Abstract
BACKGROUND:
Dysmenorrhea occurs as one of the symptoms of menstruation. While not necessarily a condition that plagues every woman, it is known to cause significant distress. Absenteeism from school and work as well as general discomfort are some of its adverse effects.
AIM:
This study aims to investigate the effects of certain diets on the prevalence and severity of dysmenorrhea.
METHODS:
Questionnaires was given to 478 women ranging from ages 1-55. The survey was centred around the age of menarche, presence and incidence of dysmenorrhea as well as how it is related to certain diets.
RESULTS:
Majority of the participants (81.74%) belonged to the age groups of 11-15 and 16-20. 45.5% of the participants attested to dysmenorrhea at each menstrual cycle. statistical correlation between diet and dysmenorrhea was insignificant (p > 0.05). Consumption of caffeinated beverages correlated with dysmenorrhea (p < 0.05). Although not statistically significant (p > 0.05), the study reported dysmenorrhea in a large proportion of participants who consumed high quantities of sugars.
CONCLUSION:
No relationship was established between diet and the incidence and severity of dysmenorrhea amongst the sample screened in Saint Vincent and the Grenadines. However, it appears that diet high in sugars might benefit from further research.
Keywords: Dysmenorrhea, Menstrual pain, Sugar, Diet, Lifestyle
Introduction
Dysmenorrhea is pain induced by menstruation which occurs at the site of the lower abdomen. Its incidence is usually within the first 6-12 months after menarche [1]. It is also observed to occur within the first 2 years of menstruation. This is when a steady ovulatory cycle is usually established in females [2]. Dysmenorrhea may be comorbid with other symptoms such as; headaches, nausea, and vomiting [3]. Dysmenorrhea is an increasingly prevalent condition among premenopausal women. Its social implications include absenteeism from places of school and work [4]. There is however a rarity of physician consultations with dysmenorrhea cited as a chief complaint [2]. The combination of these factors makes this condition one of significance.
There are two types of Dysmenorrhea; Primary and Secondary. Primary Dysmenorrhea is menstrual pain that occurs in the absence of any underlying pelvic pathology. Its aetiology is the increase of myometrial production of prostaglandins and Leukotrienes. The end of ovulation triggers the synthesis and accumulation of fatty acids in the cell membrane. Progesterone levels decrease to signal the beginning of menstruation, allowing the release of these fatty acids. One of the synthesised fatty acids is arachidonic acid which is a precursor to the production of prostaglandins like E2 and F2α and leukotrienes. Effects of the prostaglandins E2 and F2α are vasoconstriction as well as uterine contractions which induce the symptoms of dysmenorrhea [1].
Secondary Dysmenorrhea is a condition which occurs as a symptom of an existing pelvic pathology. Possible causes of this condition include but are not limited to; Endometriosis, Pelvic inflammatory diseases, adhesions, abscesses, Mullerian anomalies, and ovarian cysts [2]. A study by Harel 2008 singled Endometriosis as the more frequent cause of secondary Dysmenorrhea amongst adolescents. Endometriosis is a syndrome characterised by the presence of the uterine lining outside the uterine cavity. One of the more prevalent symptoms which enable diagnosis of endometriosis is Dysmenorrhea [2].
Possible therapeutic options for treatment of Dysmenorrhea include the use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDS), analgesics, Oral Contraceptive pills as well as injectable long-acting contraceptives (Harel, 2002) [5]. Some non-pharmacological options have also been associated with success. Some of these are herbs, acupuncture and heat therapy [5].
Several factors have been linked to an increased risk of Dysmenorrhea. A couple of these factors are: earlier age of menarche increased bleeding intensity, and longer lasting flow [5]. Some have tried to find possible relationships between diet and dysmenorrhea [1], [6], [7]. Some foods are associated with reduced risk of dysmenorrhea. Foods containing Omega-3 fatty acids are a prime example of this. It is believed that Omega-3 fatty acids competitively bind to the Omega-6 sites in the cell membrane [1].
This inhibits the production of arachidonic acid and by proxy the prostaglandins and leukotriene involved in the pathogenesis of Dysmenorrhea. Conversely, foods containing Omega-6 fatty acid have been shown to have an inverse relationship with the severity of Dysmenorrhea [1]. Other foods that may affect the severity of Dysmenorrhea are dietary fibres. Dietary fibres have been observed to decrease estrogen levels. This would, in turn, decrease the chances of the occurrence of an ovulatory cycle. Thus, decreasing the risk of Dysmenorrhea [8]. Consumption of calcium-magnesium supplements has also been observed to have a positive effect on Dysmenorrhea. It was noted to cause a decrease in the severity of pain in individuals with primary dysmenorrhea [7].
