Highlights
-
•
d-ribose can produce formaldehyde under neutral and alkaline conditions in vitro.
-
•
Administration of d-ribose induces an increase of formaldehyde in the brain of mice.
-
•
Formaldehyde in urine and serum should be monitored for a person especially an elderly individual to take d-ribose.
Keywords: d-Ribose, Formaldehyde, Retro aldol reaction
Abstract
Formaldehyde is toxic and has been implicated in the pathologies of various diseases, such as cognitive impairment and cancer. Though d-ribose is widely studied and provided as a supplement to food such as flavor and drinks, no laboratories have reported that d-ribose is involved in the formaldehyde production. Here, we show that formaldehyde is produced from d-ribose in lysine or glycine solution and Tris−HCl buffer under neutral and alkaline conditions. Intraperitoneal injection of C57BL/6J mice with d-ribose significantly increased the concentration of brain formaldehyde, compared to the injection with d-glucose or saline. These data suggest that formaldehyde levels should be monitored for the people who take d-ribose as a supplement.
1. Introduction
D-Ribose, which is a component in many important biomolecules, including riboflavin, ATP, GTP and RNA, is involved in various biological functions [[1], [2], [3]]. Due to these important issues, recently, d-ribose has been used as a supplementary nutrient [4]. d-Ribose is considered to be an energy boosting agent that improves the symptoms of diseases such as chronic fatigue syndrome, coronary artery disease, restless leg syndrome and fibromyalgia [[5], [6], [7]]. As an energetic supplement, this reduced sugar is administered to improve athletic performance [8,9] and the ability to exercise by boosting muscle energy by acting as a readily available source of energy [[10], [11], [12], [13]]. d-Ribose is also employed in surgeries to treat cardiovascular and cerebrovascular diseases, and oral d-ribose (5 g/dose, t.i.d.) is used preoperatively. Some patients presenting with acute myocardial infarction receive between 1 and 26 oral doses (mean = 8 doses) of d-ribose [14]. Thus, the average dose of 40 g of d-ribose per day is administered to patients during treatment. To treat myoadenylate deaminase deficiency, a dose of 40–60 g (daily) of d-ribose is used [15]. However, whether any side-effect occurs during the administration of d-ribose should be investigated.
Formaldehyde, the simplest aldehyde [16], is a severe cytotoxic agent [[17], [18], [19], [20]] and is implicated in aging and a variety of diseases, such as neurodegenerative diseases [[21], [22], [23], [24], [25]], diabetes [[26], [27], [28]], pulmonary fibrosis [29], chronic heart disorders and cancers [30,31]. Formaldehyde in the human body comes from endogenous and exogenous sources [32]. Endogenous formaldehyde is produced via different pathways apart from methanol metabolism [16,33,34]. The exogenous sources of formaldehyde typically include respiration and food [[35], [36], [37]]. However, whether exogenous reduced sugar can produce formaldehyde has not been determined.
D-Ribose can be synthesized through an aldol reaction from formaldehyde [38]. On the other hand, the pentose ring is not planar but occurs in one of a variety of conformations generally described as “puckered”, which causes the unstable aldofuranose ring to be vulnerable to reactions [39]. In retro aldol reaction, aldose could decompose and produce formaldehyde under alkaline conditions. That is, d-ribose may decompose and produce formaldehyde through retro aldol reaction. Whether d-ribose can also produce formaldehyde under alkaline conditions requires further investigation.
This work is concerned with production of formaldehyde from d-ribose incubated with a solution of lysine, glycine, ammonia, or Tris base. Levels of brain formaldehyde were elevated in mice who were administrated with d-ribose through intraperitoneal injection.
2. Materials and methods
2.1. Sample preparation
All of the reaction mixtures were filtered through 0.22 μm membranes (Millipore, USA) before incubation at 37 °C for different durations. d-Ribose and d-glucose came from Amresco (USA). Other standard reagents and amino acids were obtained from Sigma-Aldrich (USA).
2.2. Measurement of formaldehyde by high performance liquid chromatography
The sample (0.4 mL) was mixed with 0.1 mL of 2,4-dinitrophenylhydrazine (DNPH, final concentration of 1.0 g/l in acetonitrile), 0.1 mL of trichloroacetic acid (final concentration 10%, m/v) and 0.4 mL of acetonitrile. Then, the mixtures were centrifuged (12,000 rpm, 4 °C, 10 min). The supernatant was added to another Eppendorf tube and heated in a water bath (60 °C, 30 min). The samples were analyzed by high performance liquid chromatography (HPLC) after centrifugation (12,000 rpm, 4 °C, 10 min). The HPLC system (LC-20 A, Shimadzu, Japan) was equipped with an ultraviolet detector. The mobile phase was a mixture of acetonitrile and water (6.5 : 3.5, v/v) with a flow rate of 0.8 ml/min (column temperature, 35 °C). The formaldehyde-DNPH derivative was eluted through a C18 column (retention time, 6.5–7.5 min) and measured by UV detection at 355 nm. The concentrations of tissue protein extracts were quantified with a BCA Protein Assay Kit (Pierce, USA).
2.3. Hplc-ms
The HPLC system consisted of an Agilent 1290 Infinity Instrument coupled to a 6530 Accurate-Mass Q-TOF (Agilent technologies, Germany). The column was a LiChrospher 100 RP-18 column (5 mm, 150 × 4.6 mm, Merck, Germany). The mobile phase for formaldehyde was a 65% acetonitrile-water solution with a flow rate of 0.8 ml/min. Detection was performed in the electrospray ionization (ESI) negative mode, and the source parameters were selected as follows: gas temperature of 325 °C, capillary voltage of 3.8 kV and nozzle voltage of 1.0 kV.
2.4. Ethics statement
The handing of mice and experimental procedures were approved by the Animal Welfare and Research Ethics Committee of the Institute of Biophysics, Chinese Academy of Sciences (Permit Number: SYXK2013-77), and the methods were performed in accordance with the approved guidelines.
