Abstract
Background:
The incidence of melanoma is rising faster than that of any other preventable cancer in the United States. The American Academy of Dermatology has sponsored free skin cancer education and screenings conducted by volunteer dermatologists in the United States since 1985.
Objective:
We aimed to assess the American Academy of Dermatology’s national skin cancer screening program from 1986 to 2014 by analyzing the risk factor profile, access to dermatologic services, and examination results.
Methods:
We conducted several detailed statistical analyses of the screening population.
Results:
From 1986 to 2014, records were available for 2,046,531 screenings, 1,963,141 (96%) of which were subjected to detailed analysis. Men comprised 38% of all participants. The number of annual screenings reached approximately 100,000 in 1990 and remained relatively stable thereafter. From 1991 to 2014 (data for 1995, 1996 and 2000 were unavailable), clinical diagnoses were rendered for 20,628 melanomas, 156,087 dysplastic nevi, 32,893 squamous cell carcinomas, and 129,848 basal cell carcinomas. Only 21% of screenees had a regular dermatologist. Those with a clinical diagnosis of skin cancer were more likely than the general screening population to be uninsured.
Limitations:
Inability to verify clinical diagnoses histopathologically.
Conclusion:
Our findings suggest that the SPOTme program has detected thousands of skin cancers that may have gone undetected or experienced a delay in detection.
Keywords: access to care, clinical diagnoses, melanoma, risk factors, screening, skin cancer
To address the skin cancer epidemic in the United States, in 1985 the American Academy of Dermatology (AAD) began its national free skin cancer screening program (now known as the SPOTme skin cancer screening program), which has operated as a public health service over the past 30 years.1,2
With screening findings now extending through 2014 and by comparing trends over time, this study aimed to assess the AAD’s national skin cancer screening program spanning these 30 years by analyzing the risk factor profile, access to dermatologic services, and examination results for an estimated 1.79 million individuals and more than 2 million screenings conducted.
METHODS
The AAD has sponsored the SPOTme skin cancer screening program, in which volunteer dermatologists provide free skin cancer screenings and skin cancer education open to the general public. Screenings have been promoted in national and local media.
Over the 30 years of the screening program, answers to a specific set of standard demographic and risk factor questions were solicited yearly2 whereas others changed from year to year (Supplemental Table I; available at http://www.jaad.org). Questions related to access to dermatologic care were introduced in 1992 and asked consistently until 2010. Participants signed a consent form granting permission for the screening and for release of medical information in the event that screening revealed a lesion that was clinically suggestive for skin cancer. Screenees with such lesions were asked to consult their own dermatologist or personal physician for definitive diagnosis. For screenees without a dermatologist or personal physician, tools to find local dermatologists were offered.
Statistical analysis
Missing data for demographics, risk factor, and access to dermatologic care services variables were analyzed and are partially shown in Supplemental Table I. Observations were excluded for all subsequent analyses if any of the following criteria were met: (1) age younger than 15 years or older than 90 years (n = 28,437 [1.4%]), (2) 2 or more of the 3 standard skin cancer risk factor questions were unanswered (personal history or family history of skin cancer and recent change in mole size, shape, or color), or (3) in addition to 1 of the standard risk factors being unanswered, skin phototype, blistering sunburns before age 20, and number of moles were unanswered in available years (n = 54,953; 2.7% for criteria (2) and (3) combined). Persons younger than 15 years or older than 90 years were excluded because of a lack of century of birth (ie, date of birth was collected as MM/DD/YY), making an accurate calculation of age at these extremes tenuous. Criteria (2) and (3) portended a largely incomplete screening form, making imputation and further analysis unsuitable.