This study aims to identify possible associations between dysmenorrhea in a sample population in Saint Vincent and Grenadines.
Methods
Location
Saint Vincent and the Grenadines is a southern Caribbean nation with its main island, Saint Vincent, and a chain of smaller islands. It has an estimated population of 109, 897. The island is home to many educational parastatals including some international institutions. The study was carried out on the main island with a focus in the capital, Kingstown, as well as other major locations like Arnos Vale and Belair.
Participants
The studies were conducted between January to March 2018. A total of 600 females was approached of which only 539 agreed to participate. The criteria for the study were females aged 11 to 55 years old. Participants that attested to medical conditions such as hormonal imbalance, endometriosis, pelvic inflammatory disease and sexually transmitted infection were excluded from the study. As a result, 61 individuals were left out of the study. Four hundred seventy-eight females from different institutions, ranging from high schools to college with signed consents participated in this study. All participants were presented with self-administered questionnaires.
Data collection
Published articles in reputable journals were extensively reviewed before the development of the questionnaire. Questions spanned from eating habits, gynaecological history, age, sexual history, marital status, medical history and the onset of menstruation; these were highly considered as it relates to menstrual pain.
Menstrual Pain was graded on a scale of 1 to 10 severity, 1 being the least and 10 being the worst, while the frequency of pain was graded as yes, No, and occasionally. The menstrual flow was graded as Moderate, very heavy and very light.
Diet was categorised into cereals, diaries, proteins, veggies, and carbohydrates. Use of Sugar during and before each period was considered and graded as moderate, frequent and rarely. Though few participants were indifferent about the intake of sugar and its effects on the pain, they experienced during their periods. Women who took caffeinated beverages were also identified and were graded as frequent/regular, normal/moderate, rarely/occasionally.
The statistical package for social sciences (SPSS) version 25.0 (Chicago, IL, USA) was used to analyse the data. Descriptive analysis was used to simplify the data with the occasional use of graphs. Logistic regression analysis, chi-square and one-way ANOVA test were used for statistical analysis. A value of P < 0.05 was considered statistically significant.
Ethical approval
Necessary steps were carried out to acquire adequate permission for the study. The study was approved by the Research and Ethics committee of All Saints University, school of medicine.
Results
A total of 478 female respondents participated in this research. The age of participants ranged from 11-55 years, while a total of 81.74% of participants belonged to the age groups 11-15 and 16-20. The mean weight of participants were (61.8 ± 14.8) kg; other demographic data were also collected and categorised (Table 1).
Table 1.
Demographic summary of participants
| Count | Percentage | ||
|---|---|---|---|
| Age | 11-15 | 192 | 41.74% |
| 16-20 | 184 | 40.00% | |
| 21-25 | 59 | 12.83% | |
| 26-30 | 12 | 2.61% | |
| 31-35 | 7 | 1.52% | |
| 36-40 | 5 | 1.09% | |
| 41-50 | 0 | 0.00% | |
| 50-55 | 1 | 0.22% | |
| Marital Status | Single | 390 | 87.64% |
| Married | 16 | 3.60% | |
| Others | 39 | 8.76% | |
| Weight | 61.8 ± 14.8 (95% CI 58.60-65.10) | ||
| Height | 5.0 ± 0.40 (95%CI 4.96-5.05) | ||
A group of 23.6% of the participants reported that they were sexually active while 72.2% reported otherwise. Age at first menstruation ranged from 8-18 years with a mean of 11.81 ± 1.41 years (Figure 1).
Figure 1.
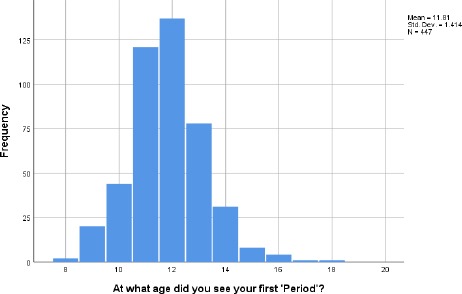
Histogram showing the summary of Menarche among participants
A group of 81.4% (381) participants experience menstrual flow every month since the first menstruation. Duration of period varied amongst the participants with most participants reporting the duration of 3-5 days (53.4%) and 5-7days (37.4%). Two thirds (75.3%) of the study participants reported having moderate menstrual flow while 77% of respondents reported pain during their period. More participants in the age class 16-20 and 21-25 had menstrual pain compared to others. Among the participants who reported pain during their period, most reported pain (31.6%) on the first day of their period (Table 2).
