Abstract
By allowing bilateral access to sound, bilateral cochlear implants (BI-CIs) or unilateral CIs for individuals with single-sided deafness (SSD; i.e., normal or near-normal hearing in one ear) can improve sound localization and speech understanding in noise. Spatial hearing in the horizontal plane is primarily conveyed by interaural time and level differences computed from neurons in the superior olivary complex that receive frequency-matched inputs. Because BI-CIs and SSD-CIs do not necessarily convey frequency-matched information, it is critical to understand how to align the inputs to CI users. Previous studies show that interaural pitch discrimination for SSD-CI listeners is highly susceptible to contextual biases, questioning its utility for establishing interaural frequency alignment. Here, we replicate this finding for SSD-CI listeners and show that these biases also extend to BI-CI listeners. To assess the testing-range bias, three ranges of comparison electrodes (BI-CI) or pure-tone frequencies (SSD-CI) were tested: full range, apical/lower half, or basal/upper half. To assess the reference bias, the reference electrode was either held fixed throughout a testing block or randomly chosen from three electrodes (basal end, middle, or apical end of the array). Results showed no effect of reference electrode randomization, but a large testing range bias; changing the center of the testing-range shifted the pitch match by an average 63 % (BI-CI) or 43 % (SSD-CI) of the change magnitude. This bias diminished pitch-match accuracy, with a change in reference electrode shifting the pitch match only an average 34 % (BI-CI) or 40 % (SSD-CI) of the expected amount. Because these effects extended to the relatively more symmetric BI-CI listeners, the results suggest that the bias cannot be attributed to interaural asymmetry. Unless the range effect can be minimized or accounted for, a pitch-discrimination task will produce interaural place-of-stimulation estimates that are highly influenced by the conditions tested, rather than reflecting a true interaural place-pitch comparison.
Keywords: cochlear implant, pitch, binaural hearing, interaural mismatch
INTRODUCTION
Spatial hearing is important for sound localization and speech understanding in background noise. Spatial hearing in the horizontal plane involves computations of interaural time differences (ITDs) and interaural level differences (ILDs) in the brainstem neurons of the superior olivary complex (Carr and Konishi 1990; Yin and Chan 1990; Joris et al. 1998). ITD- and ILD-sensitive neurons are most effective for frequency-matched inputs (e.g., Blanks et al. 2007). Frequency matching is assumed to occur near perfectly in typical acoustic hearing, but this is not necessarily the case for cochlear-implant (CI) users. This study investigated the efficacy of using a pitch-discrimination task to estimate interaural place-of-stimulation mismatch for two types of CI users likely to experience interaural mismatch: bilateral CI (BI-CI) and single-sided-deafness CI (SSD-CI) users.
Assuming standard clinical frequency-to-electrode allocation, a case of interaural mismatch must occur for BI-CI users implanted with two different array types and lengths; however, interaural mismatch can also occur for BI-CI users implanted with the same type of intracochlear array. For example, slightly different insertion depths across the ears could result in interaural mismatch. Studies have reported a large range of angular insertion depths using histology, pitch perception, computed-tomography (CT) scans, or x-rays (Baumann and Nobbe 2004; Adunka et al. 2006; Radeloff et al. 2008; Vermeire et al. 2008; van der Marel et al. 2014). Biological factors such as neuronal survival (Bierer and Nye 2014) or connective tissue growth in scala tympani could also affect interaural mismatch. Finally, the clinical programming of sound processors could produce an interaural mismatch. It is not uncommon for individual electrodes to be deactivated due to issues with loudness or non-auditory stimulation, resulting in a different number of active electrodes in each ear. With no standard clinical method to interaurally align the frequency information, a different number of active electrodes will result in a different frequency-to-place allocation in each ear.
SSD-CI users can also experience interaural mismatch, particularly at the apex (Schatzer et al. 2014). Because the electrode array is not inserted all the way into the cochlea, the most apical electrode will often not stimulate the most apical spiral ganglia. Despite this, standard clinical practice is to allocate the full device bandwidth (~ 100–8000 Hz) across the available electrodes, thereby generating an interaural mismatch. Landsberger et al. (2015) compiled insertion angles for the most apical electrode across 661 unilateral CI listeners reported in 12 different studies. Based on these reports, Wess et al. (2017) estimated that the average place-of-stimulation mismatch between electrode position and the standard frequency allocation for SSD-CI users is approximately 5 mm (1.5 octaves).
Pitch is commonly used in the BI-CI perception research literature to estimate relative place of stimulation (Kan et al. 2013; Goupell 2015; Goupell and Litovsky 2015; Kan et al. 2015), although some studies have used binaural metrics like ITD discrimination (e.g., Noel and Eddington 2013). Recent reports have shown relatively weak correspondence between the relative place pitch elicited by an electrode in one ear and several other measures, including the clinical frequency allocation associated with that electrode (Aronoff et al. 2016), the contralateral electrode yielding the best ITD sensitivity or the largest electrophysiologically measured binaural-interaction component (Hu and Dietz 2015), and the pitch elicited by an acoustic signal in the contralateral ear (Carlyon et al. 2010). It is worth noting that tasks that use pitch perception may have procedure-specific confounds because sensory judgments like pitch magnitude estimation or discrimination are affected by a number of contextual biases (e.g., Poulton 1979). To avoid such biases, an experimenter should perform some form of validation. In particular, Carlyon et al. (2010) reported that SSD-CI listeners can demonstrate a substantial pitch-range bias, whereby the interaural pitch match can change simply by changing the testing range. One possible reason that such a substantial range bias could occur for SSD-CI listeners is that the signals presented to the two ears are substantially different. If this were the case, then range bias should be smaller for more similar inputs.
The purpose of this study was to measure interaural place-of-stimulation mismatch for BI-CI and SSD-CI listeners via pitch discrimination and concurrently characterize the magnitude of contextual biases on these measurements. We hypothesized that BI-CI listeners would show a smaller range bias than SSD-CI listeners because BI-CI listeners are presented electrical stimulation to both ears in a relatively symmetric manner. In addition, BI-CI listeners that have relatively asymmetrical inputs (e.g., produced by a much longer duration of deafness in one ear and/or long inter-implant duration) would have larger range biases than those with relatively symmetrical inputs. Interaural pitch discrimination was systematically measured as a function of both reference electrode (i.e., cochlear place-of-stimulation) and comparison range. We followed the recommendations of Carlyon et al. (2010) to examine the possible influence of range effects on the measured pitch match and also included an investigation of the effect of the reference-electrode randomization within a testing block. We hypothesized that pitch discrimination would be affected by both a comparison electrode context bias (i.e., testing-range bias) and a reference-electrode context bias (i.e., reference bias).
METHODS
Seven BI-CI listeners participated in the study to examine the main question of whether BI-CI listeners would demonstrate range effects similar to those that have been previously reported for SSD-CI listeners (Carlyon et al. 2010; Schatzer et al. 2014). Three SSD-CI participants were also included in the study as a control to examine whether the previously reported range effects would replicate. Because of differences in the experimental methodologies and associated analyses for the two listener groups, the methods are presented separately for each group.
Experiment 1: BI-CI Listeners
Listeners
Seven BI-CI listeners participated in the study with the reference electrodes placed in the right ear; one of the listeners was tested twice, with the reference electrodes also placed in the left ear. Their hearing histories, demographic information, and electrode arrays are shown in Table 1. All BI-CI listeners used Cochlear-brand devices with 22 intracochlear electrodes. The internal arrays had various intra-electrode spacing depending on the generation and the curvature of the array (i.e., perimodiolar or lateral wall arrays). Despite the differences in intra-electrode spacing in linear millimeters, the manufacturer’s intent is for each array type to have a constant cochlear angle. Therefore, without precise information on electrode location, as one would receive from a CT scan, intra-electrode spacing of approximately 0.75 mm is a reasonable assumption for these types of arrays. The most apical intracochlear electrode (i.e., likely lowest place pitch) is numbered 22, and the most basal (i.e., likely highest place pitch) is numbered 1. There were two extra-cochlear ground electrodes. These CIs produce a range of 0 to 255 clinical current units (CUs), with each CU producing a logarithmically spaced change in current.
Table 1.