Schonlau’s implementation of the hot deck procedure (a form of single imputation) for Stata software3 was subsequently performed to address the remaining missing values. This type of imputation is preferable to complete case analysis and performs well when low rates of missing data are observed.4 Key demographic variables and variables related to access to care and skin cancer risk factors are presented in Table I (as well as in Supplemental Tables II and III; available at http://www.jaad.org). First, access to dermatologic care services of screenees was characterized by calculating the proportion of screenees who were uninsured, would not have otherwise seen a doctor for their skin, had no regular dermatologist, had not been to a skin cancer screening before, and/or had never had their skin checked for cancer by any doctor (Table II). Second, the definitions of melanoma risk of the US Preventive Services Task Force (USPSTF)5 were applied to SPOTme data from 2009 and 2010 (the only years in which all the requisite risk factors were solicited) to determine the proportion of the SPOTme population characterized as being at high, medium, or low risk (Table III). Using USPSTF guidelines, we defined the following groups as being at high risk for skin cancer: non-Hispanic white men and women aged[65 years, respondents who reported having been sunburned, and respondents with a family history of skin cancer. High-risk characteristics such as many moles ([50) or atypical moles could not be ascertained as they were not asked on the NHIS. The term medium-risk refers to non-Hispanic white individuals who are age 65 years or younger and who do not meet any of the high-risk criteria. The term low-risk refers to all others.5 Lastly, after exclusion of those who had previously attended a skin cancer screening or had a personal history of skin cancer, the age- and sex-specific incidences per 100,000 screenees were calculated for the 4 major clinical screening diagnoses (Fig 1).
Table I.
Demographics of persons screened in the American Academy of Dermatology’s national skin cancer screening program in 1986–2014 (N = 1,963,141)
| Variable | n | % |
|---|---|---|
| Sex | ||
| Male | 740,204 | 37.7 |
| Female | 1,222,937 | 62.3 |
| Age, y* | ||
| 15–19 | 21,406 | 1.1 |
| 20–29 | 153,501 | 7.8 |
| 30–39 | 266,139 | 13.6 |
| 40–49 | 348,164 | 17.7 |
| 50–59 | 426,308 | 21.7 |
| 60–69 | 444,937 | 22.7 |
| 70–79 | 250,281 | 12.8 |
| 80–90 | 52,405 | 2.7 |
| Education completed† | ||
| Elementary school | 35,501 | 2.9 |
| High school | 469,374 | 38.3 |
| College | 481,832 | 39.3 |
| Graduate or poste-graduate school | 239,047 | 19.5 |
| Race† | ||
| White | 960,927 | 90.3 |
| Black | 24,933 | 2.3 |
| Hispanic | 40,986 | 3.9 |
| Other | 37,297 | 3.5 |
| Access to care† | ||
| Uninsured‡ | 118,460 | 9.1 |
| No regular dermatologist§ | 1,031,836 | 79.4 |
| No prior screening|| | 945,367 | 72.7 |
| Would not have otherwise seen a doctor¶ | 853,847 | 51.4 |
| Never checked for skin cancer before# | 356,014 | 44.7 |
| Risk factors | ||
| Personal history of skin cancer | 264,074 | 13.5 |
| Family history of skin cancer | 661,707 | 33.7 |
| Recent change in a mole** | 605,765 | 30.9 |
Persons younger than 15 years or older than 90 years (1.4% of screening population) were excluded because of a lack of century of birth (ie, date of birth was collected as MM/DD/YY), making an accurate calculation of age at these extremes tenuous.
Not all variables were collected from 1986 to 2014. There are no screening forms for the years 1995, 1996, or 2000. Refer to Supplemental Table I for dates and missing data.
Do you have health insurance? (n = 1,299,765).
Do you have a regular dermatologist? (n = 1,299,765).
Have you ever been to a skin cancer screening before? (n = 1,299,765).
Without this screening, would you have seen a doctor for your skin? (n = 1,660,846).
Have you ever had your skin checked for cancer by a dermatologist or other doctor? (n = 796,354).
Do you have any moles that have recently changed in size, color, or shape?
Table II.