Table 2.
Time of onset of menstrual pain
| The onset of Menstrual Pain | Frequency | Per cent |
|---|---|---|
| 2 days before menstruation | 38 | 7.9 |
| A day before menstruation | 48 | 10.0 |
| 1st Day | 151 | 31.6 |
| 2nd Day | 39 | 8.2 |
| 3rd Day | 13 | 2.7 |
| 5th Day | 1 | 0.2 |
| Throughout my period | 123 | 25.7 |
Not all of the participants had menstrual pain during each period, 138 and 103 participants had pain occasionally and rarely with each period, respectively (Table 3). It was observed that there was a significant relationship between age and menstrual pain.
Table 3.
Age crosstabulation of participants who answered the question, “Do you always have pain each time you see your period?”
| Age | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11-15 | 16-20 | 21-25 | 26-30 | 31-35 | 36-40 | 50-55 | ||||
| Do you always have pain each time you see your period? | Yes | Frequency | 70 | 90 | 30 | 5 | 2 | 3 | 1 | 201 |
| % within Age | 38.0% | 50.8% | 52.6% | 45.5% | 28.6% | 60.0% | 100.0% | 45.5% | ||
| Occasionally | Frequency | 62 | 61 | 11 | 2 | 2 | 0 | 0 | 138 | |
| % within Age | 33.7% | 34.5% | 19.3% | 18.2% | 28.6% | 0.0% | 0.0% | 31.2% | ||
| Rarely | Frequency | 52 | 26 | 16 | 4 | 3 | 2 | 0 | 103 | |
| % within Age | 28.3% | 14.7% | 28.1% | 36.4% | 42.9% | 40.0% | 0.0% | 23.3% | ||
The respondents were asked to rate the menstrual pain on a scale of 1 to 10, and no significant difference was observed across the different grouping based on period duration however increased pain was observed with increasing duration of menstruation as shown in the box plot (Figure 2).
Figure 2.
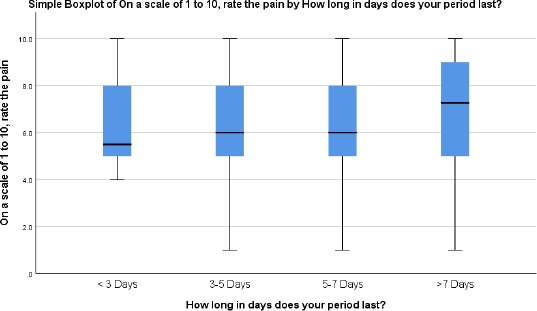
Correlating pain intensity and pain duration
Participants who reported consuming sugar frequently also reported more pain as compared to other groups; however, the difference was not statistically significant, p > 0.05 (Figure 3).
Figure 3.
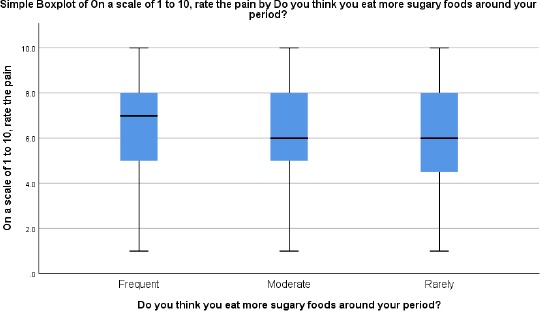
Correlating sugary food consumption and pain intensity
Individuals who reported consumption of caffeinated beverages also reported significantly higher pain than those who did not (p < 0.05). Participants who did not have sugar affected pain had a higher index of menstrual pain among the participants who reported having menstrual pain had their dominant food type collected and analysed (Table 4).
Table 4.
