decreased mitochondrial fragmentation. This suggests that NOX2 signaling modulates the exercise-response to HIIT. If and how these changes are interconnected should be clarified in future studies.
4. Materials and methods
4.1. Animals
10-week-old female B10.Q wild-type (WT) and B10.Q. p47phox mutated (ncf1*) were maintained on a 12 h light/dark cycle, group-housed with free access to water and standard rodent chow diet (Altromin no. 1324; Chr. Pedersen, Denmark).
All experiments were pre-approved by the Danish Animal Experimental Inspectorate (2015–15−0201−00477).
4.2. Maximal running capacity
Running capacity was carried as previously described [53]. Briefly, mice were acclimated to the treadmill three times (10 min at 0.16 m/s) (Treadmill TSE Systems) a week before the maximal running tests. The maximal running test started at 0.16 m/s for 300 s with 10% incline, followed by a step-wise increase (0.2 m/s every 60s) in running speed until exhaustion. Exhaustion was defined as the point at which instead of running on the treadmill, the mice fell back on the grid three times within 30 s. Maximal running speed was determined as the last completed stage during the incremental test. All tests were performed blinded.
4.3. Indirect calorimetry and body composition
Body composition was assessed by MRI-scanning (EchoMRI-4, Echo Medical System LLC, Texas, USA) according to the manufacturer's instructions.
Whole-body metabolism was assessed in a 16-chamber indirect calorimetry system after a 2-day acclimation period (PhenoMaster; TSE Systems, Frankfurt, Germany).
4.4. Acute high-intensity interval exercise (HIIE)
Fed mice performed a single HIIE bout switching between 2 min running at 100% of the maximal running speed of each group, and then 2 min of active recovery running at 30% of the maximal running speed for a total of 60 min. Tissues were harvest immediately, one and 4 h after exercise.
4.5. HIIT intervention
WT and ncf1* mice were randomly assigned to either the ctrl or HIIT group. The HIIT training was carried out as previously described [21]. Briefly, HIIT training involved treadmill running three days per week for six weeks. In each training session, mice switched between 2 min running at 100% of the maximal running speed of each group, and then 2 min of active recovery running at 30% of the maximal running speed for a total of 60 min. During the training period for the HIIT group, the ctrl mice remained sedentary in their cages.
4.6. In vivo gene transfer in adult skeletal muscle
Tibialis anterior electroporation was performed as previously described [54]. Briefly, mice were anesthetized with 2–3% isoflurane. Hyaluronidase (H3884, Sigma) dissolved in sterile saline solution (0.36 mg/ml) was injected intramuscularly in tibialis anterior (TA) muscle, followed by 40 μg plasmid injection 1 h later in re-anesthetized mice. Muscle electroporation was then performed by delivering 10 electrical pulses at an intensity of 100 V/cm, 10-ms pulse duration, 200-ms pulse interval using a caliper electrode (#45-0101 Caliper Electrode, BTX Caliper Electrodes, USA) connected to an ECM 830 BTX electroporator (BTX Harvard Apparatus). The p47-roGFP biosensor used to determine NOX2 activity was a kind gift from Dr. George G. Rodney [16].
4.7. Redox histology
Muscle freezing and sectioning was performed as previously reported [55]. In brief, TA muscles were dissected and embedded in optimum cutting temperature (OCT) medium from Tissue Tek, frozen in liquid nitrogen cooled isopentane and kept at −80 °C until processing. Redox histology was performed as previously described [56]. Briefly, p47roGFP- transfected muscles were cut in 10 μm thick cryosections followed by incubation in 50 μl of PBS containing 50 mM n-ethylmaleimide (NEM) for 10 min at 4 °C. Sections were fixed in PBS-dissolved 4% paraformaldehyde (50 mM NEM) for 10 min, washed three times in PBS (5 min) and mounted in mounting medium (Vectashield, USA).