Access to dermatologic care services by clinical screening diagnosis of AAD screening participants, 1992–2010 (N = 1,299,765)
| Uninsured (%) |
||||
|---|---|---|---|---|
| Clinical diagnosis | 1992–1999* | 2001–2006 | 2007–2010 | Total |
| Melanoma n = 14,908 | 10.7 | 12.6 | 15.6 | 12.3 |
| Dysplastic nevus n = 109,214 | 9.5 | 9.6 | 11.8 | 10.2 |
| Squamous cell carcinoma n = 20,767 | 8.4 | 8.9 | 11.6 | 9.6 |
| Basal cell carcinoma n = 92,023 | 8.7 | 9.3 | 11.2 | 9.5 |
| All screenees‡ n = 1,299,765 | 8.7 | 8.6 | 10.4 | 9.1 |
|
Never had skin checked†
(%) |
||||
| Clinical diagnosis | 1992–1999 | 2001–2006 | 2007–2010 | Total |
| Melanoma n = 7,045 | — | 46.7 | 48.8 | 47.7 |
| Dysplastic nevus n = 72,009 | — | 47.1 | 47.0 | 47.0 |
| Squamous cell carcinoma n = 13,389 | — | 36.0 | 34.7 | 35.3 |
| Basal cell carcinoma n = 51,014 | — | 36.9 | 36.6 | 36.7 |
| All screenees‡ n = 761,899 | — | 47.1 | 46.2 | 46.7 |
|
Would not have seen a doctor (%) |
||||
| Clinical diagnosis | 1992–1999 | 2001–2006 | 2007–2010 | Total |
| Melanoma n = 14,908 | 47.1 | 46.4 | 49.2 | 47.3 |
| Dysplastic nevus n = 109,214 | 49.9 | 49.9 | 50.3 | 50.0 |
| Squamous cell carcinoma n = 20,767 | 36.9 | 36.8 | 38.6 | 37.5 |
| Basal cell carcinoma n = 92,023 | 39.9 | 39.9 | 42.3 | 40.5 |
| All screenees‡ n = 1,299,765 | 51.2 | 51.4 | 52.0 | 51.5 |
|
No prior skin cancer screening (%) |
||||
| Clinical diagnosis | 1992–1999 | 2001–2006 | 2007–2010 | Total |
| Melanoma n = 14,908 | 79.2 | 71.5 | 76.4 | 76.5 |
| Dysplastic nevus n = 109,214 | 78.7 | 70.0 | 72.8 | 73.6 |
| Squamous cell carcinoma n = 20,767 | 74.1 | 65.3 | 69.2 | 69.5 |
| Basal cell carcinoma n = 92,023 | 74.6 | 65.2 | 68.2 | 69.9 |
| All screenees‡ n = 1,299,765 | 77.8 | 68.7 | 70.7 | 72.7 |
|
Have no regular dermatologist (%) |
||||
| Clinical diagnosis | 1992–1999 | 2001–2006 | 2007–2010 | Total |
| Melanoma n = 14,908 | 83.1 | 83.3 | 83.0 | 83.1 |
| Dysplastic nevus n = 109,214 | 83.1 | 82.0 | 81.3 | 82.1 |
| Squamous cell carcinoma n = 20,767 | 77.9 | 76.4 | 74.7 | 76.3 |
| Basal cell carcinoma n = 92,023 | 77.4 | 76.4 | 75.4 | 76.6 |
| All screenees‡ n = 1,299,765 | 80.1 | 79.3 | 78.4 | 79.4 |
Screen years 1986 to 1991 were not included because the access variables were not collected in these years. Years 2011 to 2014 were not included because the only access variable collected in these years was “would not have seen doctor.” There are no screening forms for the years 1995, 1996, or 2000.
“Never had skin checked for cancer” does not include individuals who answered unsure on their screening form. This variable was not included before 2001.
“All screenees” includes those with the clinical screening diagnoses.