2.5. Animals and administration of d-ribose and d-glucose
Male C57BL/6 J mice (aged 8 weeks) were obtained from the Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). According to Parigian (2016), the dose of d-ribose administered as an anti-aging nutrient was 15–30 g daily (Supplementary Information 1). According to the previous report for the dose of d-ribose taken [14,15], we calculated the dose to mice. Conversion of the human dose to a mouse dose resulted in a dose of 2.57 g/kg–5.14 g/kg daily if the human body weight is regarded as 70 kg.
After 1 week of acclimatization to the cages, mice were randomly divided into three groups and received intraperitoneal injection each day of d-ribose at doses of 3.2 g/kg (21.2 mmole/kg), d-glucose at doses of 3.82 g/kg (21.2 mmole/kg), and 0.9% saline as the control. All mice were maintained in animal facilities under pathogen-free conditions. After 10 days, formaldehyde in the serum, brain, liver and kidney was measured by HPLC coupled with DNPH as mentioned above.
2.6. Data analysis
All of the values are reported as the mean ± S.E.M.. Data analysis was performed by one-way analysis of variance (ANOVA) using Origin 8.0 statistical software. Differences with a probability level of 95% (p ≤ 0.05) were considered significant.
3. Results
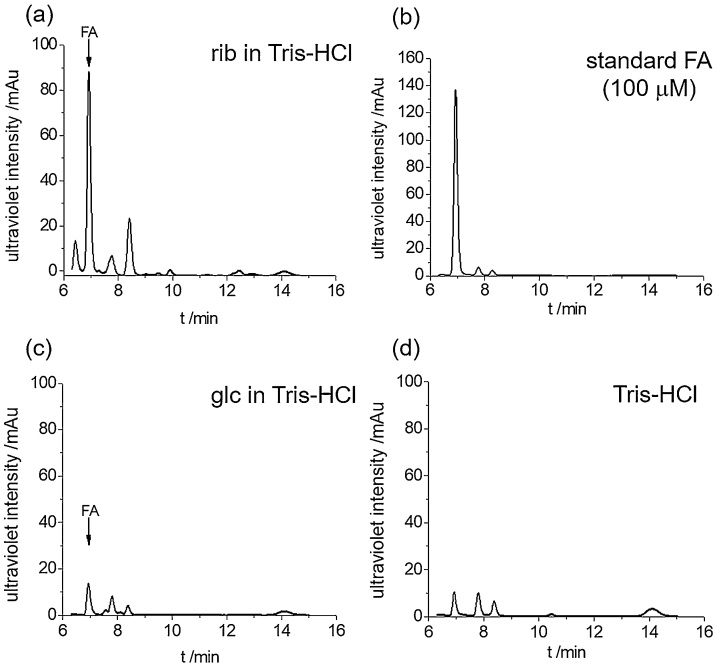
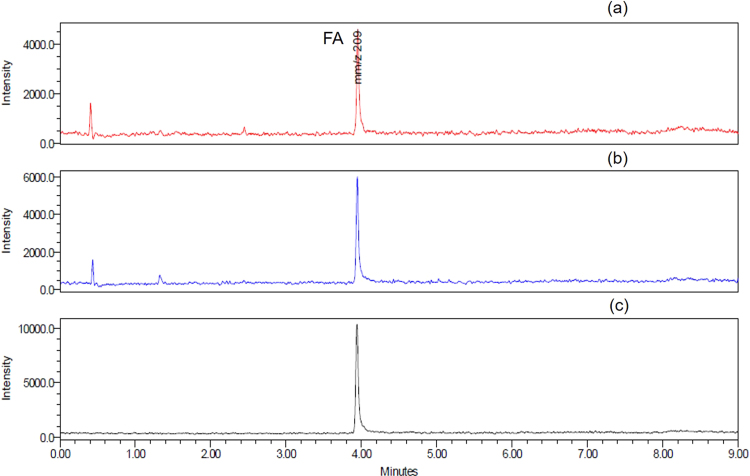
To investigate whether d-ribose produces formaldehyde under alkaline conditions, we dissolved d-ribose (100 mM final concentration) in 20 mM Tris−HCl buffer (pH = 8.0, 37 °C) for 3 days and took aliquots for measurements of formaldehyde with 1.0 g/L 2,4-dinitrophenylhydrazine (DNPH) at 60 °C for 30 min. We loaded 20 μL of the sample onto a HPLC column and monitored the absorbance at 355 nm [40]. As shown in Fig. 1, the formaldehyde peak is distinctly observed at 6.9 min via HPLC (Fig. 1a), which is identical to the formaldehyde standard reagent as a positive control (Fig. 1b). d-Glucose as a control produced little formaldehyde, similar height to Tris−HCl buffer (pH = 8.0) (Fig. 1c and 1d). That is, d-ribose can produce formaldehyde under alkaline conditions, suggesting occurrence of the retro-aldol reaction,
Fig. 1.
Formaldehyde is produced fromd-ribose incubated in Tris−HCl buffer.d-ribose (100 mM final concentration) was dissolved in 20 mM Tris−HCl buffer (pH = 8.0, at 37 °C) for 3 days, and aliquots were taken for the measurement of formaldehyde with 1 g/l 2,4-dinitrophenylhydrazine (DNPH) by HPLC. The yield of formaldehyde from a d-ribose (rib) solution (a) was greater than from a d-glucose (glc) solution (c); 100 μM formaldehyde (b) and 20 mM Tris−HCl buffer alone (c) were used as positive and negative controls under the experimental conditions, respectively.
To further demonstrate the production of formaldehyde with d-ribose in Tris−HCl buffer (pH = 8.0), fractions of formaldehyde separated by HPLC were used for mass spectrometric analysis (Supplementary Fig. 1a). The product from the formaldehyde standard reagent reacted with 2,4-dinitrophenylhydrazine (Supplementary Fig. 1b) and formaldehyde 2,4-dinitrophenylhydrazone (formaldehyde-2,4-DNPH) (Supplementary Fig. 1c) were used as controls. Based on the mass to charge ratio and retention time, the peak separated by HPLC from the product of d-ribose in 20 mM Tris−HCl buffer (pH = 8.0) was confirmed to be formaldehyde. The mass to charge ratio of the formaldehyde-2,4-DNPH derivative was 209 (M−). These data again suggest that d-ribose can produce formaldehyde under alkaline conditions.