4.8. Western blotting
Western blotting was performed as previously described [57]. Briefly, ∼40 μg of quadriceps muscle and the whole soleus were lysed for 1 min at 30 Hz on a shaking bead-mill (TissueLyser II, Qiagen, Valencia, CA, USA) in ice-cold lysis buffer (0.05 mol/L Tris Base pH 7.4, 0.15 mol/L NaCl, 1 mmol/L EDTA and EGTA, 0.05 mol/L sodium flouride, 5 mmol/L sodium pyrophosphate, 2 mmol/L sodium orthovanadate, 1 mmol/L benzamidine, 0.5% protease inhibitor cocktail (P8340, Sigma Aldrich), and (1% NP- 40). After rotating end-over-end for 30 min at 4 °C, lysate supernatants were collected by centrifugation (18,327 g) for 20 min at 4 °C. Lysate protein concentrations were determined using BSA standards (Pierce) and bicinchoninic acid assay reagents (Pierce). Total protein and phosphorylation levels of relevant proteins were determined by standard immunoblotting techniques, loading equal amounts of protein. The primary antibodies used; p-AMPK Thr172 (Cell Signaling Technology (CST)), #2535S), p-p38 MAPK Thr180/Tyr182 (CST, #9211), Hexokinase II (CST, #2867), GLUT4 p-ACC2 Ser212 (Millipore, 03-303), (ThermoFisher Scientific, PA-23052), Rac1 (BD Biosciences, #610650), NOX2 (Abcam, #Ab129068), Catalase (SCBT, sc-271803), SOD2 (Millipore, 06-984), TRX2 (SCBT, sc-50336), actin (CST, #4973) total p38 MAPK (CST, #9212), alpha 2 AMPK (a gift from D. Grahame Hardie, University of Dundee), total ERK 1/2 (CST, #9102), TBC1D1Ser231 (Millipore #07-2268), Hexokinase II (CST, #2867) MFN2 (#9482), and OPA1 (BD Biosciences #612606). A cocktail antibody (Abcam, #ab110413) was used as representative of the mitochondrial electron chain complexes (Oxphos). The optimal protein loading was pre-optimized to ensure measurements in the linear dynamic range for each antibody. Bands were visualized using a ChemiDoc imaging system (Bio-Rad, USA). Total protein staining (Coomassie) was used as a loading ctrl rather than house-keeping proteins, as previously recommended [58]. Grayscale levels range for the blots shown was minimally and linearly adjusted across entire blots, as recommended by the American Society for Biochemistry and Molecular Biology.
4.9. Single fiber immunostaining
Staining of single fibers was performed as previously described with slight modifications [23]. Briefly, ∼20 μg of quadriceps muscle was fixed by immersion in ice-cooled 4% formaldehyde for 4 h and long-term stored in 50% glycerol (diluted in PBS) at −20 °C. Single fibers were then teased from the muscles with fine forceps and transferred to immunobuffer (50 mM glycine, 0.25% bovine serum albumin (BSA), 0.03% saponin and 0.05% sodium azide in PBS) After isolation, a minimum of 30 muscle fibers were incubated overnight with an anti-COX4 antibody (#16056, Abcam, Cambridge, UK) in immunobuffer containing 0.5% saponin and, after 3 washes with immunobuffer, single muscle fibers were incubated for 2 h with a secondary antibody conjugated with Alexa Fluor 488 (Invitrogen, UK). A negative ctrl was performed with fibers not exposed to the primary antibody. The muscle fibers were mounted in Vectashield mounting medium.
4.10. Imaging acquisition
All confocal images were collected using a 63x 1.4 NA oil immersion objective lens on an LSM 780 confocal microscope (Zeiss) driven by Zen 2011. Image acquisition was performed blinded. For the p47roGFP biosensor images, raw data of the 405- and 488-nm laser lines were exported to ImageJ as 16-bit TIFFs for further analysis. Data are presented as normalized fluorescence ratio (405/488 nm) normalized to the WT ctrl group.
4.11. Statistical analyses
Results are shown as means ± S.E.M. Statistical testing was performed one-way or two-way ANOVA (repeated measures for respiration data in Fig. 4) and Holm Sidak's post hoc test for pair-wise comparisons of subgroups. Statistical analyses were performed using GraphPad Prism 8.
Author contributions
CHO and TEJ designed research; CHO, LB, ZL, LAC, JRK, SHR, TEJ performed research; RH provided the ncf1* mice and intellectual input, CHO analyzed data; CHO and TEJ wrote the paper; all authors commented on the draft, TEJ Funding Acquisition.
Acknowledgments
TEJ was supported by a Novo Nordisk Foundation Excellence project grant (#15182). CHO was supported by a CONICYT Ph.D. Scholarship. ZL was supported by a Chinese Scholarship Council Ph.D. stipend. JRK was supported by a Ph.D. stipend from the Danish Diabetes Academy funded by the Novo Nordisk Foundation. RH was supported by the Swedish Strategic Science Foundation (SSF). We thank Prof. Henriette Pilegaard for providing MFN2 and OPA1 antibodies. Imaging data were collected at the Center for Advanced Bioimaging and the Core Facility for Integrated Microscopy, University of Copenhagen, Denmark.
References
- 1.Whiteford H.A., Degenhardt L., Rehm J., Baxter A.J., Ferrari A.J., Erskine H.E. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Lee I.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebel K., Ding D., Chey T., Stamatakis E., Brown W.J., Bauman A.E. Effect of moderate to vigorous physical activity on all-cause mortality in middle-aged and older Australians. JAMA Intern. Med. 2015;175(6):970–977. doi: 10.1001/jamainternmed.2015.0541. [DOI] [PubMed] [Google Scholar]
- 4.Schnohr P., Marott J.L., Jensen J.S., Jensen G.B. Intensity versus duration of cycling, impact on all-cause and coronary heart disease mortality: the Copenhagen City heart study. Eur. J. Prev. Cardiol. 2012;19(1):73–80. doi: 10.1177/1741826710393196. [DOI] [PubMed] [Google Scholar]
- 5.Gibala M.J., Hawley J.A. Sprinting toward fitness. Cell Metabol. 2017;25(5):988–990. doi: 10.1016/j.cmet.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 6.MacInnis M.J., Gibala M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017;595(9):2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zapata-Lamana R., Henriquez-Olguin C., Burgos C., Meneses-Valdes R., Cigarroa I., Soto C. Effects of polarized training on cardiometabolic risk factors in young overweight and obese women: a randomized-controlled trial. Front. Physiol. 2018;9:1287. doi: 10.3389/fphys.2018.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa A., Henriquez-Olguin C., Jaimovich E. Reactive oxygen species and calcium signals in skeletal muscle: a crosstalk involved in both normal signaling and disease. Cell Calcium. 2016;60(3):172–179. doi: 10.1016/j.ceca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Jackson M.J., Vasilaki A., McArdle A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016;98:13–17. doi: 10.1016/j.freeradbiomed.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Parker L., Trewin A., Levinger I., Shaw C.S., Stepto N.K. Exercise-intensity dependent alterations in plasma redox status do not reflect skeletal muscle redox-sensitive protein signaling. J. Sci. Med. Sport. 2018;21(4):416–421. doi: 10.1016/j.jsams.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Merry T.L., Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016;594(18):5135–5147. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margaritelis N.V., Theodorou A.A., Paschalis V., Veskoukis A.S., Dipla K., Zafeiridis A. Adaptations to endurance training depend on exercise-induced oxidative stress: exploiting redox interindividual variability. Acta Physiol. (Oxf). 2018;222(2) doi: 10.1111/apha.12898. [DOI] [PubMed] [Google Scholar]
- 13.Jackson M.J. Recent advances and long-standing problems in detecting oxidative damage and reactive oxygen species in skeletal muscle. J. Physiol. 2016;594(18):5185–5193. doi: 10.1113/JP270657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriquez-Olguin C., Diaz-Vegas A., Utreras-Mendoza Y., Campos C., Arias-Calderon M., Llanos P. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Front. Physiol. 2016;7:282. doi: 10.3389/fphys.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakellariou G.K., Jackson M.J., Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014;48(1):12–29. doi: 10.3109/10715762.2013.830718. [DOI] [PubMed] [Google Scholar]
- 16.Pal R., Basu Thakur P., Li S., Minard C., Rodney G.G. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sareila O., Jaakkola N., Olofsson P., Kelkka T., Holmdahl R. Identification of a region in p47phox/NCF1 crucial for phagocytic NADPH oxidase (NOX2) activation. J. Leukoc. Biol. 2013;93(3):427–435. doi: 10.1189/jlb.1211588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C.K., Zhan L., Hannigan M.O., Ai Y., Leto T.L. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+ J. Leukoc. Biol. 2000;67(2):210–215. doi: 10.1002/jlb.67.2.210. [DOI] [PubMed] [Google Scholar]
- 19.Hultqvist M., Olofsson P., Holmberg J., Backstrom B.T., Tordsson J., Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc. Natl. Acad. Sci. U. S. A. 2004;101(34):12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriquez-Olguin C., Knudsen J.R., Raun S.H., Li Z., Dalbram E., Treebak J.T. Exercise-stimulated muscle ROS production and glucose uptake requires NADPH oxidase 2. bioRxiv. 2019:522805. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcinko K., Sikkema S.R., Samaan M.C., Kemp B.E., Fullerton M.D., Steinberg G.R. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol. Metab. 2015;4(12):903–915. doi: 10.1016/j.molmet.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axelrod C.L., Fealy C.E., Mulya A., Kirwan J.P. Acta physiologica; Oxford, England: 2018. Exercise Training Remodels Human Skeletal Muscle Mitochondrial Fission and Fusion Machinery towards a Pro-elongation Phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halling J.F., Ringholm S., Olesen J., Prats C., Pilegaard H. Exercise training protects against aging-induced mitochondrial fragmentation in mouse skeletal muscle in a PGC-1alpha dependent manner. Exp. Gerontol. 2017;96:1–6. doi: 10.1016/j.exger.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Cabrera M.C., Domenech E., Romagnoli M., Arduini A., Borras C., Pallardo F.V. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 25.Bjornsen T., Salvesen S., Berntsen S., Hetlelid K.J., Stea T.H., Lohne-Seiler H. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports. 2016;26(7):755–763. doi: 10.1111/sms.12506. [DOI] [PubMed] [Google Scholar]
- 26.Braakhuis A.J., Hopkins W.G., Lowe T.E. Effects of dietary antioxidants on training and performance in female runners. Eur. J. Sport Sci. 2014;14(2):160–168. doi: 10.1080/17461391.2013.785597. [DOI] [PubMed] [Google Scholar]
- 27.Paulsen G., Cumming K.T., Holden G., Hallen J., Ronnestad B.R., Sveen O. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J. Physiol. 2014;592(8):1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison D., Hughes J., Della Gatta P.A., Mason S., Lamon S., Russell A.P. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 29.Perry C.G., Lally J., Holloway G.P., Heigenhauser G.J., Bonen A., Spriet L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010;588(Pt 23):4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogdanis G.C., Stavrinou P., Fatouros I.G., Philippou A., Chatzinikolaou A., Draganidis D. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;61:171–177. doi: 10.1016/j.fct.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Cabrera M.C., Domenech E., Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Henriquez-Olguin C., Altamirano F., Valladares D., Lopez J.R., Allen P.D., Jaimovich E. Altered ROS production, NF-kappaB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim. Biophys. Acta. 2015;1852(7):1410–1419. doi: 10.1016/j.bbadis.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sogaard D., Lund M.T., Scheuer C.M., Dehlbaek M.S., Dideriksen S.G., Abildskov C.V. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol. (Oxf. Engl.) 2018;222(4) doi: 10.1111/apha.13009. [DOI] [PubMed] [Google Scholar]
- 34.Silveira L.R., Pilegaard H., Kusuhara K., Curi R., Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim. Biophys. Acta. 2006;1763(9):969–976. doi: 10.1016/j.bbamcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Pinheiro C.H., Silveira L.R., Nachbar R.T., Vitzel K.F., Curi R. Regulation of glycolysis and expression of glucose metabolism-related genes by reactive oxygen species in contracting skeletal muscle cells. Free Radic. Biol. Med. 2010;48(7):953–960. doi: 10.1016/j.freeradbiomed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Meier P., Renga M., Hoppeler H., Baum O. The impact of antioxidant supplements and endurance exercise on genes of the carbohydrate and lipid metabolism in skeletal muscle of mice. Cell Biochem. Funct. 2013;31(1):51–59. doi: 10.1002/cbf.2859. [DOI] [PubMed] [Google Scholar]
- 37.Burgomaster K.A., Howarth K.R., Phillips S.M., Rakobowchuk M., Macdonald M.J., McGee S.L. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008;586(1):151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ristow M., Zarse K., Oberbach A., Kloting N., Birringer M., Kiehntopf M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strobel N.A., Peake J.M., Matsumoto A., Marsh S.A., Coombes J.S., Wadley G.D. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2011;43(6):1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 40.Larsen S., Nielsen J., Hansen C.N., Nielsen L.B., Wibrand F., Stride N. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012;590(14):3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booth F.W., Ruegsegger G.N., Toedebusch R.G., Yan Z. Endurance exercise and the regulation of skeletal muscle metabolism. Prog. Mol. Biol. Transl. Sci. 2015;135:129–151. doi: 10.1016/bs.pmbts.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Dohlmann T.L., Hindso M., Dela F., Helge J.W., Larsen S. High-intensity interval training changes mitochondrial respiratory capacity differently in adipose tissue and skeletal muscle. Phys. Rep. 2018;6(18) doi: 10.14814/phy2.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granata C., Oliveira R.S., Little J.P., Renner K., Bishop D.J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. Offic. Publ. Federation Am. Soc. Exp. Biol. 2016;30(10):3413–3423. doi: 10.1096/fj.201500100R. [DOI] [PubMed] [Google Scholar]
- 44.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797(6–7):897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 45.Joseph L.C., Barca E., Subramanyam P., Komrowski M., Pajvani U., Colecraft H.M. Inhibition of NAPDH oxidase 2 (NOX2) prevents oxidative stress and mitochondrial abnormalities caused by saturated fat in cardiomyocytes. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroller-Schon S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxidants Redox Signal. 2014;20(2):247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.T. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jheng H.F., Tsai P.J., Guo S.M., Kuo L.H., Chang C.S., Su I.J. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell Biol. 2012;32(2):309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metabol. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen P.L. Warburg, me and Hexokinase 2: multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J. Bioenerg. Biomembr. 2007;39(3):211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- 51.Nederlof R., Gurel-Gurevin E., Eerbeek O., Xie C., Deijs G.S., Konkel M. Reducing mitochondrial bound hexokinase II mediates transition from non-injurious into injurious ischemia/reperfusion of the intact heart. J. Physiol. Biochem. 2016;73(3):323–333. doi: 10.1007/s13105-017-0555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nederlof R., Eerbeek O., Hollmann M.W., Southworth R., Zuurbier C.J. Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in heart. Br. J. Pharmacol. 2014;171(8):2067–2079. doi: 10.1111/bph.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sylow L., Moller L.L.V., Kleinert M., D'Hulst G., De Groote E., Schjerling P. Rac1 and AMPK account for the majority of muscle glucose uptake stimulated by ex vivo contraction but not in vivo exercise. Diabetes. 2017;66(6):1548–1559. doi: 10.2337/db16-1138. [DOI] [PubMed] [Google Scholar]
- 54.Knudsen J.R., Henriquez-Olguin C., Li Z., Jensen T.E. Electroporated GLUT4-7myc-GFP detects in vivo glucose transporter 4 translocation in skeletal muscle without discernible changes in GFP-patterns. Exp. Physiol. 2019 doi: 10.1113/EP087545. [DOI] [PubMed] [Google Scholar]
- 55.Li Z., Naslund-Koch L., Henriquez-Olguin C., Knudsen J.R., Li J., Madsen A.B. Chemical denervation using botulinum toxin increases Akt expression and reduces submaximal insulin-stimulated glucose transport in mouse muscle. Cell. Signal. 2018;53:224–233. doi: 10.1016/j.cellsig.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Fujikawa Y., Roma L.P., Sobotta M.C., Rose A.J., Diaz M.B., Locatelli G. Mouse redox histology using genetically encoded probes. Sci. Signal. 2016;9(419):rs1. doi: 10.1126/scisignal.aad3895. [DOI] [PubMed] [Google Scholar]
- 57.Li Z., Rasmussen M.L., Li J., Olguin C.H., Knudsen J.R., Sogaard O. Low- and high-protein diets do not alter ex vivo insulin action in skeletal muscle. Phys. Rep. 2018;6(13) doi: 10.14814/phy2.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fortes M.A., Marzuca-Nassr G.N., Vitzel K.F., da Justa Pinheiro C.H., Newsholme P., Curi R. Housekeeping proteins: how useful are they in skeletal muscle diabetes studies and muscle hypertrophy models? Anal. Biochem. 2016;504:38–40. doi: 10.1016/j.ab.2016.03.023. [DOI] [PubMed] [Google Scholar]