Table III.
USPSTF melanoma risk classification applied to SPOTme program data for 2009–2010 (N = 169,329)
| USPSTF melanoma risk classification† |
|||
|---|---|---|---|
| Clinical diagnosis* | High risk n (%) | Medium risk n (%) | Low risk n (%) |
| Melanoma (n = 1692) | 1270 (75.0) | 333 (19.7) | 89 (5.3) |
| Squamous cell carcinoma (n = 3618) | 3092 (85.5) | 459 (12.7) | 67 (1.8) |
| Basal cell carcinoma (n = 11,366) | 9536 (83.9) | 1665 (14.7) | 165 (1.4) |
| No clinical skin cancer (n = 153,693) | 109,371 (71.1) | 33,781 (22.0) | 10,541 (6.9) |
| Total 2009–2010 SPOTme population (n = 169,329) | 122,367 (72.3) | 36,123 (21.3) | 10,839 (6.4) |
USPSTF, US Preventive Services Task Force.
Multiple diagnoses allowed.
Using USPSTF guidelines, we defined the following groups as being at high risk for skin cancer: non-Hispanic white men and women aged [65 years, respondents who reported having been sunburned, and respondents with a family history of skin cancer. High-risk characteristics such as many moles ([50) or atypical moles could not be ascertained as they were not asked on the NHIS. Medium risk means non-Hispanic white individuals age 65 years or younger who did not meet the criteria for high risk. Low risk means all others.5
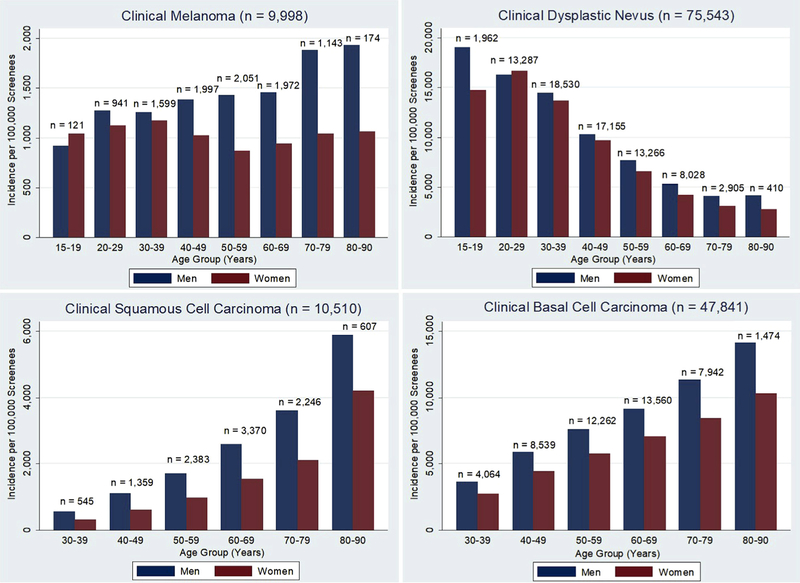
Fig 1.
Age- and sex-specific incidence of clinical screening diagnoses among American Academy of Dermatology skin cancer screening participants, from 1992 to 2010 (N = 854,641). Incidence was calculated after excluding those with a history of skin cancer and those who had been to a prior screening.
RESULTS
Demographics
From 1986 to 2014, computerized records were available for 2,046,531 skin cancer screenings, 1,963,141 (96%) of which were included for analysis. The number of annual screenings reached approximately 100,000 in 1990 and remained relatively stable through the next 2 decades. Applying the proportion of new screenees observed from 1992 to 2010 to the total number of screenings performed, we approximated that 1.79 million individuals were screened between 1986 and 2014.
Table I describes the profile of participants attending AAD programs since 1986. The changes in the variables over time were statistically significant (P = .000), though in most cases this was the result of a large sample size rather than clinically meaningful changes. Rates of participation by white men age 50 years or older remained stable throughout the duration of the program, comprising between 20% and 23% of all participants.