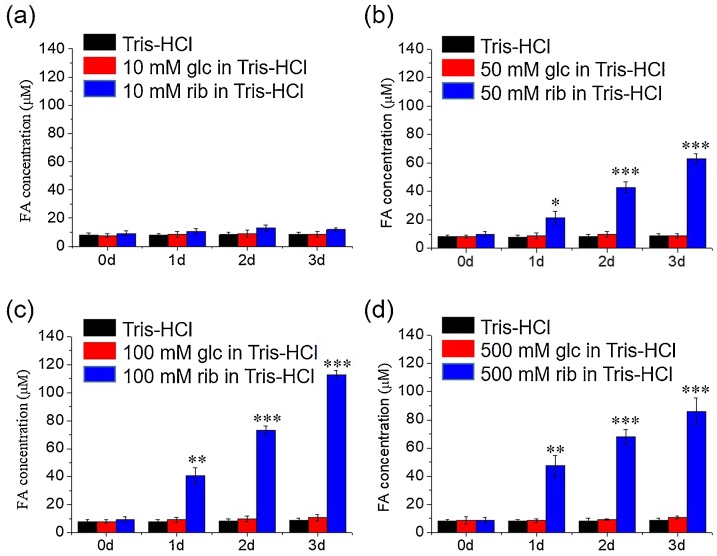
To investigate whether the yield of formaldehyde from d-ribose undergoes in concentration and time dependent manner, different concentrations of d-ribose (10 mM, 50 mM, 100 mM and 500 mM) were incubated with 20 mM Tris−HCl buffer (pH = 8.0, 37 °C), and aliquots were taken at different time intervals for measurement with HPLC (Fig. 2). The yield of formaldehyde was correlated to the concentration of d-ribose in a time-dependent manner. Under the same experimental conditions, formaldehyde was not significantly detected during incubation of either d-glucose or Tris−HCl buffer (pH = 8.0) alone. These data demonstrate that formaldehyde is resulted from d-ribose.
Fig. 2.
Yield of formaldehyde in different concentrations ofd-ribose in Tris−HCl buffer. Different concentrations of d-ribose (10, 50, 100 and 500 mM) were incubated in 20 mM Tris−HCl buffer (pH = 8.0) for 3 days. Aliquots were taken for the measurement of formaldehyde with DNPH by HPLC (a–d). d-glucose solution and Tris−HCl buffer were also measured. All of the values are expressed as the means ± S.E.M. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, n = 3.
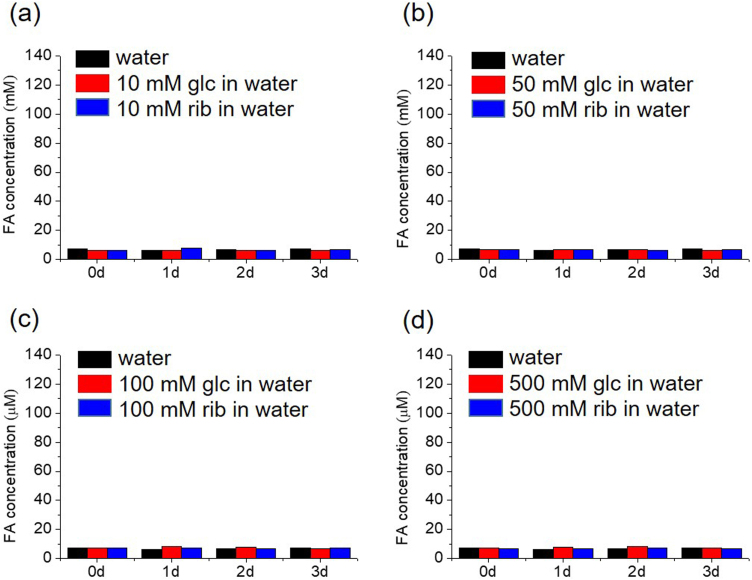
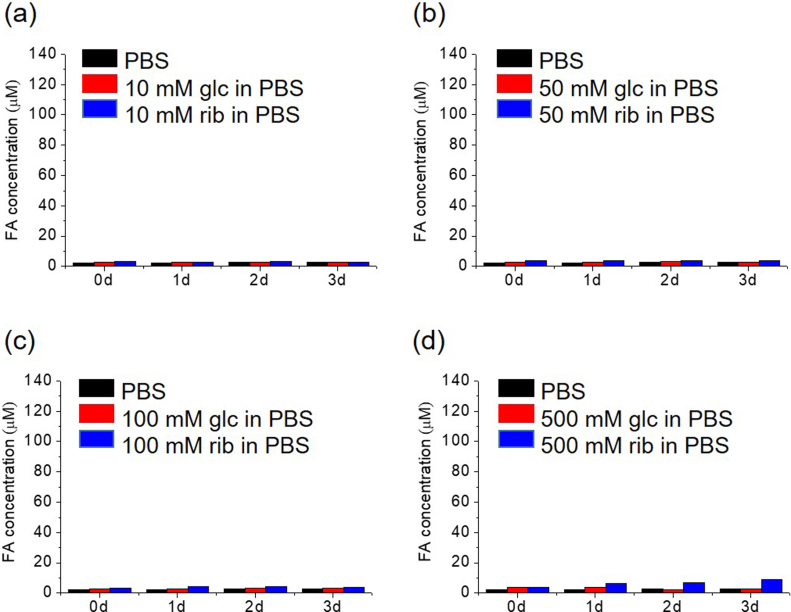
To show the role played by the amino of the Tris base in the production of formaldehyde from d-ribose, we incubated d-ribose with double-distilled water (pH = 8.0) (Supplementary Fig. 2). All of the samples showed quantities of formaldehyde similar to the background levels, and also the experiments using phosphate buffered saline (PBS, pH = 8.0) (Supplementary Fig. 3). This suggest that amino groups may be a co-factor of d-ribose for the produce of formaldehyde under alkaline conditions.