Food type among participants who reported menstrual pain
| N | P Value | ||
|---|---|---|---|
| What Foods do you eat most-Cereals | No | 256 | 0.116 |
| Yes | 222 | ||
| What Foods do you eat most-Dairies | No | 321 | 0.799 |
| Yes | 157 | ||
| What Foods do you eat most-Proteins | No | 268 | 0.93 |
| Yes | 210 | ||
| What Foods do you eat most-Vegetables | No | 360 | 0.244 |
| Yes | 118 | ||
| Yes | 144 | ||
| What Foods do you eat most-Confections | No | 322 | 0.862 |
| Yes | 156 | ||
Discussion
Primary Dysmenorrhea is common amongst young female; it is a menstrual pain without any medical conditions and impacts the quality of life of the individuals, dysmenorrhea has been linked to diet and lifestyle with an underlying physiologic mechanism of hormonal imbalance [9].
The findings from our study did not correlate diet to dysmenorrhea. Nevertheless, individuals who consumed foods high in sugar reported dysmenorrhea. A few studies have been done to assess the influence of diet on dysmenorrhea. Certain foods are said to interfere with the level of estrogen and prostaglandin in the blood. Elevated levels of Prostaglandins have been seen to be associated with dysmenorrheal [4], [8], [10]. Prostaglandins are said to cause endometrial contraction which causes menstrual cramps [10]. Individuals who consumed high vegetable diet have higher levels of Steroid-hormone binding globulins(SHBG)[4] which causes decreased estrogen and decreases stimulation of uterine endometrium and prostaglandin levels.
Though our study consisting of 478 participants was not able to show a statistical correlation between diet and dysmenorrhea, previous studies have demonstrated that after surveying 2561 females, feeding habit correlated with dysmenorrhea [11]. Another study surveying females also reported similar observations [12], [13]. The study also indicated that skipping breakfast caused dysmenorrhea when compared to participants that took breakfast irrespective of food class [12]. Some other studies show low consumption of fruit and vegetables increases dysmenorrheal, and high fibre diet with low-fat decreases dysmenorrheal [4], [14]. This study also showed that consumption of caffeinated drinks correlated significantly with dysmenorrhea (P < 0.05). Other studies have also correlated caffeine intake with dysmenorrehea [15], while it is unclear how caffeine cause dysmenorrhea, caffeine has powerful vasoconstriction effect implicated in pelvic pain, it is also associated with headaches [16].
Limitations: This study consists of across-sectional design which limits any conclusions about directionality. We also could not ascertain if people changed their diet close to their period, also if the diet affects the severity or length of pain.
We also could not assess other risk factors like smoking, family history, BMI which could be a confounding factor for primary dysmenorrhea. We could not explicitly state if non-Caribbean had their native meals or ate Caribbean food. While Caffeinated drinks were significant with dysmenorrhea, we could not ascertain the level of caffeine intake and severity of pain or specifically and if those who take caffeine also had high levels of starchy foods. Further studies will still be required.
In conclusion, while we could not identify a correlation between feeding habit and dysmenorrhea, we do know that dysmenorrhea significantly affect the quality of life of young females and also causes socio-economic stress to the nation at large, and possible ways to decrease menstrual pain via dietary interventions will positively impact not just the individual but also the nation.
Acknowledgement
The authors will like to appreciate the administration of All Saints University, School of Medicine for their support for this work.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Balbi C, Musone R, Menditto A, Di Prisco L, Cassese E, D'Ajello M, et al. Influence of menstrual factors and dietary habits on menstrual pain in adolescence age. Eur J Obstet Gynecol Reprod Biol. 2000;91(2):143–8. doi: 10.1016/s0301-2115(99)00277-8. https://doi.org/10.1016/S0301-2115(99)00277-8. [DOI] [PubMed] [Google Scholar]