Access to care
The proportion of individuals with a clinical diagnosis of melanoma or dysplastic nevus (DN) who reported having no regular dermatologist was higher than the proportion of individuals with a clinical diagnosis of squamous cell carcinoma (SCC) or basal cell carcinoma (BCC) (Table II). Nearly half of individuals with either clinical melanoma (48%) or DN (47%) reported never having had their skin checked for skin cancer, and about three-quarters of these individuals had never been to a skin cancer screening before. A lack of health insurance was reported most frequently among individuals with a clinical diagnosis of melanoma (12%) and was more common in the most recent period for all of the disease outcomes. The proportion of first-time screenees remained relatively stable throughout the program.
Findings of clinical screening
From 1991 to 2014 (data unavailable for 1995, 1996, and 2000) the number of clinical diagnoses were as follows: melanoma, 20,628; DN, 156,087; SCC, 32,893; and BCC, 129,848. The incidence of each of these diagnoses per 100,000 screenees is shown in Fig 1. For clinical diagnoses of melanoma, the incidence per 100,000 screenees increased with advancing age, with the highest incidence found in the 70 to 79 years and 80 to 90 years age groups (Fig 1). As with population-based data, clinical melanoma diagnoses were more common in men than in women and for older than for younger participants: by age 60, the incidence of clinically diagnosed melanoma was nearly 2-fold higher for men.
Risk categories
In all, 72% of SPOTme participants from 2009 and 2010 met the USPSTF criteria for being at high risk of melanoma. An additional 21% met the criteria for being at medium risk, and the remaining 6% met the criteria for classification as being at low risk of melanoma (Table III). Among those with a clinical diagnosis of melanoma, 75% met the criteria for the high-risk category; the respective rates for those with a clinical diagnosis of SCC and BCC were 86% and 84%.
DISCUSSION
The AAD’s free, volunteer-led SPOTme skin cancer screening campaign is likely one of the largest organized among screening programs for all cancer types. Of all national cancer screening efforts, only the US Centers for Disease Control and Prevention’s National Breast and Cervical Cancer Early Detection Program, which offers screening services to low-income, uninsured, and underserved women, screens more Americans (providing 12 million breast and cervical cancer screenings).6
Access to care
A significant majority of screenees with the 3 most serious findings (clinical melanoma, BCC, and SCC) reported having neither a regular dermatologist nor a prior skin cancer screening. Most telling, among those with a clinical diagnosis of melanoma, more than 80% did not have a dermatologist, and slightly less than 80% of participants had not been to a skin cancer screening previously. Nearly half of participants reported that they had not had their skin checked by a physician and had not planned to have their skin checked in the absence of the screening program. More than 12% of individuals with a clinical diagnosis of melanoma were uninsured, a rate that actually increased over time.
Findings of clinical screening
Our data indicate that male SPOTme participants had higher rates of all 3 clinical skin cancer diagnoses regardless of age (except for clinical melanoma among 15- to 19-year-old participants). Furthermore, in an earlier analysis of more than 360,000 participants screened in AAD programs, Goldberg et al described the factors associated with clinical melanoma and found that male sex was associated with increased risk, as were lack of a regular dermatologist, personal history of melanoma, age older than 50 years, and presence of a changing mole.7 In particular, white men older than 50 years disproportionately account for 60% of all melanoma deaths in the United States.8 These differences in melanoma incidence and survival have been attributed to both behavioral and, more recently, biologic differences between men and women.8–13 All of these findings suggest that screening men, and older white men in particular, is critical for early detection and would provide the greatest yield for detecting skin cancers. Nevertheless, only 38% of SPOTme screenees were men, and despite accounting for only 24% of the SPOTme population, men older than 50 years (all races) accounted for 31% of all clinical melanomas, 36% of clinical BCCs, and 44% of clinical SCCs.