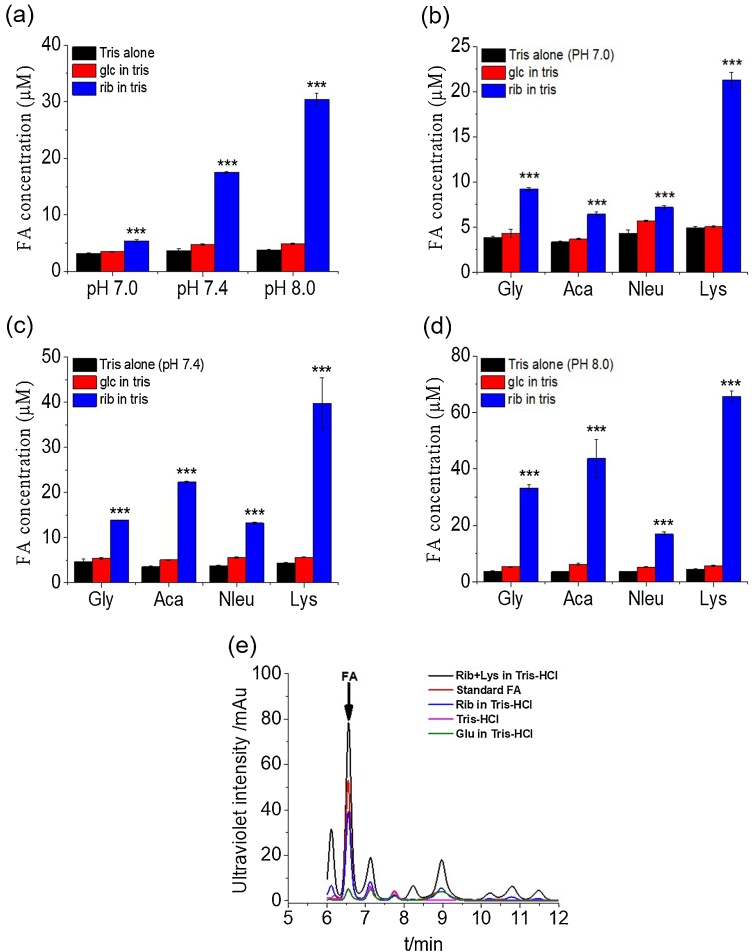
In human body, acid-base homeostasis maintains the blood pH within a narrow range (7.35˜7.45) [41]. Human serum contains many different molecules including amino acids. Their concentrations are probably between 1 mM and 10 mM [42]. Thus, to normalize the experimental conditions, we used 10 mM of the four compounds such as glycine, 6-aminocaproic acid, norleucine, and lysine to incubate in three different pH Tris−HCl buffer (pH: 7.0, 7.4, and 8.0). 500 mM d-ribose (or d-glucose) was incubated with different pH values (pH = 7.0, 7.4, and 8.0) of 20 mM Tris−HCl buffer for 3 days at 37 °C. As shown in Fig. 3a, formaldehyde in the d-ribose-incubated solution was remarkably increased with the elevation of pH values. Under the same experimental conditions, formaldehyde was not significantly detected during incubation of d-glucose in Tris−HCl buffer. Similar results were found in the presence of amino acids, four different amino acids (10 mM) containing either d-ribose or d-glucose (500 mM) were incubated with different pH values (pH = 7.0, 7.4, and 8.0) in 20 mM Tris−HCl buffer for 3 days at 37 °C (Fig. 3b, c, and d). The formaldehyde peak is distinctly observed via HPLC. That is identical to the formaldehyde standard reagent as a positive control. d-Glucose produced little formaldehyde, similar to Tris−HCl buffer alone (pH = 7.4), while d-ribose in Tris−HCl buffer and d-ribose in lysine solution produced markedly higher levels of formaldehyde than d-glucose (Fig. 3e). That is, under resemble human conditions (pH = 7.4 and contained amino), d-ribose can also produce formaldehyde.
Fig. 3.
Yield of formaldehyde in 500 mMd-ribose/d-glucose incubation with different amine acids in different pH Tris−HCl buffers. A 500 mM d-ribose/500 mM d-glucose solution was incubated in 20 mM Tris−HCl buffer (pH = 7.0, 7.4, and 8.0) for 3 days. Aliquots were taken for the measurement of formaldehyde with DNPH by HPLC (a). 10 mM lysine (Lys), norleucine (Nleu), 6-aminocaproic acid (Aca) and glycine (Gly) incubated with 500 mM d-ribose/500 mM d-glucose in 20 mM Tris−HCl buffer (pH = 7.0, 7.4, and 8.0). The peak of FA from a d-ribose series (rib), d-glucose (glc), Rib + lys, standard FA, Tris−HCl solution were figured out between 6–12 min of the real time (b–d). All of the values are expressed as the means ± S.E.M. ***p ≤ 0.001, n = 3. FA, formaldehyde.