Listener information. An extra set of listener codes are provided for the BI-CI listeners to allow comparison to previous studies
| Listener | Age (years) | Mode | Array type | Dur. deafness (years) | CI experience (years) | Etiology | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | ||||
| BCI1 | CAB | 71 | BI-CI | CI24M | CI24R | 36 | 44 | 20 | 13 | Unknown | Unknown |
| BCI2 | CAE | 65 | BI-CI | CI24RE | CI24RE | 4 | 3 | 10 | 11 | Hereditary | Hereditary |
| BCI3 | CAK | 70 | BI-CI | CI422 | CI24R(CS) | 0 | 0 | 13 | 1 | Sinus Surgery | Unknown |
| BCI4 | CAQ | 59 | BI-CI | CI24RE | CI24RE | 5 | 6 | 9 | 5 | Meniere’s | Meniere’s |
| BCI5 | CBF | 59 | BI-CI | CI24RE | CI422 | 5 | 10 | 7 | 2 | Hereditary | Hereditary |
| BCI6 | CCA | 77 | BI-CI | CI24RE | CI512 | 61 | 1 | 3 | 6 | Measles, antibiotics | Measles, antibiotics |
| BCI7 | CCI | 66 | BI-CI | CI24R(CS) | CI24RE | 2 | 8 | 15 | 4 | Possibly otoscleroses | Possibly otoscleroses |
| SSD1 | 59 | SSD-CI | n/a | Flex28 | n/a | 8 | n/a | 2 | n/a | Sudden SNHL | |
| SSD2 | 38 | SSD-CI | n/a | Flex28 | n/a | 0.25 | n/a | 4 | n/a | Sudden SNHL | |
| SSD3 | 58 | SSD-CI | CI24RE | n/a | 21 | n/a | 5 | n/a | Sudden SNHL | n/a | |
Equipment and Stimuli
Stimuli were delivered sequentially to each ear using direct stimulation with two bilaterally synchronized L34 research processors (Cochlear Ltd., Sydney, Australia). The L34s were controlled by the Nucleus Implant Communicator (NIC version 2) being run on MATLAB (Mathworks, Natick, MA). Stimuli were 300-ms, 1000-pulses-per-second (pps),1 constant-amplitude pulse trains that were perceived to be at a comfortable loudness. A monopolar grounding configuration (MP1 + 2) was used. The phase duration of the individual biphasic pulses was typically 25 μs per phase and there was an 8-μs interphase gap. Listener BCI7 needed more current to perceive a comfortable loudness and had their phase duration increased to 35 μs in the left ear.
Procedure
Testing occurred in a quiet, dedicated testing room with minimal visual distraction. The testing and procedure generally followed direct stimulation best practices as outlined by Litovsky et al. (2017).
Mapping
Most-comfortable loudness levels were found on the even-numbered electrodes in each ear. Electrodes were loudness balanced within an ear by sequentially presenting pulse trains at five different electrodes. Listeners reported if any electrodes were perceived to be a different loudness and then the experimenter adjusted current levels. Stimuli were repeated until all were perceived as equal loudness. No explicit across-ear loudness balancing was done, but listeners were instructed to use the same point on the subjective loudness scale for the mapping.
Unilateral Place-Pitch Discrimination
To determine how well BI-CI listeners could judge place pitch (i.e., intracochlear electrode location) in one ear, we performed a unilateral control experiment. Four BI-CI listeners started the testing in their right ear and completed all the right-ear trials and conditions, followed by their left ear. The other three BI-CI listeners started the testing in their left ear, followed by their right ear.
A method of constant stimuli was used where at least 20 trials per condition were collected in blocks. Within a block, there were three reference electrodes that were typically electrode 20 (apical), 12 (middle), and 4 (basal). Listeners were typically given a range of comparison electrodes that was within ± 3 electrodes of the reference electrode (e.g., for reference electrode 12, the comparison electrodes were 9, 10, 11, 12, 13, 14, and 15). Table 2 shows a list of the typical electrode comparisons tested. In some cases where the desired electrodes had been deactivated in a listener’s clinical processor settings, nearby alternative electrodes were used instead. In some cases where unilateral place-pitch discrimination was especially poor, electrodes more distant from the reference electrode were also tested.
Table 2.
List of conditions tested for the BI-CI listeners
| Condition | Listening mode | Reference electrode(s) | Presentation | Range | Comparison electrodes | Number of trials | Total trials |
|---|---|---|---|---|---|---|---|
| Right control | Unilateral | R4 | Random | ± 3 electrodes | R1–R7 | 20 | 140 |
| R12 | R9–R15 | 140 | |||||
| R20 | R17–R22 | 120 | |||||
| Left control | Unilateral | L4 | Random | ± 3 electrodes | L1–L7 | 20 | 140 |
| L12 | L9–L15 | 140 | |||||
| L20 | L17–L22 | 120 | |||||
| 1 | Bilateral | R4, R12, R20 | Blocked | Full | L2–L22, even electrodes | 30 | 990 |
| 2 | Bilateral | R4, R12, R20 | Blocked | Basal half | L2–L12, even electrodes | 30 | 540 |
| 3 | Bilateral | R4, R12, R20 | Blocked | Apical half | L12–L22, even electrodes | 30 | 540 |
| 4 | Bilateral | R4, R12, R20 | Random | Full | L2–L22, even electrodes | 30 | 990 |
| 5 | Bilateral | R4, R12, R20 | Random | Basal half | L2–L12, even electrodes | 30 | 540 |
| 6 | Bilateral | R4, R12, R20 | Random | Apical half | L12–L22, even electrodes | 30 | 540 |
Each trial in the experiment consisted of sequential single-electrode stimulation, with 300 ms of stimulation presented to the reference electrode, followed by 300 ms of no stimulation, then 300 ms of stimulation presented to the comparison electrode. Listeners responded by pressing a button to indicate whether the second stimulus had a higher or lower pitch compared to the first. No feedback was given.
Interaural Place-Pitch Discrimination
After the unilateral place-pitch-discrimination experiment, interaural place-pitch discrimination was performed using six conditions that varied the range of comparison electrodes (to measure the magnitude of the range bias) and the randomization of the reference electrodes within a block (to measure the magnitude of the reference bias). Three comparison-electrode ranges were tested. The first range encompassed the full array, including the even-numbered electrodes from the apical end (electrode 22) to the basal end (electrode 2). The second range encompassed the apical half of the array (even-numbered electrodes from 22 to 12). The third range encompassed the basal half of the array (even-numbered electrodes from 12 to 2). Three reference electrodes (apical, middle, and basal, always electrodes 20, 12, and 4, respectively) were tested, with the reference electrode always in the right ear. Listener BCI6 also repeated the experiment with the reference electrode always in the left ear. Testing within a block of trials used either a fixed reference electrode (conditions 1–3, see Table 2) or randomized reference electrodes (conditions 4–6, see Table 2).
As in the unilateral control experiment, each trial consisted of the sequential presentation of 300-ms reference- and comparison-electrode stimuli with an inter-stimulus interval of 300 ms, followed by a button-press response. No feedback was provided. The order of the six conditions [3 ranges (apical half, full range, basal half) × 2 types of reference-electrode blocking (fixed or randomized)] was determined using a Latin square design to minimize order/learning effects.2 Within a condition, the order in which the reference-comparison electrode pairs were presented was randomized, and this randomization was different across listeners and different for the second testing block for each condition (i.e., it was not counterbalanced with the first block). Fifteen trials were collected for each reference-comparison electrode pair within a condition. The listener then moved to the next condition. After completing each condition, the listener was presented the conditions in the reverse order in the Latin square, which was done to also counterbalance any order effects within a listener. The order of reference-comparison electrode pairs within a condition for the second block was randomized independently from the first block. At least 30 trials were collected for each reference-comparison electrode pair within a condition. The full list of conditions is shown in Table 2. The total number of trials was therefore 4940 (unilateral and interaural comparisons), which took at least 12 h to complete for each listener.
Data Analysis
The proportion of perceived higher responses was calculated for each combination of reference and comparison electrodes. For each reference electrode, the psychometric functions (proportion higher perceived place pitch vs comparison electrode) were fit using a maximum likelihood fit with a logistic function [ (Wichmann and Hill 2001). The slope and the “pitch-matched” comparison electrode that elicited 50 % higher responses were extracted from the fitted functions. For analysis purposes, the calculated pitch-match estimates were limited to within the range of comparison electrodes examined for a given condition. Parametric inferential statistics were performed on the pitch matches using analyses of variance (ANOVAs) in SPSS (version 23). Generalized linear mixed-model analysis was performed to examine the relationship between unilateral and interaural pitch-discrimination performance.
Experiment 2: SSD-CI Listeners
Listeners
Three SSD-CI listeners participated in the study. Their hearing histories and demographic information are shown in Table 1 and the audiometric thresholds for the ear contralateral to the CI are provided in Table 3.
Table 3.
Air-conduction hearing thresholds for the three SSD-CI listeners
| Listener | Audiometric threshold (dB HL) | Hearing aid? | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | 3000 | 4000 | 6000 | 8000 Hz | ||
| SSD1 | 15 | 10 | 10 | 10 | 30 | 30 | 20 | 15 | Yes |
| SSD2 | 10 | 15 | 15 | 0 | 10 | 20 | 20 | 25 | No |
| SSD3 | 10 | 10 | 0 | 15 | 15 | 15 | 20 | 20 | No |
One SSD-CI listener used a Cochlear-brand device with 22 intracochlear electrodes and two SSD-CI listeners used Med-El-brand devices with 12 intracochlear electrodes. The Cochlear-brand device was a perimodiolar array with the same intra-electrode spacing as the BI-CI listeners. The Med-El-brand devices were lateral wall arrays that had a larger intra-electrode spacing (about 2.1 mm) than the Cochlear-brand arrays.
Equipment and Stimuli
For the interaural pitch-discrimination measurements, pure tones were presented to both ears. The stimuli were 300 ms in duration with 10-ms raised-cosine ramps. For the CI ear, stimuli were presented to the CI sound processor via the auxiliary input. Single-electrode sound-processor maps were generated to ensure that only one electrode was stimulated at a time. This was done by setting the threshold and most comfortable levels for all electrodes other than the desired reference electrode to zero CUs in the clinical map (Bernstein et al. 2018). Then, the response bandwidth for the desired reference electrode was set to the maximum possible range (e.g., 100–8500 Hz). This resulted in an actual response band of 5049–8010 Hz for the Med-El listeners and a response band with upper and lower cutoff frequencies that varied slightly depending on the test electrode but was always 7193 Hz wide for the Cochlear-brand listener. All additional sound-processing algorithms typically available in the clinical software were disabled. The pure-tone frequency played to the CI sound processor was selected to be the center frequency of the response band for the one active electrode in the single-channel map. The resulting electrical output was examined using a Med-El-brand or Cochlear-brand implant-in-a-box, verifying the expected firing patterns—a constant-amplitude pulse train on the desired electrode.