Risk categories
Using the USPSTF’s definition of melanoma risk,5 we further analyzed the SPOTme data from 2009 and 2010, which was the only time when all of these risk factor variables (except atypical moles) were collected together. In doing so, we found that 72% of SPOTme participants would be classified as being at high risk. According to a recent analysis of 3 different National Health Interview Survey (NHIS) studies, an estimated 104.7 million US adults (or 51%) are considered at high risk of melanoma, of which only 24% had had at least 1 total body skin examination.5 Additionally, the SPOTme population is older and whiter than the general US population,14 both of which are independent risk factors for skin cancer. From the same 2010 US survey data, 3.8% of the roughly 24,000 Americans surveyed reported a family history of skin cancer and 5.9% reported a personal history of any cancer (not just skin cancer).5 In contrast, roughly 34% of SPOTme screenees reported a family history of skin cancer and 14% reported a personal history of skin cancer. Thus, although SPOTme screenees were more likely to report having been checked for skin cancer than were those in the NHIS studies (51% vs 20%), our data suggest that the SPOTme population represents a higher-risk group than the general US population on the basis of these characteristics.
In its most recent guidelines (2016), the US Preventive Services Task Force (USPSTF), which makes decisions on counseling and screening recommendations for primary care physicians, indicated that evidence is “insufficient” to assess the balance of benefits and harm of using a whole body skin examination by a primary care clinician for the early detection of cutaneous melanoma, BCC, or SCC in the adult general population.15 Many professional organizations, such as the American College of Physicians, the American College of Preventive Medicine, the American Academy of Family Physicians, and the American Cancer Society, do not recommend screening for skin cancer.16–18 Others have raised some of the unintended consequences of screening, namely, the overdiagnosis of melanoma, BCC, and SCC.18 These experts and others support implementation of risk-based skin cancer screening guidelines similar to those recommended internationally and have called for collaborations with primary care physicians, focusing on those with highest case fatality rates, as well as targeted screening of individuals with the most important phenotypic and genotypic melanoma risk factors.19 Going forward, AAD members can participate in the type of randomized and observational studies that the USPSTF seeks to determine the appropriateness of screening and provide evidence that measures benefits relative to harm.
Limitations
The first important limitation is the inability to verify clinical diagnoses. Second, there is selection bias as to who attends the AAD screening programs and other mass screening efforts, and this has been reported in numerous Journal of the American Academy of Dermatology publications.2,7,20–22 Although self-selection clearly occurs in the AAD programs, a higher rate of men attended the AAD programs relative to similar programs in Germany.23 Lastly, screening forms do not report provider-level (ie, physician-dermatologist, dermatology resident, physician assistant, or nurse practitioner) data.
Summary of new findings
Compared with the 2003 article by Geller et al,2 our study provides a number of additional points. First, the sample size has increased 2.5-fold, now totaling 2,046,531 skin cancer screenings, and data from as recently as 2014 are now available, allowing us to provide trends in screening data over a greater period of time than in the 2003 study. This study has revealed some notable trends; specifically, the proportion of men attending screenings has decreased over time, the uninsured fraction of the general screening population was greatest in the most recent years in which insurance status was solicited, and access to care has worsened over time for screenees with a clinical melanoma. Additionally, our categorization of the SPOTme population using the USPSTF’s melanoma risk definitions also revealed numerous interesting findings, in particular, the high-risk status of the significant majority of AAD screening program participants.