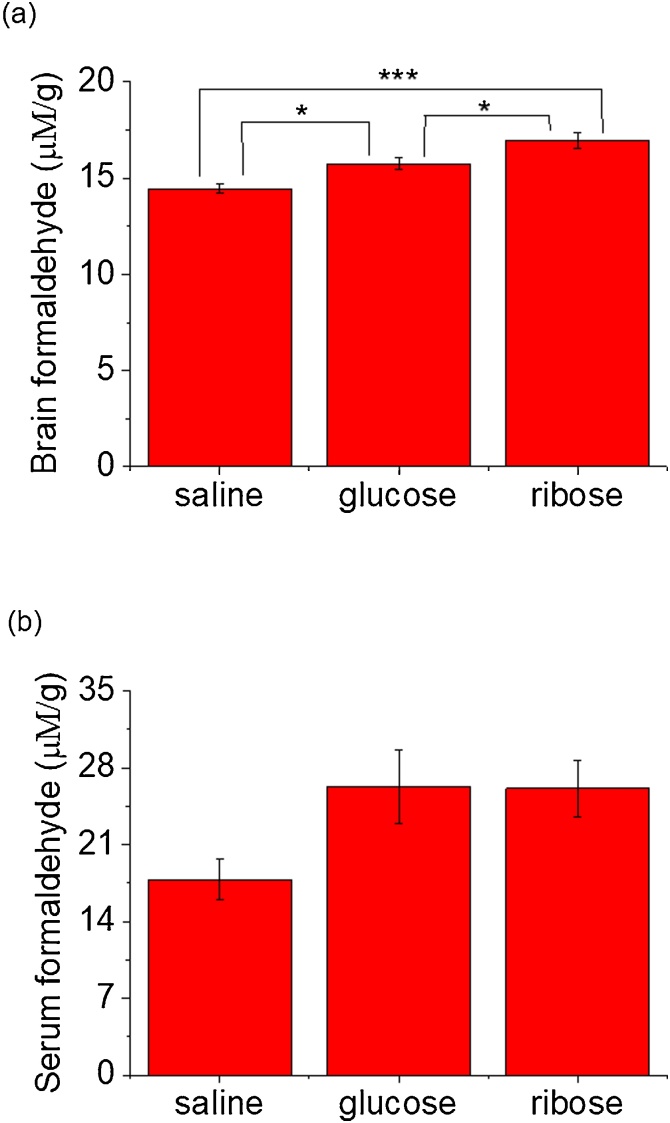
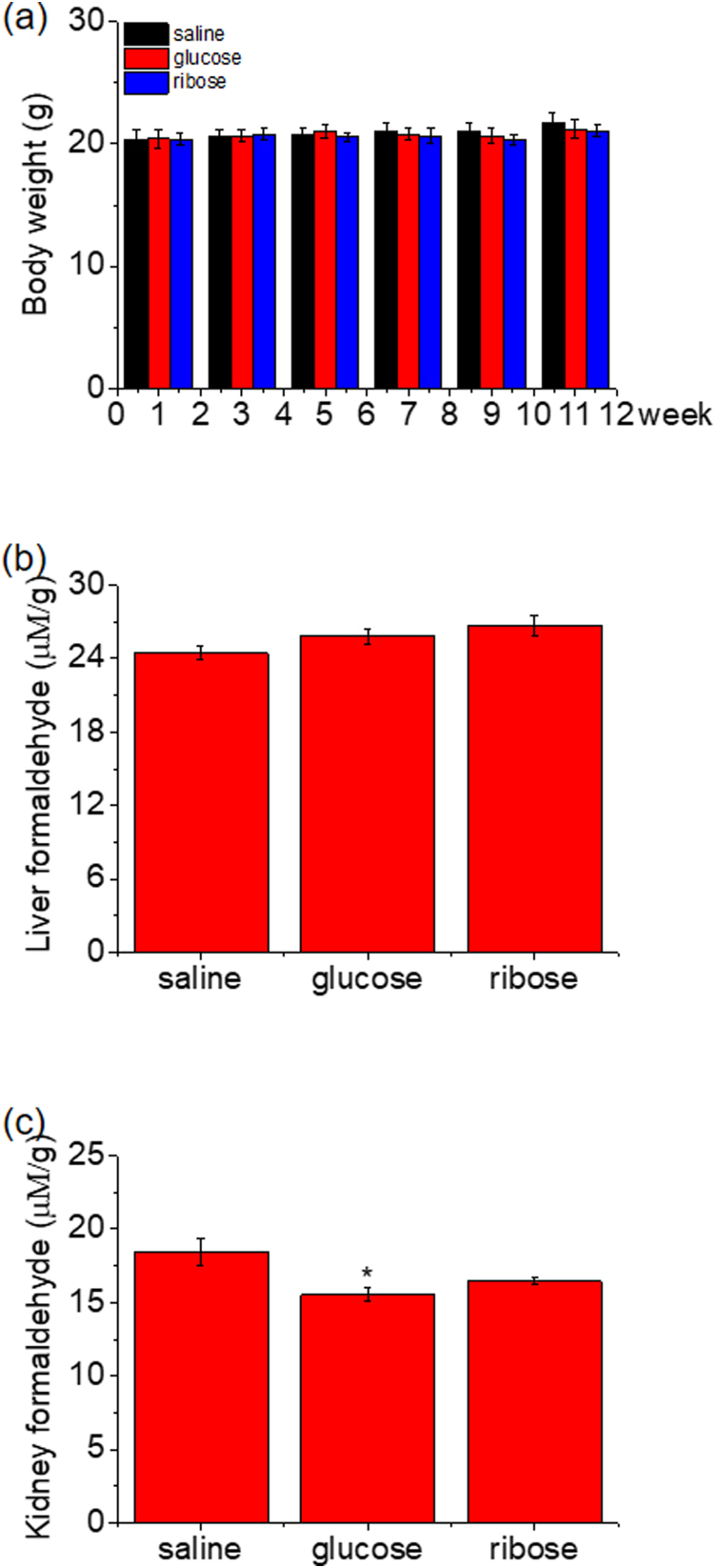
To confirm this reaction in vivo further, we intraperitoneally (i.p.) injected C57BL/6 J mice (n = 14, each group) with d-ribose (rib, 3.2 g/kg•d), d-glucose (glc, 3.82 g/kg•d), or saline (0.9% NaCl in double-distilled water as control) daily for 10 days. Mice injected with d-ribose, d-glucose or saline showed no significant effects on in their body weights from day 1 to day 11 (Supplementary Fig. 4a). Formaldehyde levels in the brain and serum were measured through HPLC with 1.0 g/L DNPH (Fig. 4). The results showed that d-ribose-treated mice had significantly higher levels of formaldehyde in the brain than those in the saline group and d-glucose group (p < 0.05) (Fig. 4a). Neither d-ribose-injected nor d-glucose-injected mice got a significant elevation of serum formaldehyde levels (p > 0.05), though elevation was observable under the same neutral experimental conditions (Fig. 4 b). No marked changes in the formaldehyde levels of liver and kidney in the three groups could be observed except a significant decline of kidney formaldehyde in d-glucose-injected rats (Supplementary Fig. 4 b, c). However, whether formaldehyde is produced from decomposition of d-ribose through retro aldol reaction or from another metabolic pathway of d-ribose in vivo requires further investigating.
Fig. 4.
Formaldehyde levels in C57BL/6 J mice after administration ofd-ribose. Mice (n = 14 in each group) were injected (i.p.) with d-ribose series (rib, 3.2 g/kg•d), d-glucose (glc, 3.82 g/kg•d), and saline (0.9% NaCl in double-distilled water) as a control (ctrl) daily for 10 days, followed by the measurement of formaldehyde in the brain (a), serum (b), with 1.0 g/l 2,4-dinitrophenylhydrazine (DNPH) by HPLC, as described in the text. All of the values are expressed as the mean ± S.E.M. *p < 0.05, n = 8.
4. Discussion
The current work provided information that d-ribose produces formaldehyde under neutral and alkaline conditions. Yield of formaldehyde occurred in solutions of lysine, glycine or Tris base. The human body is rich in the 20 types of amino acids. Each amino acid contains an amino group. Consequently, brain formaldehyde levels of d-ribose-injected mice were significantly elevated, suggesting that d-ribose intake induces formaldehyde production.
As early as 1861, Butlerow and research group studied the direct synthesis of sugars from formaldehyde under alkaline condition [43]. Breslow proposed a mechanism for the aldol reaction [38]. In fact, the aldol reaction is reversible overall [44]. Consequently, if an aldol, the product of an aldol reaction, is treated with an acid or base catalyst in an aqueous medium, the reverse reaction occurs [45,46] and formaldehyde can synthesize simple sugars under an early earth environment [47]. We observed the yield of formaldehyde when d-ribose was incubated with either Tris−HCl buffer or amino acid solution. The yield of formaldehyde underwent the d-ribose concentration and pH value dependent manner. However, classical retro aldol reaction occurs under strong alkali condition [43,48]. The reason why formaldehyde yield was not so high may be due to the reaction of d-ribose under relatively mild conditions. These data indicate that the retro-aldol reaction is involved in the production of formaldehyde from d-ribose.
For the animal experiment, mice were administered 3.2 g/kg d-ribose. We believe that a dose of 3.2 g/kg d-ribose (once daily) is reasonable compared to the dose of d-ribose used in humans. d-Ribose was orally administered or intravenously over at least 5 h to eight healthy volunteers and five patients with myoadenylate deaminase deficiency [49]. The administered dose was approximately 1.0 g/kg daily, and the conversion of this dose to a mouse was 12.3 g/kg according to the Guidance for Industry, Center for Drug Evaluation and Research, U.S. (CDER, 2005, Supplementary Information 2). Oral administration of 15–20 g of d-ribose per hour (50–60 g, daily) was performed to prevent pain and stiffness of the muscles [15]. On the basis of the work by Bayram and coworkers, each patient received oral d-ribose (5 g, three times a day, tid) for 6 weeks. Each dose of d-ribose (5 g) was added to their chosen beverage during a meal or d-ribose was added directly to the meal itself [50]. Oral d-ribose (5 g/dose) was given preoperatively. On average, 8 doses of d-ribose were employed once the patient was a surgical candidate for coronary artery bypass, and d-ribose was continued postoperatively following extubation [14]. Thus, an average dose of 40 g of d-ribose was administered to the participants during the treatment. The usage dose is approximately ˜0.57 g/kg when a person’s weight is 70 kg. In this work, the conversion of the mouse dose to a human dose should be ˜0.26 mg/kg daily. Administration of this dose of d-ribose significantly elevated the formaldehyde in mouse brain.
The concentration of endogenous formaldehyde is positively correlated with the severity of cognitive impairments in Alzheimer’s disease (AD) patients [21], which is due to the metabolic imbalance between synthesis and degradation of formaldehyde [16,34]. The average pathological concentration of formaldehyde in the urine of AD patients is 13.70 ± 5.17 μM, i.e., the pathological urine formaldehyde concentration for AD is ˜1.4 times as much as the physiological concentration (9.61 ± 2.90 μM) in age-matched normal participants [24]. Accumulation of a pathological concentration of endogenous formaldehyde (15 μM) induced chronic damage to N2a cells [24,51], especially the impairment of neuronal processes and neurites, which may result from Tau hyperphosphorylation [51]. As described previously [52], the elevation of brain formaldehyde levels resulted from the administration of d-ribose may lead to cognitive impairment. According to the current results, administration of d-ribose elevated the brain formaldehyde level to greater than 15 μM/g. However, increases of liver formaldehyde levels could not be significantly observed though it has hepatotoxicity, similar to other toxicants such as carbon tetrachloride [53] and imazamox [54]. In spite of these data from mice, they reminds us that humans should have their serum and urine formaldehyde measured when they receive d-ribose as a supplement [55], especially a high dosage of pentose for a long period.
In conclusion, we demonstrated that d-ribose can produce formaldehyde under neutral and alkaline conditions [[17], [18], [19]]. Formaldehyde levels were showed to be significantly increased in the brain of mice after administration of d-ribose. Thus, formaldehyde in urine and serum should be monitored for a person especially an elderly individual who takes d-ribose as a supplement. However, whether formaldehyde is produced from the retro aldol reaction or from other metabolic pathways of d-ribose in vivo should be further investigated.
Funding
This work was supported by the Beijing Municipal Science and Technology Project (Z161100000217141; Z161100000216137); the NSFC (81573763, 31670805); the National Key Research and Development Program of China (2016YFC1306300); the Chinese Academy of Sciences (CAS-20140909); and the Queensland-Chinese Academy of Sciences Biotechnology Fund (GJHZ201302).