- 2.Harel Z. Dysmenorrhea in adolescents. Ann N Y Acad Sci. 2008;1135:185–95. doi: 10.1196/annals.1429.007. https://doi.org/10.1196/annals.1429.007 PMid:18574224. [DOI] [PubMed] [Google Scholar]
- 3.Aktaş D. Prevalence and Factors Affecting Dysmenorrhea in Female University Students:Effect on General Comfort Level. Pain Manag Nurs. 2015;16(4):534–43. doi: 10.1016/j.pmn.2014.10.004. https://doi.org/10.1016/j.pmn.2014.10.004 PMid:26256218. [DOI] [PubMed] [Google Scholar]
- 4.Tavallaee M, Joffres MR, Corber SJ, Bayanzadeh M, Rad MM. The prevalence of menstrual pain and associated risk factors among Iranian women. J Obstet Gynaecol Res. 2011;37(5):442–51. doi: 10.1111/j.1447-0756.2010.01362.x. https://doi.org/10.1111/j.1447-0756.2010.01362.x PMid:21208343. [DOI] [PubMed] [Google Scholar]
- 5.Harel Z. A Contemporary Approach to Dysmenorrhea in Adolescents. Springer Link. 2002;4(12):797–805. doi: 10.2165/00128072-200204120-00004. https://doi.org/10.2165/00128072-200204120-00004. [DOI] [PubMed] [Google Scholar]
- 6.Nagata C, Hirokawa K, Shimizu N, Shimizu H. Associations of menstrual pain with intakes of soy, fat and dietary fiber in Japanese women. Eur J Clin Nutr. 2005;59(1):88–92. doi: 10.1038/sj.ejcn.1602042. https://doi.org/10.1038/sj.ejcn.1602042 PMid:15340367. [DOI] [PubMed] [Google Scholar]
- 7.Zarei S, Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Javadzadeh Y, Effati-Daryani F. Effects of calcium-vitamin D and calcium alone on pain intensity and menstrual blood loss in women with primary dysmenorrhea:A randomized controlled trial. Pain Med (United States) 2016;18(1):3–13. doi: 10.1093/pm/pnw121. https://doi.org/10.1093/pm/pnw121 PMid:27296057. [DOI] [PubMed] [Google Scholar]
- 8.Nagata C, Hirokawa K, Shimizu N, Shimizu H. Soy, fat and other dietary factors in relation to premenstrual symptoms in Japanese women. BJOG An Int J Obstet Gynaecol. 2004;111(6):594–9. doi: 10.1111/j.1471-0528.2004.00130.x. https://doi.org/10.1111/j.1471-0528.2004.00130.x PMid:15198788. [DOI] [PubMed] [Google Scholar]
- 9.Osayande AS, Mehulic S. Diagnosis and initial management of dysmenorrhea. Am Fam Physician. 2014;89(5):341–6. PMid:24695505. [PubMed] [Google Scholar]
- 10.Iacovides S, Avidon I, Bentley A, Baker FC. Reduced quality of life when experiencing menstrual pain in women with primary dysmenorrhea. Acta Obstet Gynecol Scand. 2014;93(2):213–7. doi: 10.1111/aogs.12287. https://doi.org/10.1111/aogs.12287 PMid:24266425. [DOI] [PubMed] [Google Scholar]
- 11.Gagua T, Tkeshelashvili B, Gagua D. Primer dismenore:Tiflis, Gürcistan'in adolesan populasyonunda prevalans ve risk faktörleri. J Turkish Ger Gynecol Assoc. 2012;13(3):162–8. doi: 10.5152/jtgga.2012.21. https://doi.org/10.5152/jtgga.2012.21 PMid:24592031 PMCid:PMC3939234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helwa HAA, Mitaeb AA, Al-Hamshri S, Sweileh WM. Prevalence of dysmenorrhea and predictors of its pain intensity among Palestinian female university students. 2018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5769430/pdf/12905_2018_Article_516.pdf . [DOI] [PMC free article] [PubMed]
- 13.Najafi N, Khalkhali H, Moghaddam Tabrizi F, Zarrin R. Major dietary patterns in relation to menstrual pain:a nested case control study. BMC Womens Health. 2018;18(1):69. doi: 10.1186/s12905-018-0558-4. https://doi.org/10.1186/s12905-018-0558-4 PMid:29783972 PMCid:PMC5963185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiologic reviews. 2013 doi: 10.1093/epirev/mxt009. PMid:24284871. [DOI] [PubMed] [Google Scholar]
- 15.Ozerdogan N, Sayiner D, Ayranci U, Unsal A, Giray S. Prevalence and predictors of dysmenorrhea among students at a university in Turkey. Prevalence and predictors of dysmenorrhea among students at a university in Turkey. 2009 doi: 10.1016/j.ijgo.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Faramarzi M, Hajar S, Salmalian H. Association of Psychologic and Nonpsychologic Factors With Primary Dysmenorrhea. Iran Red Crescent Med J. 2014;16(8):16307. doi: 10.5812/ircmj.16307. https://doi.org/10.5812/ircmj.16307 PMid:25389482 PMCid:PMC4222008. [DOI] [PMC free article] [PubMed] [Google Scholar]