Implications
Our results indicate that many screenees had not seen a specialist before this screening. We note a nearly 20-year upward trend in the rates of uninsurance among AAD screenees; such findings have strong relevance to the changing state of health care reform in the United States and underscores the need for free screening programs such as SPOTme to provide care for those who may not have the means to afford or access to regular health care. Third, there are important clinical findings outside of the screening setting. Using the USPSTF’s definitions of risk, we found that 93% of SPOTme participants from 2009 and 2010 would be classified as being at high (72%) or medium (21%) risk of melanoma. Therefore, it is important to note that the SPOTme program is primarily screening individuals who many experts would agree should undergo routine skin cancer screening.19 Nonetheless, we contend that stronger efforts to attract men, particularly those age 50 years or older, are warranted, as these men currently make up a minority of the screening population yet disproportionately suffer the greatest mortality burden of disease. Although several potential barriers may exist in recruiting men to skin cancer screenings (including limited health literacy, inconvenient times, or lack of awareness and concern about skin lesions),12,24,25 some barriers may be lifted by conducting more public awareness and educational campaigns targeted toward men.
Conclusion
For 30 years, the AAD’s SPOTme skin cancer screening program has conducted more than 2 million free screenings of a predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants. When one considers that roughly half of those receiving a clinical diagnosis of skin cancer reported that they would not have otherwise seen a physician to have their skin examined, it is likely that the SPOTme program has detected thousands of skin cancers that may have gone undetected or not been detected in a timely manner.
Our finding of consistently high rates of multiple risk factors for skin cancer among new screenees is consistent with NHIS study data,5 suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer. Although the voluntary AAD program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened.
The authors thank the Melanoma/Skin Cancer Community Outreach Programs Committee and the chair of the Council on Community, Corporate and Philanthropic Relations at the American Academy of Dermatology for their thoughtful reading and approval of the manuscript.
Supplementary Material
CAPSULE SUMMARY.
The SPOTme program has provided free skin cancer screenings since 1985.
In all, 72% of screenees met the US Preventive Services Task Force criteria for high risk of melanoma. Among individuals with clinically diagnosed melanoma, 47% would not have otherwise seen a doctor for a skin examination.
Further efforts to attract men and the uninsured are warranted.
Acknowledgments
Funding sources: Supported by the American Academy of Dermatology’s SPOTme program, which is made possible through a donation from the Bristol-Myers Squibb Foundation. Dr Beaulieu was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (award No. TL1TR001062), and Dr Tsao was supported by the National Institutes of Health (award No. K24CA149202).
Abbreviations used:
- AAD
American Academy of Dermatology
- BCC
basal cell carcinoma
- DN
dysplastic nevus
- NHIS
National Health Interview Survey
- SCC
squamous cell carcinoma
- USPSTF
US Preventive Services Task Force
Footnotes
Conflicts of interest: None disclosed.
REFERENCES
- 1.American Academy of Dermatology. 30 years of free skin cancer screenings Available at: https://www.aad.org/public/spot-skin-cancer/programs/screenings/30-years-of-skin-cancer-awareness. Accessed August 10, 2016.
- 2.Geller AC, Zhang Z, Sober AJ, et al. The first 15 years of the American Academy of Dermatology skin cancer screening programs: 1985–1999. J Am Acad Dermatol 2003;48:34–41. [DOI] [PubMed] [Google Scholar]
- 3.Schonlau M Stata software package, hotdeckvar.pkg, for Hot deck Imputation Available at: http://www.schonlau.net/. Accessed August 10, 2016.
- 4.van der Heijden GJ, Donders AR, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol 2006; 59:1102–1109. [DOI] [PubMed] [Google Scholar]
- 5.Lakhani NA, Saraiya M, Thompson TD, King SC, Guy GP Jr. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med 2014;61:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Centers for Disease Control and Prevention. National Breast and Cervical Cancer Early Detection Program (NBECCEDP) Available at: https://www.cdc.gov/cancer/nbccedp/index.htm. Accessed August 8, 2017.
- 7.Goldberg MS, Doucette JT, Lim HW, Spencer J, Carucci JA, Rigel DS. Risk factors for presumptive melanoma in skin cancer screening: American Academy of Dermatology National Mel-anoma/Skin Cancer Screening Program experience 2001–2005. J Am Acad Dermatol 2007;57:60–66. [DOI] [PubMed] [Google Scholar]
- 8.The Skin Cancer Foundation. Why do men have worse melanoma survival than women? Is it behavioral, biology, or both? Available at: https://www.skincancer.org/publications/the-melanoma-letter/summer-2014-vol-32-no-2/men. Accessed July 2, 2018.
- 9.Joosse A, Collette S, Suciu S, et al. Superior outcome of women with stage I/II cutaneous melanoma: pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials. J Clin Oncol 2012;30: 2240–2247. [DOI] [PubMed] [Google Scholar]
- 10.Joosse A, de Vries E, Eckel R, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol 2011;131:719–726. [DOI] [PubMed] [Google Scholar]
- 11.Shellenberger R, Nabhan M, Kakaraparthi S. Melanoma screening: a plan for improving early detection. Ann Med 2016;48:142–148. [DOI] [PubMed] [Google Scholar]
- 12.Swetter SM, Layton CJ, Johnson TM, Brooks KR, Miller DR, Geller AC. Gender differences in melanoma awareness and detection practices between middle-aged and older men with melanoma and their female spouses. Arch Dermatol 2009;145: 488–490. [DOI] [PubMed] [Google Scholar]
- 13.Swetter SM, Pollitt RA, Johnson TM, Brooks DR, Geller AC. Behavioral determinants of successful early melanoma detec-tion: role of self and physician skin examination. Cancer 2012; 118:3725–3734. [DOI] [PubMed] [Google Scholar]
- 14.QuickFacts United States. United States Census Bureau. Population estimates, July 1, 2017 Available at: https://www.census.gov/quickfacts/fact/table/US/PST045216. Accessed January 4, 2018.
- 15.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316:429–435. [DOI] [PubMed] [Google Scholar]
- 16.Nahar VK, Mayer JE, Grant-Kels JM. The case for skin cancer screening with total-body skin examinations. JAMA Oncol 2016;2:999–1001. [DOI] [PubMed] [Google Scholar]
- 17.Swetter SM, Geller AC, Halpern AC. What the USPSTF “insufficient” skin cancer screening recommendation means for primary care clinicians and dermatologists. JAMA Dermatol 2016;152:973–975. [DOI] [PubMed] [Google Scholar]
- 18.Tsao H, Weinstock MA. Visual inspection and the US Preven-tive Services Task Force recommendation on skin cancer screening. JAMA 2016;316:398–400. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guide-lines and a review of the US Preventive Services Task Force controversy. Melanoma Manag 2017;4:13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geller AC, Halpern AC, Sun T, et al. Participant satisfaction and value in American Academy of Dermatology and American Cancer Society skin cancer screening programs in Massachu-setts. J Am Acad Dermatol 1999;40:563–566. [DOI] [PubMed] [Google Scholar]
- 21.Koh HK, Geller AC, Miller DR, Caruso A, Gage I, Lew RA. Who is being screened for melanoma/skin cancer? Characteristics of persons screened in Massachusetts. J Am Acad Dermatol 1991; 24:271–277. [DOI] [PubMed] [Google Scholar]
- 22.Koh HK, Norton LA, Geller AC, et al. Evaluation of the American Academy of Dermatology’s National Skin Cancer Early Detection and Screening Program. J Am Acad Dermatol 1996;34:971–978. [DOI] [PubMed] [Google Scholar]
- 23.Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol 2012;66:201–211. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Education Sciences. National Center for Education Statistics National Assessment of Health Literacy; Available at: https://nces.ed.gov/naal/health_results.asp. Accessed August 8, 2017. [Google Scholar]
- 25.Bureau of Labor Statistics. Time spent working by full-time and part-time status, gender, and location in 2014 Available at: https://www.bls.gov/opub/ted/2015/time-spent-working-by-full-and-part-time-status-gender-and-location-in-2014.htm. Accessed August 8, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.