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
We thank Ms. Zhensheng Xie, who works in the Institute of Biophysics (IBP), Chinese Academy of Sciences (CAS), for technical assistance with the mass spectrometry. We also thank Beibei Wu (IBP, CAS), and Tao Su (IBP, CAS) for their performing the animal experiments, and discussing the reactive mechanism, respectively.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2019.02.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Broom A.D., Townsend L.B., Jones J.W., Robins R.K. Purine nucleosides. Vi. Further methylation studies of naturally occurring purine nucleosides. Biochemistry. 1964;3:494–500. doi: 10.1021/bi00892a005. [DOI] [PubMed] [Google Scholar]
- 2.Keller P.J., Le Van Q., Kim S.U., Bown D.H., Chen H.C., Kohnle A., Bacher A., Floss H.G. Biosynthesis of riboflavin: mechanism of formation of the ribitylamino linkage. Biochemistry. 1988;27(4):1117–1120. doi: 10.1021/bi00404a006. [DOI] [PubMed] [Google Scholar]
- 3.Mauser M., Hoffmeister H.M., Nienaber C., Schaper W. Influence of ribose, adenosine, and "AICAR" on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circul. Res. 1985;56(2):220–230. doi: 10.1161/01.res.56.2.220. [DOI] [PubMed] [Google Scholar]
- 4.Kerksick C.M., Wilborn C.D., Roberts M.D., Smith-Ryan A., Kleiner S.M., Jager R., Collins R., Cooke M., Davis J.N., Galvan E., Greenwood M., Lowery L.M., Wildman R., Antonio J., Kreider R.B. ISSN exercise & sports nutrition review update: research & recommendations. J. Int. Soc. Sports Nutr. 2018;15(1):38. doi: 10.1186/s12970-018-0242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teitelbaum J.E., Johnson C., St Cyr J. The use of D-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study. J. Alternat. Complement. Med. (New York, N.Y.) 2006;12(9):857–862. doi: 10.1089/acm.2006.12.857. [DOI] [PubMed] [Google Scholar]
- 6.Perlmutter N.S., Wilson R.A., Angello D.A., Palac R.T., Lin J., Brown B.G. Ribose facilitates thallium-201 redistribution in patients with coronary artery disease. J. Nucl. Med. 1991;32(2):193–200. [PubMed] [Google Scholar]
- 7.Shecterle L., Kasubick R., St Cyr J. D-ribose benefits restless legs syndrome. J. Altern. Complement. Med. 2008;14(9):1165–1166. doi: 10.1089/acm.2008.0227. [DOI] [PubMed] [Google Scholar]
- 8.Dhanoa T.S., Housner J.A. Ribose: more than a simple sugar? Curr. Sports Med. Rep. 2007;6(4):254–257. doi: 10.1007/s11932-007-0041-8. [DOI] [PubMed] [Google Scholar]
- 9.Bishop D. Dietary supplements and team-sport performance. Sports Med. 2010;40(12):995–1017. doi: 10.2165/11536870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Gross M., Kormann B., Zollner N. Ribose administration during exercise: effects on substrates and products of energy metabolism in healthy subjects and a patient with myoadenylate deaminase deficiency. Klin. Wochenschr. 1991;69(4):151–155. doi: 10.1007/BF01665856. [DOI] [PubMed] [Google Scholar]
- 11.Murphy S.P., Allen L.H. Nutritional importance of animal source foods. J. Nutr. 2003;133(11 Suppl 2):3932s–3935s. doi: 10.1093/jn/133.11.3932S. [DOI] [PubMed] [Google Scholar]
- 12.Shecterle L.M., Terry K.R., St Cyr J.A. The patented uses of D-ribose in cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2010;5(2):138–142. doi: 10.2174/157489010791515241. [DOI] [PubMed] [Google Scholar]
- 13.Seifert J.G., Subudhi A.W., Fu M.X., Riska K.L., John J.C., Shecterle L.M., St Cyr J.A. The role of ribose on oxidative stress during hypoxic exercise: a pilot study. J. Med. Food. 2009;12(3):690–693. doi: 10.1089/jmf.2008.0065. [DOI] [PubMed] [Google Scholar]
- 14.Perkowski D.J., Wagner S., Schneider J.R., St Cyr J.A. A targeted metabolic protocol with D-ribose for off-pump coronary artery bypass procedures: a retrospective analysis. Ther. Adv. Cardiovasc. Dis. 2011;5(4):185–192. doi: 10.1177/1753944711412421. [DOI] [PubMed] [Google Scholar]
- 15.Zollner N., Reiter S., Gross M., Pongratz D., Reimers C.D., Gerbitz K., Paetzke I., Deufel T., Hubner G. Myoadenylate deaminase deficiency: successful symptomatic therapy by high dose oral administration of ribose. Klin. Wochenschr. 1986;64(24):1281–1290. doi: 10.1007/BF01785710. [DOI] [PubMed] [Google Scholar]
- 16.Xiao R., He R. Springer Publisher; 2017. Metabolism of Formaldehyde in Vivo, in Formaldehyde and Cognition; pp. 21–46. [Google Scholar]
- 17.Pino R., Lyles G.A. Effect of activity of semicarbazide-sensitive aminooxidases and cellular glutathione on the cytotoxic effect of allylamine, acrolein, and formaldehyde in human cultured endothelial cells. Vopr. Med. Khim. 1997;43(6):537–547. [PubMed] [Google Scholar]
- 18.Lovschall H., Eiskjaer M., Arenholt-Bindslev D. Formaldehyde cytotoxicity in three human cell types assessed in three different assays. Toxicol. In Vitro. 2002;16(1):63–69. doi: 10.1016/s0887-2333(01)00093-5. [DOI] [PubMed] [Google Scholar]
- 19.He R., Lu J., Miao J. Formaldehyde stress, Science China. Life Sci. 2010;53(12):1399–1404. doi: 10.1007/s11427-010-4112-3. [DOI] [PubMed] [Google Scholar]
- 20.Mo W., He R. Formaldehyde and Cognition. Springer Publisher; 2017. The role of formaldehyde in cell proliferation and death, in formaldehyde and cognition; pp. 79–97. [Google Scholar]
- 21.Tong Z., Han C., Qiang M., Wang W., Lv J., Zhang S., Luo W., Li H., Luo H., Zhou J., Wu B., Su T., Yang X., Wang X., Liu Y., He R. Age-related formaldehyde interferes with DNA methyltransferase function, causing memory loss in Alzheimer’s disease. Neurobiol. Aging. 2015;36(1):100–110. doi: 10.1016/j.neurobiolaging.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Qiang M., Xiao R., Su T., Wu B.B., Tong Z.Q., Liu Y., He R.Q. A novel mechanism for endogenous formaldehyde elevation in SAMP8 mouse. J. Alzheimers Dis. 2014;40(4):1039–1053. doi: 10.3233/JAD-131595. [DOI] [PubMed] [Google Scholar]
- 23.Tulpule K., Dringen R. Formaldehyde in brain: an overlooked player in neurodegeneration? J. Neurochem. 2013;127(1):7–21. doi: 10.1111/jnc.12356. [DOI] [PubMed] [Google Scholar]
- 24.Li T., Su T., He Y.G., Lu J.H., Mo W.C., Wei Y., He R.Q. Brain formaldehyde is related to water intake behavior. Aging Dis. 2016;7(5):561–584. doi: 10.14336/AD.2016.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J., He R. Formaldehyde and Cognition., Formaldehyde and Cognition. Springer Publisher; 2017. Tau phosphorylation and amyloid-β deposition in the presence of formaldehyde; pp. 167–189. [Google Scholar]
- 26.Gronvall J.L.E., Garpenstrand H., Oreland L., Ekblom J. Autoradiographic imaging of formaldehyde adducts in mice: possible relevance for vascular damage in diabetes. Life Sci. 1998;63(9):759–768. doi: 10.1016/s0024-3205(98)00331-2. [DOI] [PubMed] [Google Scholar]
- 27.Gu Y., Hsu H.T., Zhu J., Zheng X., Jiang H., Fan H., Yang T. A systematic survey on the diagnosis strategy and patient management of type 1 diabetes by Chinese physicians, Science China. Life Sci. 2018;61(3):318–327. doi: 10.1007/s11427-017-9052-4. [DOI] [PubMed] [Google Scholar]
- 28.Yin W., Qin W., Gao Y. Urine glucose levels are disordered before blood glucose level increase was observed in Zucker diabetic fatty rats, Science China. Life Sci. 2018;61(7):844–848. doi: 10.1007/s11427-017-9134-6. [DOI] [PubMed] [Google Scholar]
- 29.Leal M.P., Brochetti R.A., Ignacio A., Camara N.O.S., da Palma R.K., de Oliveira L.V.F., de Fatima Teixeira da Silva D., Lino-Dos-Santos-Franco A. Effects of formaldehyde exposure on the development of pulmonary fibrosis induced by bleomycin in mice. Toxicol. Rep. 2018;5:512–520. doi: 10.1016/j.toxrep.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshita D., Nakajima-Takenaka C., Shimizu J., Hattori H., Nakashima T., Kikuta A., Matsuyoshi H., Takaki M. Effects of formaldehyde on cardiovascular system in in situ rat hearts. Basic Clin. Pharmacol. Toxicol. 2009;105(4):271–280. doi: 10.1111/j.1742-7843.2009.00442.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida I., Ibuki Y. Formaldehyde-induced histone H3 phosphorylation via JNK and the expression of proto-oncogenes. Mutat. Res. 2014;770:9–18. doi: 10.1016/j.mrfmmm.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Su T., Monte W.C., Hu X., He Y., He R. Formaldehyde as a trigger for protein aggregation and potential target for mitigation of age-related, progressive cognitive impairment. Curr. Top. Med. Chem. 2016;16(5):472–484. doi: 10.2174/1568026615666150813142215. [DOI] [PubMed] [Google Scholar]
- 33.Skrzydlewska E. Toxicological and metabolic consequences of methanol poisoning. Toxicol. Mech. Methods. 2003;13(4):277–293. doi: 10.1080/713857189. [DOI] [PubMed] [Google Scholar]
- 34.Liu K.-L., He Y.-G., Yu L.-X., Chen Y., He R.-Q. Elevated formaldehyde in the cecum of APP/PS1 mouse. Microbiology China. 2017;44(8):1761–1766. [Google Scholar]
- 35.Aydin S., Ogeturk M., Kuloglu T., Kavakli A., Aydin S. Effect of carnosine supplementation on apoptosis and irisin, total oxidant and antioxidants levels in the serum, liver and lung tissues in rats exposed to formaldehyde inhalation. Peptides. 2015;64:14–23. doi: 10.1016/j.peptides.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Trezl L., Csiba A., Juhasz S., Szentgyorgyi M., Lombai G., Hullan L. Endogenous formaldehyde level of foods and its biological significance. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung a-Food Research And Technology. 1997;205(4):300–304. [Google Scholar]
- 37.Qu M., Lu J., He R. Formaldehyde and Cognition. Springer Publisher; 2017. formaldehyde from environment, in formaldehyde and cognition; pp. 1–19. [Google Scholar]
- 38.Breslow R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959;(21):22–26. [Google Scholar]
- 39.Nelson D.L., Cox M.M. 3rd ed. Worth Publishers; New York, America: 2004. Lehninger Principle of Biochemistry. [Google Scholar]
- 40.Su T., Wei Y., He R.-Q. Assay of brain endogenous formaldehyde with 2, 4-dinitrophenylhydrazine through UV-HPLC. Prog. Biochem. Biophys. 2011;38(12):1171–1177. [Google Scholar]
- 41.Celotto A.C., Capellini V.K., Baldo C.F., Dalio M.B., Rodrigues A.J., Evora P.R. Effects of acid-base imbalance on vascular reactivity. Braz. J. Med. Biol. Res. 2008;41(6):439–445. doi: 10.1590/s0100-879x2008005000026. [DOI] [PubMed] [Google Scholar]
- 42.Frame E.G. The levels of individual free amino acids in the plasma of normal man at various intervals after a high-protein meal. Clin. Chem. 1958;4(6):559–560. doi: 10.1172/JCI103763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butlerow A. Ueber die aethylmilchsäure. Ann. