Abstract
The traditional healthcare system is at the doorstep for entering into the arena of molecular medicine. The enormous knowledge and ongoing research have now been able to demonstrate methodologies that can alter DNA coding. The techniques used to edit or change the genome evolved from the earlier attempts like nuclease technologies, homing endonucleases, and certain chemical methods. Molecular techniques like meganuclease, transcription activator-like effector nucleases (TALENs), and zinc-finger nucleases (ZFNs) initially emerged as genome-editing technologies. These initial technologies suffer from lower specificity due to their off-targets side effects. Moreover, from biotechnology’s perspective, the main obstacle was to develop simple but effective delivery methods for host cell entry. Later, small RNAs, including microRNA (miRNA) and small interfering RNA (siRNA), have been widely adopted in the research laboratories to replace lab animals and cell lines. The latest discovery of CRISPR/Cas9 technology seems more encouraging by providing better efficiency, feasibility, and multi-role clinical application. This later biotechnology seem to take genome-engineering techniques to the next level of molecular engineering. This review generally discusses the various gene-editing technologies in terms of the mechanisms of action, advantages, and side effects.
Keywords: transcription activator-like effector nucleases, TALENs, zinc-finger nucleases, ZFNs, RNAi, CRISPR/Cas9 technology
Main Text
Over the last half century after post-DNA helical structure discovery, the world has seen a continuous staircase outburst of various molecular technologies, which are now heading forward toward translation into clinical and laboratory practice.1 Given the availability of sequencing platforms, acquired wisdom about the micro-mechanics at work within the genetic apparatus, and the introduction of user-friendly nanotechnologies, it was possible for next-generation scientists to manipulate the genetic codes at various levels.2 Over the last two decades we saw a plethora of molecular techniques, which allowed us to edit genes or their alter pathways, allowing humans for the first time to micro-edit the DNA codes and further to alter the mRNA fate through post-transcriptional modifications.3
Principally, genome-wide editing techniques can be interpreted as methods where DNA sequences are changed by deletions, mRNA processing, and post-transcriptional modifications to result in altered gene expression, leading to functional behavior of proteins.4, 5 Common to these methods are three basic steps, including mechanisms for genetic tool entry into the cell and later nucleus; altering gene transcription and onward processing function; and, finally, the end-output in the shape of a suppressed, overexpressed, or simply an altered protein product.6, 7 From a holistic point of view, the techniques involve an apparently simplistic concept involving multiple receptor-ligand interactions; varying cell entry modes like lipofection, sonification, and transfection; and further downstream pathway effects. Furthermore, these technologies are variable in terms of their specificity and sensitivity, off-target effects, finances, and technique expertise. The body’s immune response to accept the foreign genetic elements within the cells can lead to the rejection of foreign tissues.
Moreover, molecular knowledge, in terms of methodology differences, defining targetable diseases, innovative nanotechnology tools for gene editing, and ethical aspects, also needs to be understood. The platforms for these technologies are improving every day, with a plethora of new data appearing due to technology miniaturization and automation and newer discoveries to improve the yield and specificity of an edited product. Alongside the developmental improvement in genome-wide engineering the regulatory work-up, standardization protocols need to be devised to reduce inter and intra-method imprecision, defining the indications and contraindications of every technique to help improve the concept of personalized medicine.
This review briefly explains the available technologies, provides comparison and contrast between different genome-editing methods, and identifies some newer versions of genome editing with possible bioethical concerns.
Review Methodology
PubMed searches with the keywords genome-editing techniques or gene-editing techniques in the last 10 and 5 years yielded a total of 4,466 and 4,054 references, except some historical and related references. Specific searches for articles dealing with specific genome-editing methods included conventional genome-editing systems (n = 100), chemical methods (n = 252), meganucleases (n = 83), zinc-finger nucleases (ZFNs) (n = 890), transcription activator-like effector nucleases (TALENs) (n = 1,136), homing endonucleases (n = 265), and CRISPR (n = 11,421). The search was therefore limited to reviews showing conceptual information of common techniques and comparative information about ZFNs, TALENSs, and CRISPR technologies. Finally, the literature was searched for learning newer and advanced gene-editing methods and bioethical concerns associated with genome biotechnologies.
Genome-Editing Techniques
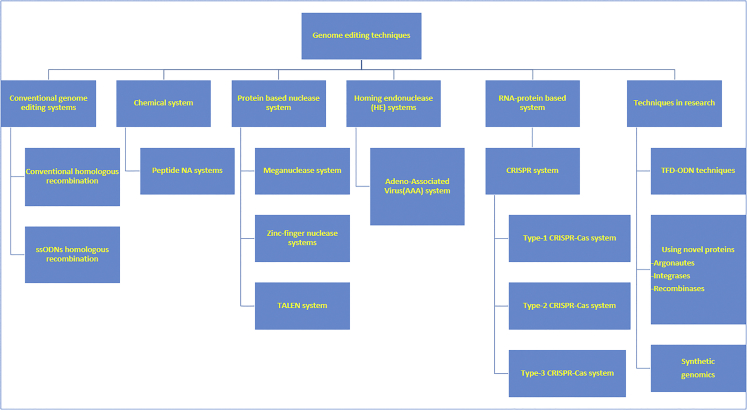
The recent expansion and advancements in the field of biotechnology provided us with information and insight into the biochemical and molecular mechanisms to edit DNA and, thus, modify downstream pathways. To date, multiple biotechnologies have shown promise for clinical use, but the field of genome-editing technologies is rapidly evolving and improving. The new techniques seem promising, but the earlier ones have also been updated and improved. For simplicity and consolidation, an overview of genome-editing techniques is presented in Figure 1.
Figure 1.
A Consolidated Overview of Genome-Editing Techniques
Representative genome-editing techniques are discussed below.
-
(1)
Conventional genome-editing technique. In the true sense, the technique may not relate with evolving genome-editing techniques. As highlighted in Figure 1, it includes homologous recombination related with gene intervention. While not much in vogue or lab use today, the technique is based on physiological processes involving a double-stranded repair system. However, some recent data have shown RAD52 protein to be important in mediating homologous recombination, and this protein therefore has been considered as a therapy target in certain cancers like BRCA 1 and 2 repair pathways.6, 7 However, the technique as of now could not gain widespread introduction due to the emergence of newer techniques.
-
(2)
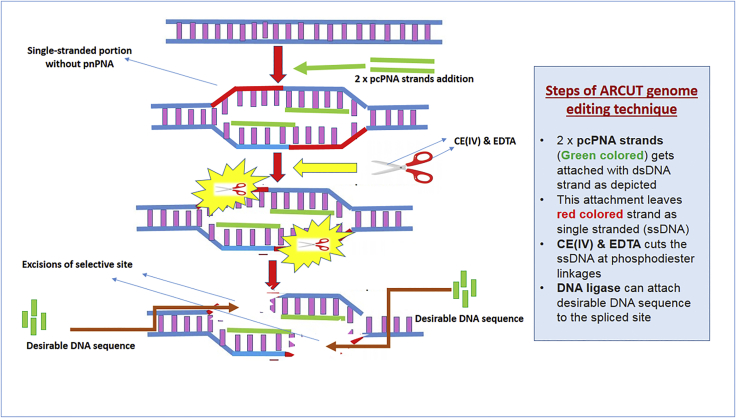
Chemical modalities of genome editing. Komiyama8 utilized non-restriction enzyme methodology termed artificial restriction DNA cutter (ARCUT). This method uses pseudo-complementary peptide nucleic acid (pcPNA), whose job is to specify the cleavage site within the chromosome or the telomeric region. Once pcPNA specifies the site, excision here is carried out by cerium (CE) and EDTA (chemical mixture), which performs the splicing function.8 Furthermore, the technology uses a DNA ligase that can later attach any desirable DNA within the spliced site. The advantage of this particular technique is that it can be used in high salt concentrations. Upon initial introduction, the technique looked quite appealing to the clinical market; however, later issues like increased turnaround time and specifically the manufacturing of site-specific pcPNA became huge hurdles (Figure 2).8, 9
-
(3)
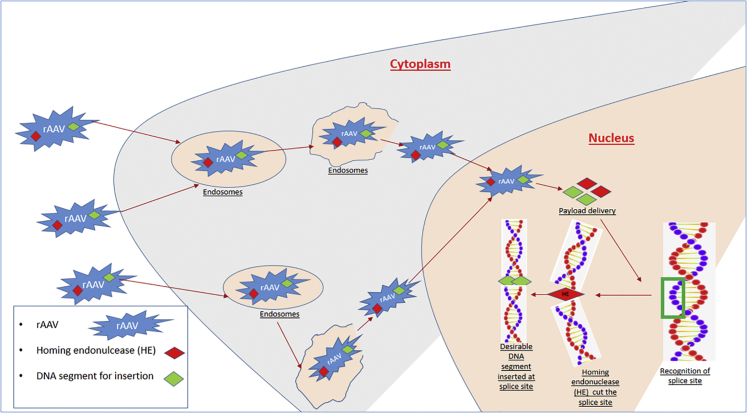
Homing endonuclease systems. Homing endocucleases (HEs) with this word “homing” practically is interpreted as lateral transmission of a genome DNA sequence. The general concept involves a DNA segment where a site is removed by the endocnulceases, which thus results in the formation of 2 segments of DNA fragment.10 So what are these HEs? They are nucleases that occur naturally, with a size almost equivalent to 14 bp, and they are capable of splicing slightly larger DNA sequences.11 Recently, the introduction of recombinant adeno-associated viruses (rAAVs) have allowed them as efficient vehicles for transporting genetic tools of genome engineering into the cell, as depicted in Figure 3.12 Issues pertaining to this technology include engineering difficulties in the preparation of these nucleases as well as developing vectors for their entry into cells.13 Another issue with rAAV, though improving with better biotechnology, was off-target effects like reducing site specificity, less DNA integration, and possible host genome mutations.14
-
(4)
Protein-based nuclease systems. These systems incorporate nuclease proteins for DNA sequence editing. The common techniques are described below.