For the acoustic ear, stimuli were delivered via circumaural headphones (HD280 Pro; Sennheiser, Hannover, Germany). Seventeen different pure-tone frequencies were tested in the experiment, ranging from 263 to 11,114 Hz, in steps equivalent to a 1.5-mm distance along the basilar membrane as defined by Greenwood (1990).
Procedure
Testing occurred in a double-walled sound-attenuating booth (Industrial Acoustics Inc., Bronx, NY, or Eckel Industries, Cambridge, MA). For each listener, three or four single-electrode maps were generated and loaded into a research sound processor. Before the pitch-discrimination experiment began, loudness-balanced stimulus levels were set for each combination of three or four single-electrode maps and 17 acoustic comparison frequencies. For each single-electrode map, the volume control on the sound processor was set to its maximum level. The stimulus in the CI ear was adjusted to a comfortable level by controlling the level of the acoustic stimulus that was delivered to the sound processor. Then the stimuli in the acoustic ear were loudness balanced to the stimulus in the CI ear at each frequency. For each acoustic frequency, the experimenter repeated the sequential presentation of the stimuli to the two ears, adjusting the level of the tone in the acoustic ear upward or downward in steps of 1, 2, or 3 dB on each presentation until the listener reported that the stimuli were matched in loudness. This process was then repeated for each of the 17 acoustic comparison frequencies.
No unilateral control measurements were performed for the SSD-CI listeners because the acoustic ear (i.e., the ear for which the frequency varied from trial to trial for comparison with a fixed reference electrode) was assumed to yield no peripheral limitation to the ordering of pitches from low to high. The testing procedure was similar to the interaural place-pitch comparisons for the BI-CI listeners with the exception that only the non-randomized reference-electrode (i.e., blocked) conditions were tested. Each block consisted of a fixed reference electrode in the CI ear and one of three possible frequency ranges in the acoustic ear. The full range included 17 frequencies (263–11,114 Hz), the apical half included nine frequencies equal to or lower than 2000 Hz, and the basal half included the nine frequencies equal to or greater than 2000 Hz. Thus, the 2000-Hz tone was included in all three testing ranges, similar to how electrode 12 was included in all three testing ranges for the BI-CI listeners. Each listener completed 20 or 30 trials for each combination of reference electrode, testing range, and comparison-tone frequency. The trials were divided into two blocks for each condition, with the order of presentation randomized to minimize possible learning and order effects. Each block consisted of half of the total number of trials (i.e., 10 or 15 trials) for each of the comparison-tone frequencies for a given reference electrode and testing range. One block was completed for each combination of reference electrode and testing range before the same combinations were presented a second time, but with the test blocks presented in reversed order. The full list of conditions tested is shown in Table 4.
Table 4.
List of conditions tested for the SSD-CI listeners
| Listener | Listening mode | Reference electrodes | Presentation | Range | Comparison frequencies (Hz) | Number of trials |
|---|---|---|---|---|---|---|
| SSD1 | Bilateral | R10, R8, R6, R2 | Blocked | Full | 263–11,114 | 20 |
| Blocked | Basal half | 2000–11,114 | 20 | |||
| Blocked | Apical half | 263–2000 | 30 | |||
| SSD2 | Bilateral | R11, R6, R2 | Blocked | Full | 263–11,114 | 20 |
| Blocked | Basal half | 2000–11,114 | 30 | |||
| Blocked | Apical half | 263–2000 | 30 | |||
| SSD3 | Bilateral | L8, L12, L15 | Blocked | Full | 263–11,114 | 30 |
| Blocked | Basal half | 2000–11,114 | 30 | |||
| Blocked | Apical half | 263–2000 | 30 |
As for the BI-CI listeners, single trials consisted of stimuli presented sequentially to the two ears. A 300-ms reference pure tone was presented to CI sound processor, followed by a 300-ms of inter-stimulus silence, then a 300-ms comparison-tone stimulus presented over headphones to the acoustic ear. Listeners responded by indicating if the second stimulus had a higher or lower pitch compared to the first. No feedback was given.
Data Analysis
As for the BI-CI listeners, the proportion of perceived higher responses was calculated for each combination of reference electrode, comparison frequency, and testing range. Psychometric functions were fit to the data, and both the slope and the matched comparison electrode that elicited 50 % higher responses (i.e., the pitch match) were extracted from the fitted functions. Because of the limited sample size, inferential statistics were not performed for the SSD-CI listeners.
RESULTS AND DISCUSSION
Experiment 1: BI-CI Listeners
Unilateral Place-Pitch Discrimination
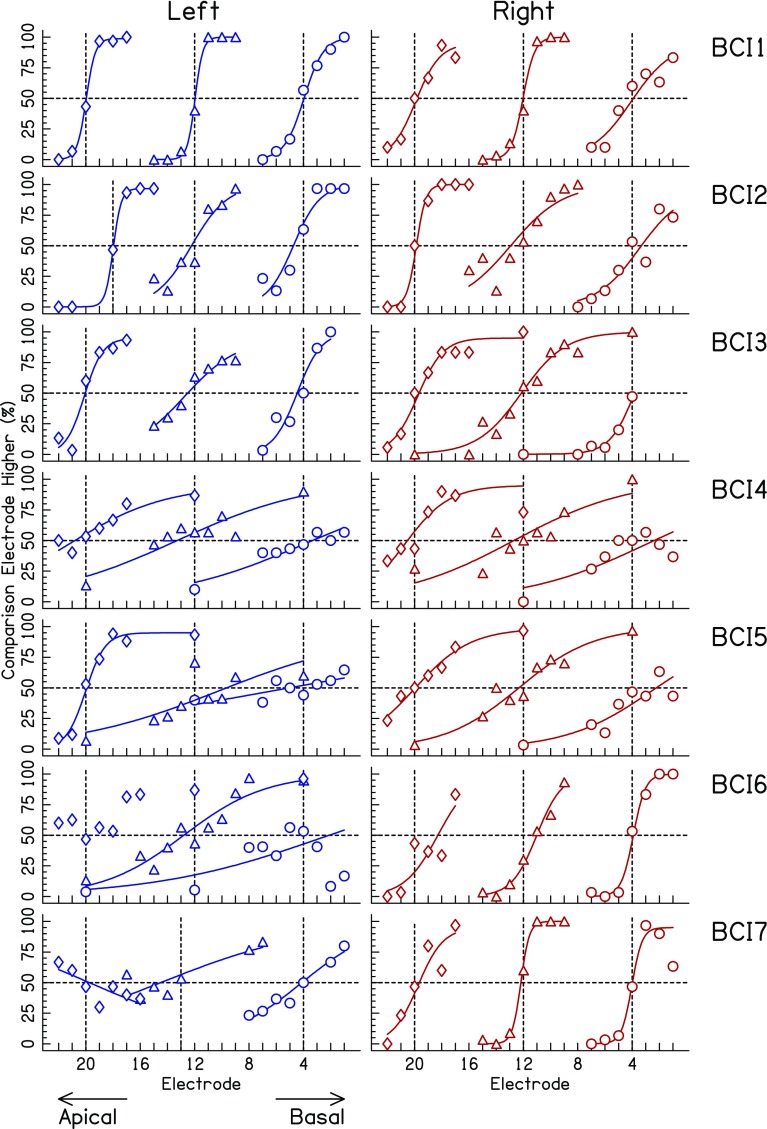
Figure 1 shows the raw unilateral electrode- or place-pitch-discrimination results and fitted psychometric functions (curves) for each ear and reference electrode. The most important feature of these data is that they indicate that the BI-CI listeners could distinguish lower from higher place pitch, and therefore they should be able to apply this perceptual concept to an interaural comparison of place pitch. Most listeners could reliably discriminate reference electrodes unilaterally in either ear, as indicated by psychometric functions with steep slopes and 50 % crossover points (i.e., pitch matches) that generally fell within ± 1 electrode of the reference electrodes. The root-mean-square (RMS) deviation between reference electrodes and pitch matches was 0.84 electrodes. There was only one instance where a sigmoidal curve could not be fit to the data (BCI6 in the left ear at electrode 20).
Fig. 1.
Psychophysical response functions for the unilateral place-pitch discrimination task for the left ear (left column) and right ear (right column) for the BI-CI listeners. Vertical dashed lines indicate the reference electrodes. Reference electrodes 4, 12, and 20 were used for all listeners except electrodes for BCI2 (left ear E20) and BCI7 (left ear E12) were not available and therefore replaced with nearby electrodes. The minimal set of comparison electrodes are reported in Table 2; some listeners were tested on extra comparison electrodes, particularly in cases of shallow psychometric functions. Fits to the data are shown by the solid curves. The fitting procedure did not converge for BCI6, reference electrode E20 in the left ear
The data were sorted into better and poorer ear based on the average slope of the fitted unilateral pitch-discrimination functions (Fig. 1) across the three reference-electrode conditions in each ear. For the one case that did not produce a fit, the slope was set to a value of zero. Listeners BCI1 and BCI2 had the best overall unilateral pitch-discrimination ability as measured by the average slope, and both listeners were relatively better in the left ear. Listeners BCI3, BCI4, and BCI5 had relatively shallower slopes, and both ears had about the same slope. Listeners BCI6 and BCI7 had relatively steep average slopes in the right ear, but poor pitch discrimination with the left ear and much shallower average slopes. The slope for BCI7 on electrode 20 in the left ear had the only negative slope from the curve fits; however, performance was so poor that this appears to be simply an electrode with no place-pitch perception.
A repeated-measures ANOVA with factors ear (two levels: better or poorer) and reference electrode (three levels: apical, middle, and basal) was performed on the slopes. There was no effect of reference electrode on the slope [F(2,12) = 0.49; p = 0.63; =0.08] and the ear × reference interaction was not significant [F(2,12) = 0.039; p = 0.96; =0.01]. There was a main effect of ear, whereby the better ear had steeper slopes (34.8 ± 21.0 %/electrode) than the poorer ear (16.3 ± 13.1 %/electrode) [F(1,6) = 6.94; p = 0.039; =0.54], but this is not a meaningful result given that the better and poorer ears were assigned based on the average slope.
Interaural Place-Pitch Discrimination
The interaural pitch-discrimination data were examined in three ways. First, the raw data were plotted to examine the effect of reference electrode and testing range on the psychometric functions (Fig. 2). Second, interaural pitch-match estimates were derived from the psychometric functions by determining the comparison electrode number equally likely to be perceived as higher or lower in pitch than the reference electrode (Fig. 3). Third, the slopes of the interaural pitch-discrimination functions (Fig. 2) were compared to the slopes of the unilateral pitch-discrimination functions (Fig. 1) to investigate whether inter-subject variability in pitch-discrimination performance could be explained by unilateral performance.
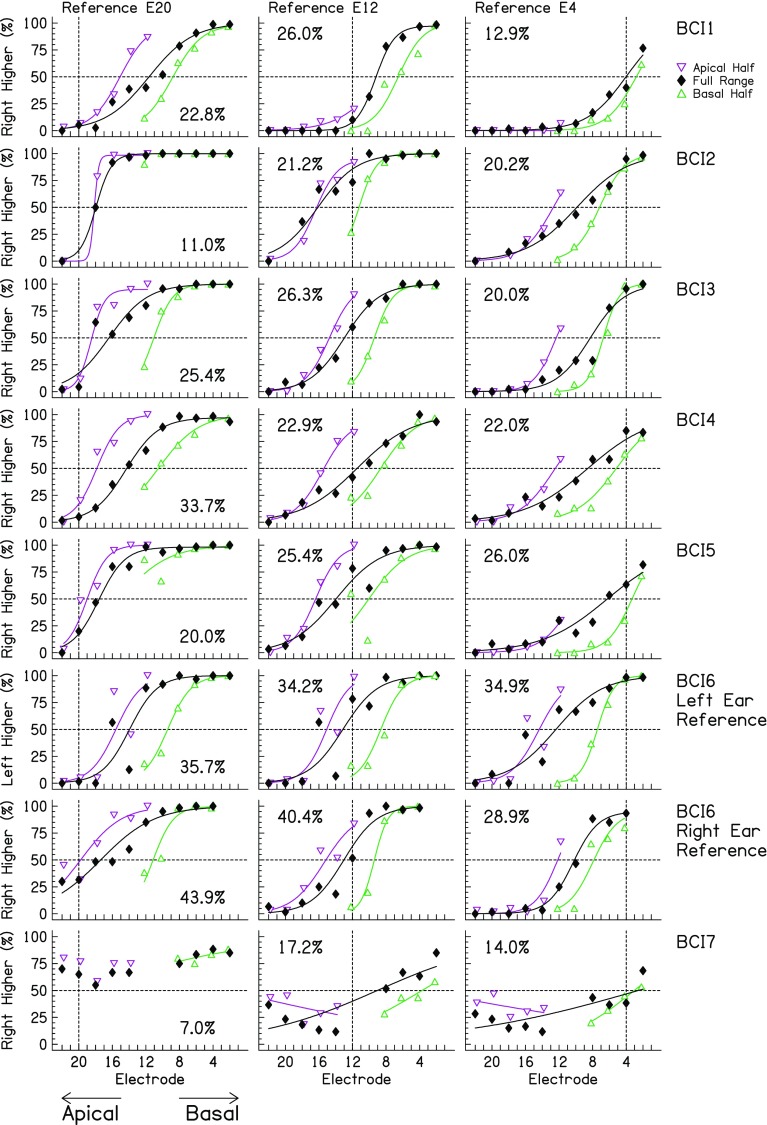
Fig. 2.
Psychophysical response functions for the interaural place-pitch discrimination task for the BI-CI listeners. Data are averaged over the blocked and randomized reference-electrode conditions. Each row has three panels: conditions where the apical (E20, left column), middle (E12, middle column), and basal (E4, right column) reference electrode was used. Vertical dashed lines indicate the reference electrodes. Each panel shows data for the three testing ranges (downward-pointing open purple triangles = apical half, closed black diamonds = full range, upward-pointing open green triangles = basal half). Fits to the data are shown by the solid curves; groups of data without fits occurred when the fitting procedure did not converge. The RMS difference in RAU-transformed data for common comparison electrodes tested in the various range conditions is reported in each panel (see text)
Fig. 3.
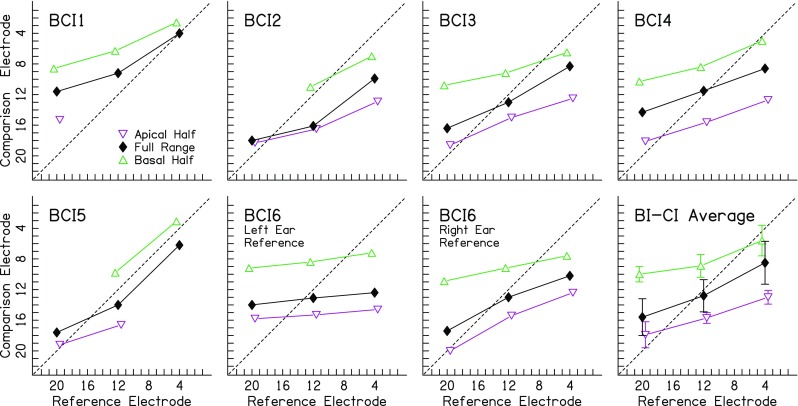
Summary of interaural place-pitch matches (i.e., the 50 % crossover points in Fig. 2) for the BI-CI listeners. Diagonal lines represent the unitary slope that would be expected if the pitch matches followed the change in reference-electrode position
Pitch-Discrimination Psychometric Functions
The raw interaural pitch-discrimination data are plotted in Fig. 2. Different listeners (and testing ears for listener BCI6) are shown in each row, different reference electrodes are shown in each column (left column = reference electrode 20, middle column = reference electrode 12, right column = reference electrode 4), and different testing ranges are indicated by different symbols in each panel (downward-pointing open purple triangles = apical half, closed black diamonds = full range, upward-pointing open green triangles = basal half). The data from the fixed and randomized reference conditions were combined because there was no significant difference between the conditions (see statistical analysis below).
Three important trends are evident in these data. First, for most of the listeners and conditions, the psychometric functions were “well-behaved,” with monotonically increasing pitch-discrimination performance as the comparison-electrode number decreased (recall that the most apical, lowest-pitched electrode is 22). One exception was listener BCI7, who did not produce reliable monotonic pitch-discrimination functions. Even though interaural pitch-discrimination performance was generally monotonic, as expected, closer examination in some of the panels showed that pitch reversals sometimes occurred. For example, BCI5 consistently reported electrode 12 to be higher in pitch than electrode 10, and BCI6 (left-ear reference) consistently reported electrode 16 to be higher than electrode 14. These pitch reversals, however, were infrequent. Second, the interaural pitch-match estimates (i.e., the 50 % correct point on the psychometric function) increased as place of stimulation moved from more apical to basal (i.e., decreasing electrode number), as expected. Third, and most importantly for this study, the data clearly show that the comparison-stimulus range had a substantial effect on the psychometric function. If the pitch-match estimates were completely determined by the perception of place pitch and were unaffected by context biases like testing range, then the individual points for each electrode condition in each panel should produce the same percentage of higher responses. In contrast, as the testing range shifted basally (i.e., apical half to full range to basal half), pitch matches also shifted basally.
To quantify the inconsistency in pitch perception across testing ranges, the percentage values were transformed to rationalized arcsine units (RAU; Studebaker 1985), and the RMS difference between comparison electrodes common to each testing range were calculated and reported in each panel. (The RMS differences in Figs. 2 and 4 are described as percentage points instead of RAU, even though this is technically incorrect.) The overall RMS difference was 26.3 % with a range of 7.0–43.9 %. The smallest effects of comparison range were observed for BCI1 for reference electrode 4 (RMS difference = 12.9 %), BCI2 for reference electrode 20 (RMS difference = 11.0 %), and for BCI7 for all three reference electrodes. All other reference electrodes had RMS differences ≥ 20 %. This means that for the vast majority of cases, shifting the testing range changed the likelihood that a listener would report a given comparison electrode as higher in pitch than a given reference electrode by 20 % or more.
Fig. 4.
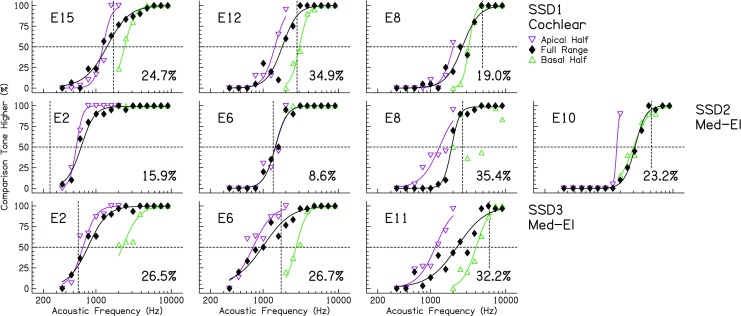
Psychophysical response functions for the interaural place-pitch discrimination task for the SSD-CI listeners. Conventions are the same as in Fig. 2, except that the horizontal axis represents the comparison-tone frequency in the acoustic ear, and the vertical dashed lines indicate the center frequency allocated to the reference electrode in a listener’s everyday sound processor map
Pitch-Match Estimates
Figure 3 plots the interaural place-pitch matches (i.e., the 50 % point of the psychometric functions in Fig. 2) as a function of reference electrode and testing range for each BI-CI listener (except BCI7 for whom pitch matches could not be derived). These data can be examined with respect to two hypotheses. First, if the data were unaffected by the testing range, then all three curves should produce the same interaural place-pitch match. Second, if the pitch matches reflected the relative cochlear places of stimulation in the two ears, the place-pitch matches should produce a line that is roughly parallel to the one-to-one diagonal (dashed lines). As a first-order approximation assuming the same electrode array types and uniform neural survival, equal insertion depth in the two ears should yield a pitch match at the same electrode number in the two ears, while differences in insertion depth should produce an approximately linear shift away from the dashed diagonal line in each panel, with no change in slope. The data in Fig. 3 show that neither hypothesis was supported. The interaural place-pitch matches were rarely consistent across testing ranges, and the slopes of the pitch-match functions were consistently less than one, meaning that changing the reference electrode by a certain distance along the array yielded a much smaller change in the pitch-matched electrode in the other ear.
The testing-range effect in Fig. 3 was quantified in terms of the difference between the pitch matches for any two testing ranges, divided by the difference between the centers of the testing ranges. The idea was to normalize the range effect by the maximum effect that would be expected if listeners were completely ignoring the pitch of the reference electrode and instead making unilateral judgements of the relative pitch of a given comparison electrode relative to the center of the testing range in a given experimental block (Carlyon et al. 2010). The mean normalized range effect across listeners was 63 %, meaning that changing the testing range moved the pitch-match estimate by about two thirds of the distance between the centers of any two testing ranges. Carlyon et al. (2010) suggested that pitch matches that change by less than 50 % of the change between the centers of two testing ranges can be considered reliable. Using this criterion, 75 % of our pitch matches would be considered unreliable (not shown).
The slopes of the pitch-match functions in Fig. 3 were clearly shallower than unity (diagonal dashed line); on average, the slope was 0.34 electrodes/electrode. This means that changing the reference electrode yielded a much smaller change in pitch match than would be expected if the pitch match correctly reflected relative cochlear place of stimulation in the two ears. For example, changing from reference electrode 20 to 12 would be expected to change the corresponding pitch match by 8 electrodes, but the pitch match changed on average by only 0.34 × 8 = 2.7 electrodes. Changing the reference electrode did play some role in the place-pitch estimate because the slopes were greater than zero. This means that listeners were not completely ignoring the place pitch associated with the reference ear.
To confirm the observations in Figs. 2 and 3, inferential statistics were performed on the pitch matches excluding listener BCI7. A three-way ANOVA was performed on the pitch matches with fixed factors reference electrode (three levels: electrode 20, 12, and 4), testing range (three levels: apical half, full range, and basal half), and blocking type (two levels: blocked or random). Conditions that did not have a pitch match were omitted from the analysis yielding a non-full rank set of test conditions. Pitch matches increased with increasing reference electrode [F(2,93) = 56.0; p < 0.0001; =0.55]; Tukey post-hoc tests showed significant differences across all three reference electrodes (p < 0.0001 for all six comparisons). Pitch matches changed significantly with testing range [F(2,93) = 109.5; p < 0.0001; =0.70]; Tukey post hoc tests showed significant differences across all three testing ranges (p < 0.0001 for all six comparisons). The reference electrode × testing range interaction was significant [F(4,93) = 2.75; p = 0.033; =0.11]. Post hoc unpaired t tests assuming equal variances that were Bonferroni corrected for multiple (nine) comparisons revealed that this interaction occurred because the apical- and full-range matches for reference electrode 20 were not significantly different (p = 0.37) and the basal- and full-range matches for reference electrode 4 were not significantly different (p = 0.18); the other seven comparisons were significantly different (p < 0.05 for all). In other words, the pitch matches for the full testing range had a steeper slope than the other two testing ranges (Fig. 3). Neither the main effect of blocking type [F(1,93) = 1.19; p = 0.28; =0.01] nor any of the interactions involving blocking type were found to be significant (p > 0.05 for all). For this reason, the data plotted in Figs. 2 and 3 were collapsed across blocking types.
Relationship Between Bilateral and Unilateral Pitch-Discrimination Performance
Sensitivity to changes in electrode position can be characterized in terms of the slopes of the pitch-discrimination functions in Figs. 1 and 2. A steep slope means that a listener perceived the pitch associated with adjacent electrodes as substantially different. It was hypothesized that cases of poor interaural pitch-discrimination performance (e.g., listener BCI7, Fig. 2) might be explained by poor unilateral pitch-discrimination performance (Fig. 1). A generalized linear mixed-model analysis examined the relationship between the slopes for the unilateral and bilateral pitch-discrimination functions. Only the bilateral slopes for the full comparison range condition were included in the analysis. The bilateral pitch-discrimination slope was considered to be the dependent variable with unilateral slope entered into the model as a covariate, subject entered into the model as a random effect, and each test electrode treated as a repeated measure. Electrode 20 was excluded from the analysis for listeners BCI6 and BCI7 because of poorly behaved pitch-discrimination functions that could not be fit with a psychometric curve. Two analyses were conducted. The first analysis considered the unilateral slopes estimated for the poorer ear (i.e., the ear with the shallowest slope), while the second considered the average unilateral slopes across the two ears (Fig. 1). Unilateral slope was found to be a significant predictor of bilateral slope in both analyses [poorer ear slope: χ2(1) = 142, p < 0.0001; average slope across ears: χ2(1) = 13.5, p = 0.0002]. Further inspection of the data revealed that the significant relationship between unilateral and bilateral pitch-discrimination slopes was completely driven by two data points: electrode 12 for listener BCI1 and electrode 20 for listener BCI2. These two cases had very sharp pitch-discrimination functions for both the unilateral and interaural pitch tasks.
Experiment 2: SSD-CI Listeners
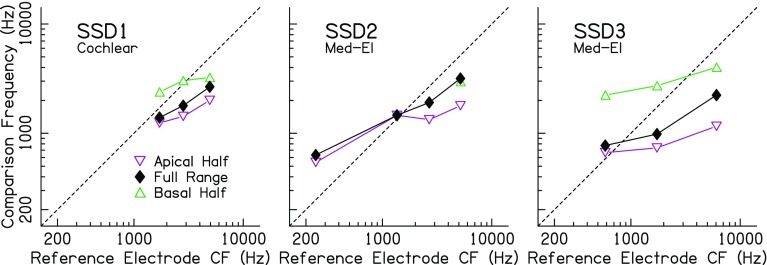
The raw pitch-discrimination results for the three SSD-CI listeners are plotted in Fig. 4, and the interaural pitch matches based on the fitted psychometric functions are plotted in Fig. 5. The horizontal axis in Fig. 4 represents the frequency of the acoustic comparison tone, and the vertical dashed lines represent the reference electrode center frequency (CF) associated with the listener’s clinical sound processor frequency allocation. In Fig. 5, the vertical axis represents the frequency of the pitch-matched comparison tone while the horizontal axis represents the reference electrode sound processor CF. Examining the data in this way allows for an assessment of the extent to which a given pitch match corresponds to the electrode normally assigned to that frequency. Because of the small number of subjects, inferential statistics were not performed for the SSD-CI listeners.
Fig. 5.
Summary of interaural place-pitch matches for the SSD-CI listeners from Fig. 4. Conventions are the same as in Fig. 3, except that the vertical axis represents the pitch-matched comparison-tone frequency in the acoustic ear, and the horizontal axis represents the center frequency allocated to the reference electrode in a listener’s everyday sound processor map
The data in Figs. 4 and 5 show very similar trends to the BI-CI listeners. There was a clear effect of testing range for most of the subjects and reference electrodes. The range effect was demonstrated by the horizontal shift between psychometric functions in Fig. 4. As in Fig. 2, the percentages reported in each panel reflect the RMS differences (in RAU) between common reference acoustic frequencies in each testing-range condition. These RMS differences were generally in the same range as for the BI-CI listeners (overall RMS = 26.0 %; range = 8.6–35.4 %). There was also a clear vertical shift in pitch-match estimates (Fig. 5) for the different testing ranges for listeners SSD1 and SSD3. Listener SSD2 was an exception, with almost no range effect for the two most-apical electrodes tested (electrodes 2 and 6). Finally, the slopes of the pitch-match results (Fig. 5) were consistently less than unity (mean = 0.40, range = 0.24–0.61 octaves/octave). This indicates that the difference in the perceived pitch match between reference electrodes was on average less than half than would be expected based on the clinical sound-processor frequency allocation. At the same time, the slopes were greater than zero, indicating that changing the reference did have some impact on the perceived pitch match.
As was done for the BI-CI listeners, the magnitude of the range effect was computed (in millimeters based on Greenwood 1990) for each reference electrode tested relative to the shift that would be expected if listeners were basing their responses on the center of the comparison range. On average across the three SSD-CI listeners, the mean normalized range effect was 43 %, somewhat smaller than the mean range effect of 63 % that was observed for the BI-CI listeners. SSD1 (35 %) and SSD2 (24 %) had a relatively small normalized range effect, whereas the range effect for SSD3 (69 %) was of similar magnitude to the average BI-CI listener. According to Carlyon’s (2010) reliability criterion (i.e., the pitch match should change no more than 50 % of the change in the midpoint of the comparison range), 42 % of the pitch matches for SSD-CI listeners were unreliable. Overall, these data suggest that SSD-CI listeners are susceptible to range biases like the BI-CI listeners and replicate findings from previous studies.
GENERAL DISCUSSION
To achieve optimal binaural hearing with CIs, one should match interaural place of stimulation on individual electrodes (Kan et al. 2013; Hu and Dietz 2015; Kan et al. 2015; Williges et al. 2018). Current clinical practice does not presently employ such a strategy. One method used commonly in research to address potential interaural mismatch is to perform pitch comparisons. The primary finding of this study is that for most of the seven BI-CI and three SSD-CI listeners presented with single-electrode pulse trains or tones, interaural place-of-stimulation comparisons were much more strongly affected by the comparison place-pitch range than by the reference-electrode place pitch (Figs. 2, 3, 4, and 5). In other words, the measured interaural pitch matches provided little information about the perceived place pitch of the reference electrode. Instead, the results suggest that listeners were mostly attending to the comparison ear and were unilaterally making judgements about the pitch relative to the overall range for a given condition (Carlyon et al. 2010). As a result, the observed pitch-match estimates gravitated towards the middle of the testing range (Poulton 1979). For an ideal pitch-matching metric, changing the reference electrode should shift the pitch match by the same amount (i.e., 100 % of the change in the reference electrode), while changing the comparison electrode testing range should have no effect (i.e., 0 % of the change in the comparison-range midpoint). In contrast, changing the reference electrode only shifted the pitch match by 34 % of the change for BI-CI listeners (Fig. 3) and 40 % of the change for SSD-CI listeners (Fig. 5), while changing the testing range shifted the pitch match by 63 % of the change in range midpoint for BI-CI listeners and by 43 % for SSD-CI listeners. The small contribution of place of stimulation of the reference electrode in both populations is striking. We hypothesized that the range effect previously observed in SSD-CI listeners was caused primarily by the dissimilarity of interaural inputs. In contrast to this hypothesis, the range effect was large for both BI-CI and SSD-CI listeners. Thus, we conclude that there exists a broader and more general issue with performing interaural place-pitch comparisons with electrical stimulation that cannot simply be ascribed to asymmetric hearing.
Unilateral control data collected for the BI-CI listeners (Fig. 1) verified that they were able to discriminate higher and lower place pitch (i.e., they understood the concept of place pitch and could perceive it) when presented with pairs of electrodes in the same ear. The rationale was that if the BI-CI listeners could perform the unilateral place-pitch task, they should be able to perform the interaural place-pitch task. Listeners BCI1 and BCI2 had a relatively good ability to evaluate unilateral place pitch, where in most cases they responded nearly perfectly and demonstrated relatively steep slopes when the data were fit. In addition, the fits to the data show the pitch matches were within one electrode of the reference for these listeners. Listeners BCI3, BCI4, and BCI5 showed relatively shallower pitch-discrimination slopes. Listeners BCI1-BCI5 had psychometric function slopes that were relatively similar across the ears. Listeners BCI6 and BCI7 showed relatively good discrimination and steep slopes in the right ear and relatively poorer discrimination and shallow slopes in the left ear, which seems consistent with their hearing histories (see Table 1). This large range of unilateral pitch-discrimination abilities is consistent with the literature (e.g., McKay et al. 1999; Chatterjee and Yu 2010).
Figures 2 and 3 show the interaural place-pitch matches for BI-CI listeners. For each reference electrode, the testing range greatly affected the pitch match. Listener BCI4, for example, demonstrated some of the largest range effects in the study. We also varied whether a blocked or randomized test design would affect responses. We hypothesized that a randomized design would produce much shallower slopes because the reference electrode changed from trial to trial. In fact, we found this manipulation to leave the pattern of data relatively unaltered. Together, we interpret these two findings as evidence that the reference ear was mostly ignored, where listeners instead learned the range of electrodes in the comparison ear and responded to each comparison stimulus relative to that range. Listener BCI7 was also a noteworthy example. This listener had excellent place-pitch discrimination abilities in the right ear (Fig. 1). However, many electrodes were deactivated in their left ear (they had undergone an ex-plantation and re-implantation) and their left-ear unilateral place-pitch-discrimination abilities were the poorest in the study. This listener also had poorly behaved interaural pitch-discrimination functions, suggesting that their interaural pitch comparisons were adversely affected by their inability to discriminate pitch in their left ear. A mixed-model regression analysis of the data confirmed the association between unilateral and bilateral pitch-discrimination ability: those subjects and electrodes with the steepest unilateral pitch-discrimination slopes (BCI1, electrode 12 and BCI2, electrode 20) also had the steepest bilateral pitch-discrimination slopes.
Another possible interpretation of the observed range effects is that listeners might have been inclined to use both higher and lower response buttons fairly equally. In extreme cases where the pitch of the reference stimulus fell outside of the comparison range, the listener would have had to respond using only one button for the entire block. If they were biased against doing so, this would have resulted in what appeared to be a range effect. This explanation, however, is unlikely to explain the lack of significant differences in responses between the blocked and randomized conditions for the BI-CI listeners. For a given comparison range, the overall proportion of higher responses would have been substantially different in the random condition, where the reference electrode changed from trial to trial, than in the blocked condition where it was held fixed. Yet the estimated pitch matches were the same for these two conditions. Therefore, the observed range effects are more consistent with the idea that listeners were considering the context of the available comparison range (Carlyon et al. 2010), rather than simply performing interaural pitch judgments. We cannot, however, dismiss a possible bias toward an equal proportion of higher and lower responses for the SSD-CI listeners who were only tested in the blocked condition.
Substantial range effects were also observed for the SSD-CI listeners in Figs. 4 and 5 confirming the results of previous studies (Carlyon et al. 2010; Schatzer et al. 2014) and suggesting that this problem is not limited to BI-CI listeners. A noteworthy exception was listener SSD2, who demonstrated some of the smallest range effects in the entire study. The fact that range effects were observed for both BI-CI and SSD-CI listeners suggests that these effects cannot be attributed solely to the different sound percepts associated with asymmetric hearing. In fact, Carlyon et al. (2010) also found range effects in normal-hearing listeners when tones were presented in one ear and narrowband noises were presented in the other. This suggests that range effects are a pervasive problem and it may be that these effects would extend to all populations, including bimodal CI users (CI in one ear and low-frequency aided acoustic hearing in the other ear).
Subjective attributes of sounds like pitch or loudness are susceptible to such contextual biases (e.g., Poulton 1979), particularly when sounds differ in multiple dimensions (Carlyon et al. 2010). This is a worrying problem for the field of CIs because pitch comparisons are a standard procedure for interaural place-of-stimulation alignment (e.g., van Hoesel et al. 2009; Litovsky et al. 2010; Lu et al. 2011; Goupell and Litovsky 2015; Kan et al. 2015). The range-effect bias is insidious. An experimenter with a preconceived notion of where the pitch match should be may choose a testing range with the goal of placing a pitch match near the center of the testing range. In doing so, they would likely achieve the desired result because of the tendency for listeners to provide pitch matches in the center of the testing range. In other words, the combination of the experimenter having an assumption of where a pitch match should be combined with the range bias produces a self-fulfilling prophecy. In fact, many of the purportedly pitch-matched electrode pairs examined in BI-CI single-electrode direct-stimulation binaural-processing experiments may have been biased by preconceived notions on what the match should be. On the other hand, for monopolar stimulation using single electrodes, there appears to be a large tolerance to interaural mismatch for binaural discrimination, lateralization, and fusion—approximately ± 3 mm (Kan et al. 2013; Goupell 2015; Kan et al. 2015)—suggesting that even if the pitch-match estimates used to choose electrode were biased by range effects, these binaural measurements were likely still valid. Note, however, that tolerance to interaural mismatch may be limited to single-electrode stimulation. Multi-electrode stimulation (particularly when attempting to transmit low-rate ITD information) may be less tolerant to interaural mismatch (Williges et al. 2018). Overall, the current results strongly support at least following the recommendations of Carlyon et al. (2010), who advised that an experimenter should test multiple ranges to determine the veracity of the pitch match. At the same time, these results suggest that at least for the pitch-discrimination task employed here, the pitch match is unreliable in most cases. Alternatively, an experimenter could employ a different pitch-matching method that is more resistant to such biases—if it can systematically be demonstrated that such a method exists—or use another electrode-pairing measure such as ITD discrimination (Francart et al. 2008; Hu and Dietz 2015; Kan et al. 2015; Bernstein et al. 2018).
The current results can be compared with two previous studies that examined range effects for SSD-CI listeners (Carlyon et al. 2010; Schatzer et al. 2014). Carlyon et al. (2010) tested four SSD-CI listeners using three different interaural place-pitch comparison tasks. They also showed range effects in their results, consistent with the results of the current study. For their two-interval interaural pitch-discrimination task, they tested 30 trials/condition, the electrode in the CI ear was the reference, and the tone or pulse train in the acoustic ear was varied in frequency, similar to our study. Because of the way the testing was conducted, there were limited cases that can be directly compared to our study. For example, one SSD-CI listener demonstrated strong range effects (see Fig. 1a in Carlyon et al. 2010) and one listener demonstrated consistent matches for two ranges and one reference electrode (see Fig. 1b in Carlyon et al. 2010). The latter case with consistent matches and minimal comparison-range bias would be most comparable to our listener SSD2 for reference electrodes 2, 6, and perhaps 10. It is important, however, to point out that this consistency in pitch matches was the exception in the current study, where large range biases were readily observed for most BI-CI and SSD-CI listeners. Another difference between the two studies is that the current study had a large range of conditions, including relatively large differences ranges of comparison electrodes or frequencies. Carlyon et al. (2010) also suggested an arbitrary rule that pitch matches that change by less than 50 % of the change in the center of the testing ranges could be considered as valid. By such a metric, we estimated that 75 % of the BI-CI and 42 % of the SSD-CI pitch matches were invalid. It is important to note, however, that the rule suggested by Carlyon et al. has not been formally evaluated, meaning that it is unclear if the rule is strict enough. For example, a criterion stricter than the proposed 50 % may be more appropriate for interaural place-of-stimulation matching, where precise interaural matching is critical for maximum binaural functioning.
Carlyon et al. (2010) also pointed out that inconsistent pitch matches occurred across three different types of interaural place-pitch tasks. That study, however, did not systematically compare pitch-matching methods. The current results clearly demonstrate that a pitch-discrimination task employing a method of constant stimuli generates results that are unacceptably biased to be useful as a method of evaluating the relative place of stimulation in two ears for CI listeners. It remains unknown whether other methods of pitch matching would yield less biased results. For example, another common pitch-matching task involves the adjustment of the electrode in one ear to match the place pitch of the other ear (e.g., Aronoff et al. 2016). Other less common methods, such the midpoint comparison (Long et al. 2005; Carlyon et al. 2010) may also be relatively more resistant to range effects. It will be necessary to undertake a comprehensive study to directly compare pitch-matching methods if such methods are used in the future to measure interaural place-of-stimulation mismatch with CI listeners.
Schatzer et al. (2014) also examined range effects in a study that measured pitch matches associated with all 12 electrodes for each of eight SSD-CI listeners. They were unable to measure reliable pitch matches for two of the listeners, while 22 % of the electrodes for the remaining six listeners showed range effects according to the definition of Carlyon et al. (2010). Thus, across the eight SSD-CI listeners, reliable range-independent pitch matches were obtained for about 59 % of the electrodes tested, comparable to the 58 % of reliable matches observed in our smaller sample of SSD-CI listeners. Interestingly, Schatzer et al. (2014) found that when the unreliable pitch matches were discarded from the analysis, the correspondence between pitch match and electrode location (obtained from x-ray images) improved. While this finding suggests that erroneous results may be avoided by discarding unreliable pitch matches, it is important to note that 75 % of the pitch-match estimates were deemed unreliable for the BI-CI listeners in our study. Discarding these estimates would leave very few reliable data points to work with.
The current results could have implications for the interpretation of previous studies suggesting that the pitch percept associated with the CI place of stimulation is relatively plastic. For example, Reiss et al. (2007) tested 18 short-array (hybrid or electro-acoustic hearing with acoustic and electric stimulation in the same ear) CI users longitudinally over 5 years. They found that the place pitch of an individual electrode would often adapt to better match the CF of the frequency allocation of that electrode over the course of the first few years after activation. Three methods were employed: a manual method and two computerized methods (adaptive and method of constant stimuli) that involved a forced-choice discrimination of higher and lower pitch perception. The computerized constant-stimuli method is most similar to that employed in the current study. On one hand, because they reported consistent pitch matches using both manual and adaptive methods, pitch plasticity seems plausible and has been reproduced in other studies (e.g., Reiss et al. 2014; Reiss et al. 2015; Tan et al. 2017). Furthermore, the longitudinal design may be more robust to range biases as long as the range is fixed across testing sessions. On the other hand, consistent pitch matches might also be expected if all three methods suffered from the same bias. We would like to note that the listeners that could not reliably and consistently perform the interaural pitch task in Reiss et al. (2007; see Fig. 4) may have been particularly affected by range biases (a similar idea was suggested by Schatzer et al. 2014), which would be consistent with the data from the current study broadly showing that interaural pitch comparisons are very difficult for CI users. Carlyon et al. (2010) explicitly controlled for range-bias effects and found little evidence for pitch plasticity; pitch matches were generally within 0.5 octaves of the CT scan estimates for three SSD-CI listeners with reliable pitch matches. This result is consistent with other longitudinal studies that did not find evidence for pitch plasticity in bimodal CI users (Vermeire et al. 2008; Reiss et al. 2015). To date, the only pitch plasticity that has been observed using acoustic and electric stimulation has been found in hybrid (electro-acoustic-stimulation) CI users (Reiss et al. 2007), which suggests a different outcome depending on the type of stimulation and amount of residual acoustic hearing.
The possible plasticity of the pitch percept (Reiss et al. 2007) raises another important question with respect to the utility of pitch-matching methods. Even if an experiment could reliably measure an interaural pitch match, would the pitch percept align with the best binaural functioning? Binaural computations first occur in the brainstem; the effects of interaural mismatch therefore occur at such a low level that they should be relatively more resistant to adaptation than the assumed more central computation of pitch. Pitch comparisons are performed with sequential comparisons of stimuli in each ear, whereas ITD analysis involves the comparison of simultaneous stimuli. Even if pitch extraction took place at the level of the brainstem, the interaural judgment nevertheless requires a comparison across time, thereby relying on a more central comparison of the stimuli that could be subject to plasticity. Differential plasticity across the pitch and binaural mechanisms could be a worst-case scenario, whereby interaural pitch matches would change over time much more than binaural matches, thus producing a situation where pitch provides relatively little guidance about optimizing binaural function.
Finally, it should also be noted that optimal binaural function is not the only goal that should be considered when programming a CI for BI-CI and SSD-CI listeners. Speech understanding in quiet, fusion of the sounds across the ears, and other percepts may differ in how a clinician would program a frequency-allocation table. Clarifying a broader landscape of speech understanding and binaural functioning is critical for the field if we are to provide the best guidance in providing hearing to both ears and maximizing the effectiveness of BI-CIs and SSD-CIs.
Acknowledgements
We thank Cochlear Ltd. and Med-El for providing the testing equipment and technical support. We thank Danielle King, Emily Waddington, and Tori Levi who helped collect data for this study. We thank the Department of Hearing and Speech Sciences at University of Maryland, College Park (Dr. Nicole Nguyen), the Cochlear Implant Center at Greater Baltimore Medical Center (Dr. Regina Presley), the University of Maryland Medical School (Dr. David Eisenman and Dr. Ronna Hertzano), and Walter Reed National Military Medical Center (Dr. Gerald Schuchman) for their assistance with recruiting listeners. We thank Ginny Alexander for the managerial help and Kenneth Jensen for helpful comments on a previous version of this paper. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency.
Funding
The research reported in this publication was supported by the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Number R01 DC015798 (M.J.G. and J.G.W.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
We performed pilot tests using a very low rate, 25 pps, for several BI-CI listeners, because Carlyon et al. (2010) suggested that 25 pps might yield less biased results than 1000 pps. The main reason for using very low-rate pulse trains around 25 pps is to reduce the influence of temporal pitch cues on the pitch perception, because some electrodes have a different upper limit of temporal pitch than others (e.g., Kong and Carlyon 2010). We found no apparent difference in using 25 or 1000 pps in our pilot tests for listeners who could perform the task. For a few of our BI-CI listeners, the low rate was so distracting that they had substantial difficulty or could not perform the interaural pitch-discrimination task. Changing to the higher rate allowed them to better attend to the pitch of the pulse train. We therefore opted for the higher stimulation rate for this study. Note, that this finding is in contrast to other studies where BI-CI listeners appeared to have no problem with interaural pitch discrimination using 25-pps pulse trains (e.g., Ihlefeld et al. 2015). The reason for the discrepancy is unclear and motivates further investigation on the use of very low rates in interaural pitch-discrimination tasks.
The Latin square design applied to listeners BCI1, 2, 4, 5, 6 and 7. We recruited one extra BI-CI listener for the study, BCI3, who was tested on the same order of conditions as listener BCI5. Listener BCI6, who was tested with the reference electrodes in both the right and left ears, was assigned a unique order for the left ear, but the same order as BCI1 for the right ear.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adunka OF, Pillsbury HC, Kiefer J. Combining perimodiolar electrode placement and atraumatic insertion properties in cochlear implantation—fact or fantasy? Acta Otolaryngol. 2006;126:475–482. doi: 10.1080/00016480500437393. [DOI] [PubMed] [Google Scholar]
- Aronoff JM, Padilla M, Stelmach J, Landsberger DM (2016) Clinically paired electrodes are often not perceived as pitch matched. Trends Hear 20:2331216516668302 [DOI] [PMC free article] [PubMed]
- Baumann U, Nobbe A. Pitch ranking with deeply inserted electrode arrays. Ear Hear. 2004;25:275–283. doi: 10.1097/00003446-200406000-00008. [DOI] [PubMed] [Google Scholar]
- Bernstein JGW, Stakhovskaya OA, Schuchman GI, Jensen KK, Goupell MJ (2018) Interaural-time-difference discrimination as a measure of place of stimulation for cochlear-implant users with single-sided deafness. Trends Hear 22:2331216518765514 [DOI] [PMC free article] [PubMed]
- Bierer JA, Nye AD. Comparisons between detection threshold and loudness perception for individual cochlear implant channels. Ear Hear. 2014;35:641–651. doi: 10.1097/AUD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks DA, Roberts JM, Buss E, Hall JW, Fitzpatrick DC. Neural and behavioral sensitivity to interaural time differences using amplitude modulated tones with mismatched carrier frequencies. J Assoc Res Otolaryngol. 2007;8:393–408. doi: 10.1007/s10162-007-0088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon RP, Macherey O, Frijns JH, Axon PR, Kalkman RK, Boyle P, Baguley DM, Briggs J, Deeks JM, Briaire JJ, Barreau X, Dauman R. Pitch comparisons between electrical stimulation of a cochlear implant and acoustic stimuli presented to a normal-hearing contralateral ear. J Assoc Res Otolaryngol. 2010;11:625–640. doi: 10.1007/s10162-010-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Yu J. A relation between electrode discrimination and amplitude modulation detection by cochlear implant listeners. J Acoust Soc Am. 2010;127:415–426. doi: 10.1121/1.3257591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francart T, Brokx J, Wouters J. Sensitivity to interaural time differences with combined cochlear implant and acoustic stimulation. J Assoc Res Otolaryngol. 2008;10:131–141. doi: 10.1007/s10162-008-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupell MJ. Interaural correlation-change discrimination in bilateral cochlear-implant users: effects of interaural frequency mismatch, centering, and age of onset of deafness. J Acoust Soc Am. 2015;137:1282–1297. doi: 10.1121/1.4908221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupell MJ, Litovsky RY. Detection of changes in envelope correlation in bilateral cochlear-implant users. J Acoust Soc Am. 2015;137:335–349. doi: 10.1121/1.4904491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Hu H, Dietz M (2015) Comparison of interaural electrode pairing methods for bilateral cochlear implants. Trends Hear 2331216515617143 [DOI] [PMC free article] [PubMed]
- Ihlefeld A, Carlyon RP, Kan A, Churchill TH, Litovsky RY. Limitations on monaural and binaural temporal processing in bilateral cochlear implant listeners. J Assoc Res Otolaryngol. 2015;16:641–652. doi: 10.1007/s10162-015-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Smith PH, Yin TC. Coincidence detection in the auditory system: 50 years after Jeffress. Neuron. 1998;21:1235–1238. doi: 10.1016/S0896-6273(00)80643-1. [DOI] [PubMed] [Google Scholar]
- Kan A, Stoelb C, Litovsky RY, Goupell MJ. Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. J Acoust Soc Am. 2013;134:2923–2936. doi: 10.1121/1.4820889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A, Litovsky RY, Goupell MJ. Effects of interaural pitch-matching and auditory image centering on binaural sensitivity in cochlear-implant users. Ear Hear. 2015;36:e62–e68. doi: 10.1097/AUD.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YY, Carlyon RP. Temporal pitch perception at high rates in cochlear implants. J Acoust Soc Am. 2010;127:3114–3123. doi: 10.1121/1.3372713. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Svrakic M, Roland JT, Jr, Svirsky M. The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear Hear. 2015;36:e207–e213. doi: 10.1097/AUD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Jones GL, Agrawal S, van Hoesel R. Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans. J Acoust Soc Am. 2010;127:400–414. doi: 10.1121/1.3257546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Goupell MJ, Kan A, Landsberger DM (2017) Use of research interfaces for psychophysical studies with cochlear-implant users. Trends Hear 21:2331216517736464 [DOI] [PMC free article] [PubMed]
- Long CJ, Nimmo-Smith I, Baguley DM, O'Driscoll M, Ramsden R, Otto SR, Axon PR, Carlyon RP. Optimizing the clinical fit of auditory brain stem implants. Ear Hear. 2005;26:251–262. doi: 10.1097/00003446-200506000-00002. [DOI] [PubMed] [Google Scholar]
- Lu T, Litovsky R, Zeng FG. Binaural unmasking with multiple adjacent masking electrodes in bilateral cochlear implant users. J Acoust Soc Am. 2011;129:3934–3945. doi: 10.1121/1.3570948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, O'Brien A, James CJ. Effect of current level on electrode discrimination in electrical stimulation. Hear Res. 1999;136:159–164. doi: 10.1016/S0378-5955(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Noel VA, Eddington DK. Sensitivity of bilateral cochlear implant users to fine-structure and envelope interaural time differences. J Acoust Soc Am. 2013;133:2314–2328. doi: 10.1121/1.4794372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton EC. Models for biases in judging sensory magnitude. Psychol Bull. 1979;86:777–803. doi: 10.1037/0033-2909.86.4.777. [DOI] [PubMed] [Google Scholar]
- Radeloff A, Mack M, Baghi M, Gstoettner WK, Adunka OF Variance of angular insertion depths in free-fitting and perimodiolar cochlear implant electrodes. Otol Neurotol. 2008;29:131–136. doi: 10.1097/MAO.0b013e318157f0ea. [DOI] [PubMed] [Google Scholar]
- Reiss LA, Turner CW, Erenberg SR, Gantz BJ. Changes in pitch with a cochlear implant over time. J Assoc Res Otolaryngol. 2007;8:241–257. doi: 10.1007/s10162-007-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Turner CW, Karsten SA, Gantz BJ. Plasticity in human pitch perception induced by tonotopically mismatched electro-acoustic stimulation. Neuroscience. 2014;256:43–52. doi: 10.1016/j.neuroscience.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Ito RA, Eggleston JL, Liao S, Becker JJ, Lakin CE, Warren FM, McMenomey SO. Pitch adaptation patterns in bimodal cochlear implant users: over time and after experience. Ear Hear. 2015;36:e23–e34. doi: 10.1097/AUD.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzer R, Vermeire K, Visser D, Krenmayr A, Kals M, Voormolen M, Van de Heyning P, Zierhofer C. Electric-acoustic pitch comparisons in single-sided-deaf cochlear implant users: frequency-place functions and rate pitch. Hear Res. 2014;309:26–35. doi: 10.1016/j.heares.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Studebaker GA. A ‘rationalized’ arcsine transform. J Speech Hear Res. 1985;28:455–462. doi: 10.1044/jshr.2803.455. [DOI] [PubMed] [Google Scholar]
- Tan CT, Martin B, Svirsky MA. Pitch matching between electrical stimulation of a cochlear implant and acoustic stimuli presented to a contralateral ear with residual hearing. J Am Acad Audiol. 2017;28:187–199. doi: 10.3766/jaaa.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Marel KS, Briaire JJ, Wolterbeek R, Snel-Bongers J, Verbist BM, Frijns JH. Diversity in cochlear morphology and its influence on cochlear implant electrode position. Ear Hear. 2014;35:e9–e20. doi: 10.1097/01.aud.0000436256.06395.63. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJM, Jones GL, Litovsky RY. Interaural time-delay sensitivity in bilateral cochlear implant users: effects of pulse rate, modulation rate, and place of stimulation. J Assoc Res Otolaryngol. 2009;10:557–567. doi: 10.1007/s10162-009-0175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire K, Nobbe A, Schleich P, Nopp P, Voormolen MH, Van de Heyning PH. Neural tonotopy in cochlear implants: an evaluation in unilateral cochlear implant patients with unilateral deafness and tinnitus. Hear Res. 2008;245:98–106. doi: 10.1016/j.heares.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Wess JM, Brungart DS, Bernstein JGW. The effect of interaural mismatches on contralateral unmasking with single-sided vocoders. Ear Hear. 2017;38:374–386. doi: 10.1097/AUD.0000000000000374. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63:1293–1313. doi: 10.3758/BF03194544. [DOI] [PubMed] [Google Scholar]
- Williges B, Jurgens T, Hu H, Dietz M (2018) Coherent coding of enhanced interaural cues improves sound localization in noise with bilateral cochlear implants. Trends Hear 22:2331216518781746 [DOI] [PMC free article] [PubMed]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]