Meganucleases. Also termed molecular DNA scissors, these are large base pair structures that are sometimes found in the genome. Their potential to excise large pieces of DNA sequences was recently recognized as a genetic tool to modify DNA. This genetic potential has been manipulated in labs by modifying the recognition sites to create nicks, as required for DNA sequence change. These meganucleases are sometimes joined by proteins to create large variants like DmoCre and E-Drel, which can further provide nucleotide site-specific cleavage.15 The technique revolves around two basic steps: first is the recognition of a cleavage site, and then endonucleases splice out the region.16 The positive aspect related to meganucleases is less toxicity, as they are naturally occurring and provide very specific site cleavage. However, there are newer techniques now in the clinical arena that have not allowed them to flourish more.
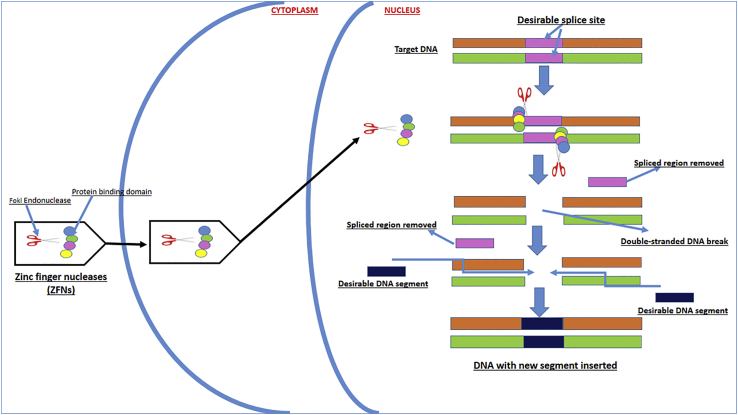
ZFNs. ZFNs are purely artificial structures generated by a combinatorial approach where restriction endonucleases are joined with zinc-finger-binding domain protein. Figure 4 explains their mechanism of action in detail, where a binding protein domain identifies after reaching the desirable splice site, which is then cut at a specific codon by special restriction endonucleases called FokI. The biotechnology is restricted in terms of attachment with 3 codons on either side of the DNA chain. The technique in recent years has gained widespread popularity due to its simplicity and specificity, and it is being employed in clinical usage for certain diseases.17, 18
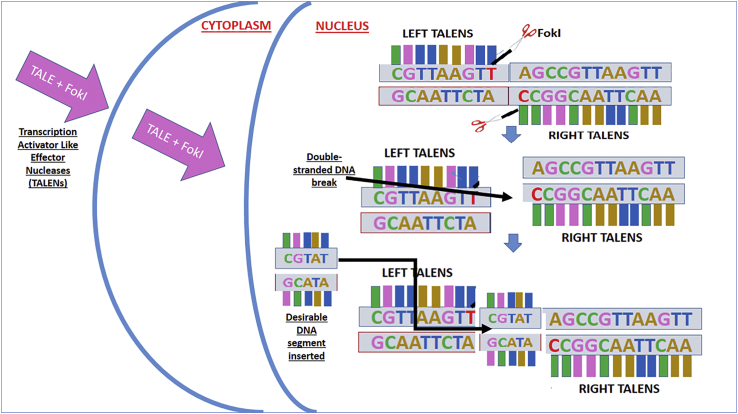
TALENs. TALENS almost resemble ZFNs in terms of manufacturing and mode of action. They are made by a similar principle where a restriction nuclease is bound to a DNA-binding protein domain called TAL effector.19 The difference between TALENs and ZFNs is that the former can target 3 nt in one go and the latter can only address 1 nt, thus making TALENs slightly more site specific with fewer off-target effects.20 However, the techniques share many similarities (Figure 5).
-
(5)
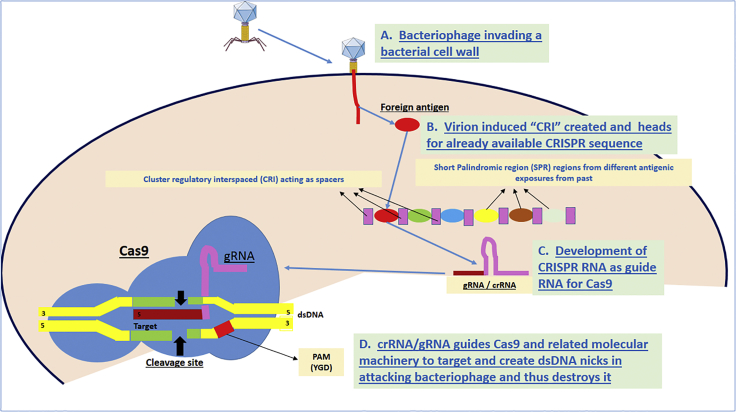
RNA DNA systems. These systems primarily include the different types of CRISPR methods. The concept of CRISPR is primitive and has been derived from an ancient immunity system, adopted in nature by some prokaryotic cells like Archea and probably some bacteria.21 CRISPR in itself has two components, including SPR termed sometimes as spacers, which are hallmarked by varying and differing nucleotide sequences, and probably each one of them represents a past exposure to foreign antigen. The CRI may represent the genetic memory for a bacterium and can be re-activated once encountered with a similar foreign antigen. CRI has similar nucleotides (repeats) representing like separators between different CRIs.22 Figure 6 attempts to provide a basic overview of the CRISPR/Cas9 concept.
Cas especially Cas9 as depicted in Figure 6 has a nuclease function. Whenever CRISPR RNA (crRNA; also termed guide RNA [gRNA]) guides the Cas9 protein regarding a possible antigenic threat, like a bacteriophage, it with the help of gRNA creates double-stranded DNA (dsDNA) nicks at the guided selected sites, causing a site-specific cleavage and, thus, destruction of the antigen.23 Moreover, the memory from the antigen is stored as spacer within CRISPR.24
This physiological role of Cas9/CRISPR as explained above had recently been extensively utilized for multiple clinical conditions.25, 26, 27 At the time of writing this review, the news broke about Lulu and Nana being claimed to be the first genetically modified babies, where the human genome was edited to create resistance against HIV infection.28 Specific crRNA/gRNA has been engineered, which can be introduced into cell nuclei and later Cas9 where the non-desirable dsDNA is associated with the Cas9 after guidance provided by the specific crRNA/gRNA. This complementary binding between gRNA and the non-desirable segment allows Cas9 to destroy the DNA fragment. In clinical and research practice, the created nick can be specifically filled by inserting the sequence of choice to change the non-desirable sequence of nucleotides.29
Over the last few years, CRISPR/Cas9 technology has gained widespread popularity on account of its simplicity and specificity, with different versions of the original now under research. Multiple experimentations and biotechnologies have been re-defining the CRISPR/Cas technologies into 3 distinct types of CRISPR-Cas types, based on crRNA processing and further action, including the following:
Type 1 CRISPR/Cas system. This version utilized Cas5 or Cas6 for pre-processing of crRNA; further cleavage function needs Cas3, Cascade, and crRNA for interference.
Type 2 CRISPR/Cas system. Though Cas9 typically functions under the guidance of crRNA to target DNA, RNase III, trans activating RNA (tracrRNA), and a yet-to-be-identified protein factor are involved in trimming at the 5′ end.
Type 3 CRISPR/Cas system. Like the type 1 system, this category uses Cas6 for processing crRNA 3′ end trimming. The uniqueness of this technique is its targeting of RNA, which is done by a specific complex called type III Csm/Cmr complex.30
Apart from the aforementioned conventional style classification of CRISPR/Cas classification, the data review provided multiple other biotechnologies now being utilized. Some examples include photo-activating CRISPR system,31 Intein-inducible split Cas9,32 and modifications like hybrid crRNA-tracrRNA.33
-
(6)
Gene-silencing techniques. These methods may not fall truly under genome editing, but they still are capable of modifying the DNA sequence. These technologies include RNAi, CRISPR interference (CRISPRi), and morpholino oligonucleotide techniques.34, 35, 36
Figure 2.
Excision of Selective Site of dsDNA by Utilizing Artificial Restriction DNA Cutter
Figure 3.
Schematic Showing rAAV Entry, Movement within Cytoplasm, Attachment with DNA, and Integration with DNA Segment for Possible Genome Modification
The steps include the following: (1) entry of rAAV into cell, (2) uptake by exosome and transport within cytoplasm, (3) release of rAAV for entry into nucleus, (4) rAAV delivery of homing endocnulease (HE) and desirable DNA segment, (5) HE cut of the non-desirable DNA code, and (6) rAAV-delivered desirable DNA code replacement of the DNA.
Figure 4.
Schematic Showing Step by Step Zinc-Finger Nuclease-Induced Genome Editing
The mechanisms include the following: (1) ZFNs containing FokI endonucleases and protein-binding domains are introduced into the cell, (2) FokI and protein-binding domains are released to enter the nucleus, (3) protein-binding domains attach with DNA fragment to be removed, (3) FokI cuts out the identified DNA segment by creating double-stranded DNA break, and (4) the desirable DNA segment is inserted and integrated into the DNA sequence.
Figure 5.
Diagram Showing Mechanisms of Transcription Activator-like Effector Nucleases
The steps of gene editing include the following: (1) TALENs containing FokI endonucleases and TALE domains are introduced into the cell, (2) FokI and TALE domains are released to enter the nucleus, (3) TALE recognizes the non-desirable DNA segments and attaches with them, (4) FokI cleaves the non-desirable DNA segments, and (5) after the non-desirable DNA segments are cleaved, the desirable segment of DNA is incorporated into the DNA.
Figure 6.
Schematic Demonstrating the Concept of CRISPR/Cas9 Interactions Leading to the Destruction of Viral Genome at the Selected Splice Site by the crRNA/gRNA
Comparative Analysis
The above provides a gist of the various commonly used genome-editing techniques. Though there is enormous development, innovation, and design of newer ways to edit the genome, we focus our further discussion on the comparison of common techniques, including ZFN, TALEN, and CRISPR methods. Tables 1, 2, and 3 provide a comparative assessment among these methods.
Table 1.
Biotechnology Differences among Prototype Genome-Editing Techniques
| Serial No. | Parameter | ZFN | TALEN | CRISPER/Cas | Reference |
|---|---|---|---|---|---|
| 1 | design simplicity | moderate (ZFNs need customized protein for every DNA sequence) | slightly complex (identical repeats are multiple, which creates technical issues of engineering and delivery into cells) | simpler (available versions for crRNA can be easily designed) | 48 |
| 2 | engineering feasibility | low | higher | highest | 24, 49 |
| 3 | multiplex genome editing | few models | few models | high-yield multiplexing available (no need for obtaining embryonic stem cells) | 48, 50 |
| 4 | large-scale library preparation | not much progress (need individual gene tailoring) | not much progress (need individual gene tailoring) | progress demonstrated (CRISPR only requires plasmid containing small oligonucleotides) | 51 |
| 5 | specificity | low | higher | highest | 24 |
| 6 | efficiency | normala | normalb | high | 24, 48, 52 |
| 7 | cost | low | high | low | 53 |
Table 2.
Side Effect Profiles for Genome-Editing Methods
| Serial No. | Parameter | ZFN | TALEN | CRISPER/Cas | Reference |
|---|---|---|---|---|---|
| 1 | off-target effect incidence | – | – | – | 54 |
| a | homologous recombination rate frequency | + | + | + | – |
| b | non-homologous end joining (NHEJ) mutation rates | + | + | ++ (only with earlier versions) | 55, 56 |
| c | immune reaction susceptibility | less | less | more | 57, 58 |
| d | RNA-guided endonuclease (RGEN)-induced off-target mutatagenesis | − | − | ++ | 59 |
| 2 | cytotoxicity chances | ++ | + | + | – |
Table 3.
Clinical and Research Applications across Important Genome-Editing Techniques
| Serial No. | Parameter | ZFN | TALEN | CRISPER/Cas | Reference |
|---|---|---|---|---|---|
| 1 | diagnostic utility | + | + | +++ | 60 |
| 2 | clinical trial use | ++ | + | +++ | 61 |
| 3 | utility as epigenetic marker | ++ | +++ | ++++ | 62 |
| 4 | making gene-knockout models for research | no | no | yes (CRISPRi) | 63 |
| 5 | capacity for modification of mitochondrial DNA | no | no | probable | 64 |
| 6 | genetic editing in human babies | no | no | yes | 65 |
| 7 | RNA editing | no | no | yes | 66 |
Advancements in Genome Engineering
The biotechnology is booming with a lot of newer modalities to edit the genome. Oligodeoxyribonucleotide (ODN) can be utilized with double-stranded transcription factor decoy (TFD) to act as a therapeutic target for multiple diseases, which can affect the transcription factor and thus bring in the requisite change in transcription and further downstream protein actions.37 Papaioannou et al.38 have utilized single-stranded ODNs to precisely cut genomes for repairing very small point mutations, giving a footprint-free genome-editing modality. This concept involves a drug (doxycycline)-induced Cs9 transgene, which is carried into the cell by a specific transposon, providing us with very specific and efficient Cas9-mediated editing of the genome. This technique does not need the conventional donor template, and, thus, it is termed footprint-free genome editing.38 The technique seems to have minimal off-target effects and is considered to be a safer version.
Other novel modalities of genome editing are also appearing in the literature, with slight modifications of existing techniques. Martínez-Gálvez et al.39 used single-stranded DNA (ssDNA) and argonautes in gene editing and helped improved gene editing. Some researchers have utilized certain enzymes like integrases and in the future may obviate the need for nucleases.40
The most interesting part of the genome-editing technique, which may be the game changer in genome editing, is the whole genome engineering by synthesis that in fact would re-create the genome from scratch as per the given designed DNA code. This probably will become the synthetic genomics of the future.41 Though research work in this domain stands preliminary, over time it is anticipated that this technology may overtake the concept of genome editing.
Bioethical Issues and Genome-Editing Techniques
Genome-editing tools are powerful in terms of their potential to not only bring biotechnological revolution in the field of crop development and human pathology but also, in the wrong hands, lead to abuse and misuse in multiple ways, including manipulation of germline genetics. Genuine bioethical concerns have been raised by many experts.42 While time will be the actual judge of these technologies as boon or bane, still the methods can impact the human race probably in the most nuclear ways, and our incoming human race may be victimized in ways we do not yet understand.43 Principal concerns apart from illegal germline mutation include the morality, the eugenics helping the fittest to survive, ongoing clinical debates about informed consent, religious debate, the possible rise of clones, designer babies, and possibly superhumans.44, 45, 46 Moreover, the current literature also rules in the possibility of genome editing as a future weapon of war.47
While the quest for a healthy baby and right of best possible treatment choice have been acknowledged in many societies, the approaching biotechnological revolution seems imminent and undeniable. The pressing need, therefore demands a harmonious and regulated translation of needed aspects of genome-editing-related technologies for molecular medicine and other non-clinical crop and food industries. This will need consensus in public opinion, debates among experts, involvement of biotechnologists, opinions of bioethical experts, regulatory frameworks within legislatures, and final guidelines and oversight for the finally allowed limited application.
Conclusions
This review discussed multiples aspects of genome-editing technologies, including a classification; some basic explanatory concepts on mechanisms; and comparison between methods, newer advancements, and bioethical concerns. It seems that CRISPR/Cas technologies are probably superseding ZFNs and TALENS. However, the CRIPSR/Cas methods are also being improvised, and newer additions have further enhanced its functional capabilities with reduced off-target effects. Furthermore, the process of engineering better gene modification technologies is evolving and can one day replace even CRISPR/Cas, possibly shifting to synthetic genomics. Among all these revolutionary developments, bioethical concerns need serious attention.
References
- 1.Friedrich M. Ueber die chemische Zusammensetzung der Eiterzellen [On the chemical composition of pus cells] Medicinisch-chemische Untersuchungen. 1871;4:441–460. [Google Scholar]
- 2.Watson J.D., Crick F.H.C. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 3.Sanger F., Coulson A.R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 1975;94:441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- 4.Mullis K.B. 2016. https://www.karymullis.com/
- 5.Pavletich N.P., Pabo C.O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991;252:809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 6.Claussin C., Chang M. Multiple Rad52-Mediated Homology-Directed Repair Mechanisms Are Required to Prevent Telomere Attrition-Induced Senescence in Saccharomyces cerevisiae. PLoS Genet. 2016;12:e1006176. doi: 10.1371/journal.pgen.1006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai N., Kagawa W. [Molecular mechanisms of homologous recombination promoted by budding yeast Rad52] Seikagaku. 2014;86:693–697. [PubMed] [Google Scholar]
- 8.Komiyama M. Chemical modifications of artificial restriction DNA cutter (ARCUT) to promote its in vivo and in vitro applications. Artif. DNA PNA XNA. 2014;5:e1112457. doi: 10.1080/1949095X.2015.1112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito K., Komiyama M. Site-selective scission of human genome using PNA-based artificial restriction DNA cutter. Methods Mol. Biol. 2014;1050:111–120. doi: 10.1007/978-1-62703-553-8_9. [DOI] [PubMed] [Google Scholar]
- 10.Schultz B.R., Chamberlain J.S. Recombinant adeno-associated virus transduction and integration. Mol. Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith J., Grizot S., Arnould S., Duclert A., Epinat J.C., Chames P., Prieto J., Redondo P., Blanco F.J., Bravo J. A combinatorial approach to create artificial homing endonucleases cleaving chosen sequences. Nucleic Acids Res. 2006;34:e149. doi: 10.1093/nar/gkl720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon Y., Wang D., Tai P.W.L., Riley J., Gao G., Rivera-Pérez J.A. Streamlined ex vivo and in vivo genome editing in mouse embryos using recombinant adeno-associated viruses. Nat. Commun. 2018;9:412. doi: 10.1038/s41467-017-02706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rey-Rico A., Cucchiarini M. Controlled release strategies for rAAV-mediated gene delivery. Acta Biomater. 2016;29:1–10. doi: 10.1016/j.actbio.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Jin L., Lange W., Kempmann A., Maybeck V., Günther A., Gruteser N., Baumann A., Offenhäusser A. High-efficiency transduction and specific expression of ChR2opt for optogenetic manipulation of primary cortical neurons mediated by recombinant adeno-associated viruses. J. Biotechnol. 2016;233:171–180. doi: 10.1016/j.jbiotec.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Zaslavskiy M., Bertonati C., Duchateau P., Duclert A., Silva G.H. Efficient design of meganucleases using a machine learning approach. BMC Bioinformatics. 2014;15:191. doi: 10.1186/1471-2105-15-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurrough T.A., Brown C.M., Zhang K., Hausner G., Junop M.S., Gloor G.B., Edgell D.R. Active site residue identity regulates cleavage preference of LAGLIDADG homing endonucleases. Nucleic Acids Res. 2018;46:11990–12007. doi: 10.1093/nar/gky976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochiai H., Yamamoto T. Construction and Evaluation of Zinc Finger Nucleases. Methods Mol. Biol. 2017;1630:1–24. doi: 10.1007/978-1-4939-7128-2_1. [DOI] [PubMed] [Google Scholar]
- 18.Ji H., Lu P., Liu B., Qu X., Wang Y., Jiang Z., Yang X., Zhong Y., Yang H., Pan H. Zinc-Finger Nucleases Induced by HIV-1 Tat Excise HIV-1 from the Host Genome in Infected and Latently Infected Cells. Mol. Ther. Nucleic Acids. 2018;12:67–74. doi: 10.1016/j.omtn.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensel G., Kumlehn J. Genome Engineering Using TALENs. Methods Mol. Biol. 2019;1900:195–215. doi: 10.1007/978-1-4939-8944-7_13. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasegaran S., Carroll D. Origins of Programmable Nucleases for Genome Engineering. J. Mol. Biol. 2016;428(5 Pt B):963–989. doi: 10.1016/j.jmb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer S., Maier L.K., Stoll B., Brendel J., Fischer E., Pfeiffer F., Dyall-Smith M., Marchfelder A. An archaeal immune system can detect multiple protospacer adjacent motifs (PAMs) to target invader DNA. J. Biol. Chem. 2012;287:33351–33363. doi: 10.1074/jbc.M112.377002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eid A., Mahfouz M.M. Genome editing: the road of CRISPR/Cas9 from bench to clinic. Exp. Mol. Med. 2016;48:e265. doi: 10.1038/emm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F., Wen Y., Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 2014;23(R1):R40–R46. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J.H., Adikaram P., Pandey M., Genis A., Simonds W.F. Optimization of genome editing through CRISPR-Cas9 engineering. Bioengineered. 2016;7:166–174. doi: 10.1080/21655979.2016.1189039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain W., Mahmood T., Hussain J., Ali N., Shah T., Qayyum S., Khan I. CRISPR/Cas system: A game changing genome editing technology, to treat human genetic diseases. Gene. 2019;685:70–75. doi: 10.1016/j.gene.2018.10.072. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Zheng W., Liu S., Li B., Jiang X. Delivery of CRISPR/Cas9 by novel strategies for gene therapy. ChemBioChem. 2019;20:634–643. doi: 10.1002/cbic.201800629. [DOI] [PubMed] [Google Scholar]
- 27.Cai B., Sun S., Li Z., Zhang X., Ke Y., Yang J., Li X. Application of CRISPR/Cas9 technologies combined with iPSCs in the study and treatment of retinal degenerative diseases. Hum. Genet. 2018;137:679–688. doi: 10.1007/s00439-018-1933-9. [DOI] [PubMed] [Google Scholar]
- 28.Regalado A. Exclusive: Chinese scientists are creating CRISPR babies. A daring effort is under way to create the first children whose DNA has been tailored using gene editing. MIT Technology Review. 2018. https://www.technologyreview.com/s/612458/exclusive-chinese-scientists-are-creating-crispr-babies/
- 29.Damian M., Porteus M.H. A crisper look at genome editing: RNA-guided genome modification. Mol. Ther. 2013;21:720–722. doi: 10.1038/mt.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rath D., Amlinger L., Rath A., Lundgren M. The CRISPR-Cas immune system: biology, mechanisms and applications. Biochimie. 2015;117:119–128. doi: 10.1016/j.biochi.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Polstein L.R., Gersbach C.A. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat. Chem. Biol. 2015;11:198–200. doi: 10.1038/nchembio.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zetsche B., Volz S.E., Zhang F. A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat. Biotechnol. 2015;33:139–142. doi: 10.1038/nbt.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mali P., Aach J., Stranges P.B., Esvelt K.M., Moosburner M., Kosuri S., Yang L., Church G.M. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 2013;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer S.E. RNA Interference and MicroRNA-Mediated Silencing. Curr. Protoc. Mol. Biol. 2015;112 doi: 10.1002/0471142727.mb2601s112. 26.1.1–5. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M., Yokota E., Sakuma T., Yamatsuji T., Takigawa N., Ushijima T., Yamamoto T., Fukazawa T., Naomoto Y. Development of an integrated CRISPRi targeting ΔNp63 for treatment of squamous cell carcinoma. Oncotarget. 2018;9:29220–29232. doi: 10.18632/oncotarget.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul S., Caruthers M.H. Synthesis of Phosphorodiamidate Morpholino Oligonucleotides and Their Chimeras Using Phosphoramidite Chemistry. J. Am. Chem. Soc. 2016;138:15663–15672. doi: 10.1021/jacs.6b08854. [DOI] [PubMed] [Google Scholar]
- 37.Hecker M., Wagner A.H. Transcription factor decoy technology: A therapeutic update. Biochem. Pharmacol. 2017;144:29–34. doi: 10.1016/j.bcp.2017.06.122. [DOI] [PubMed] [Google Scholar]
- 38.Papaioannou I., Simons J.P., Owen J.S. Oligonucleotide-directed gene-editing technology: mechanisms and future prospects. Expert Opin. Biol. Ther. 2012;12:329–342. doi: 10.1517/14712598.2012.660522. [DOI] [PubMed] [Google Scholar]
- 39.Martínez-Gálvez G., Ata H., Campbell J.M., Ekker S.C. ssDNA and the Argonautes: The Quest for the Next Golden Editor. Hum. Gene Ther. 2016;27:419–422. doi: 10.1089/hum.2016.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledford H. Beyond CRISPR: A guide to the many other ways to edit a genome. Nature. 2016;536:136–137. doi: 10.1038/536136b. [DOI] [PubMed] [Google Scholar]
- 41.Luo Z., Yang Q., Geng B., Jiang S., Yang S., Li X., Cai Y., Dai J. Whole genome engineering by synthesis. Sci. China Life Sci. 2018;61:1515–1527. doi: 10.1007/s11427-018-9403-y. [DOI] [PubMed] [Google Scholar]
- 42.Krishan K., Kanchan T., Singh B. Human Genome Editing and Ethical Considerations. Sci. Eng. Ethics. 2016;22:597–599. doi: 10.1007/s11948-015-9675-8. [DOI] [PubMed] [Google Scholar]
- 43.Krishan K., Kanchan T., Singh B., Baryah N., Puri S. Germline Editing: Editors Cautionary. Clin. Ter. 2018;169:e58–e59. doi: 10.7417/T.2018.2053. [DOI] [PubMed] [Google Scholar]
- 44.Shinwari Z.K., Tanveer F., Khalil A.T. Ethical Issues Regarding CRISPR Mediated Genome Editing. Curr. Issues Mol. Biol. 2018;26:103–110. doi: 10.21775/cimb.026.103. [DOI] [PubMed] [Google Scholar]
- 45.Liao S.M. Designing humans: A human rights approach. Bioethics. 2019;33:98–104. doi: 10.1111/bioe.12519. [DOI] [PubMed] [Google Scholar]
- 46.Hofmann B. The gene-editing of super-ego. Med. Health Care Philos. 2018;21:295–302. doi: 10.1007/s11019-018-9836-z. [DOI] [PubMed] [Google Scholar]
- 47.Fraser C.M., Dando M.R. Genomics and future biological weapons: the need for preventive action by the biomedical community. Nat. Genet. 2001;29:253–256. doi: 10.1038/ng763. [DOI] [PubMed] [Google Scholar]
- 48.Wang H., La Russa M., Qi L.S. CRISPR/Cas9 in Genome Editing and Beyond. Annu. Rev. Biochem. 2016;85:227–264. doi: 10.1146/annurev-biochem-060815-014607. [DOI] [PubMed] [Google Scholar]
- 49.Salsman J., Dellaire G. Precision genome editing in the CRISPR era. Biochem. Cell Biol. 2017;95:187–201. doi: 10.1139/bcb-2016-0137. [DOI] [PubMed] [Google Scholar]
- 50.Sakuma T., Nishikawa A., Kume S., Chayama K., Yamamoto T. Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci. Rep. 2014;4:5400. doi: 10.1038/srep05400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poirier J.T. CRISPR Libraries and Screening. Prog. Mol. Biol. Transl. Sci. 2017;152:69–82. doi: 10.1016/bs.pmbts.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Sun B., Yang J., Yang S., Ye R.D., Chen D., Jiang Y. A CRISPR-Cpf1-Assisted Non-Homologous End Joining Genome Editing System of Mycobacterium smegmatis. Biotechnol. J. 2018;13:e1700588. doi: 10.1002/biot.201700588. [DOI] [PubMed] [Google Scholar]
- 53.Qiu X.Y., Zhu L.Y., Zhu C.S., Ma J.X., Hou T., Wu X.M., Xie S.S., Min L., Tan D.A., Zhang D.Y., Zhu L. Highly Effective and Low-Cost MicroRNA Detection with CRISPR-Cas9. ACS Synth. Biol. 2018;7:807–813. doi: 10.1021/acssynbio.7b00446. [DOI] [PubMed] [Google Scholar]
- 54.Koo T., Lee J., Kim J.S. Measuring and Reducing Off-Target Activities of Programmable Nucleases Including CRISPR-Cas9. Mol. Cells. 2015;38:475–481. doi: 10.14348/molcells.2015.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Symington L.S., Gautier J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- 56.Yin H., Xue W., Chen S., Bogorad R.L., Benedetti E., Grompe M., Koteliansky V., Sharp P.A., Jacks T., Anderson D.G. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat. Biotechnol. 2014;32:551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jordan B. Les débuts de CRISPR en thérapie génique - Chroniques génomiques [First use of CRISPR for gene therapy] Med. Sci. (Paris) 2016;32:1035–1037. doi: 10.1051/medsci/20163211024. [DOI] [PubMed] [Google Scholar]
- 58.Chew W.L. Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018;10 doi: 10.1002/wsbm.1408. [DOI] [PubMed] [Google Scholar]
- 59.Cho S.W., Kim S., Kim Y., Kweon J., Kim H.S., Bae S., Kim J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shang W., Wang F., Fan G., Wang H. Key elements for designing and performing a CRISPR/Cas9-based genetic screen. J. Genet. Genomics. 2017;44:439–449. doi: 10.1016/j.jgg.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Wassef M., Luscan A., Battistella A., Le Corre S., Li H., Wallace M.R., Vidaud M., Margueron R. Versatile and precise gene-targeting strategies for functional studies in mammalian cell lines. Methods. 2017;121-122:45–54. doi: 10.1016/j.ymeth.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Vojta A., Dobrinić P., Tadić V., Bočkor L., Korać P., Julg B., Klasić M., Zoldoš V. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016;44:5615–5628. doi: 10.1093/nar/gkw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang B., Yang Q., Chen J., Wu L., Yao T., Wu Y., Xu H., Zhang L., Xia Q., Zhou D. CRISPRi-Manipulation of Genetic Code Expansion via RF1 for Reassignment of Amber Codon in Bacteria. Sci. Rep. 2016;6:20000. doi: 10.1038/srep20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fogleman S., Santana C., Bishop C., Miller A., Capco D.G. CRISPR/Cas9 and mitochondrial gene replacement therapy: promising techniques and ethical considerations. Am. J. Stem Cells. 2016;5:39–52. [PMC free article] [PubMed] [Google Scholar]
- 65.Callaway E. Gene-editing research in human embryos gains momentum. Nature. 2016;532:289–290. doi: 10.1038/532289a. [DOI] [PubMed] [Google Scholar]
- 66.Zimmer C. Scientists Find Form of Crispr Gene Editing With New Capabilities. The New York Times, June 3, 2016. 2016. https://www.nytimes.com/2016/06/04/science/rna-c2c2-gene-editing-dna-crispr.html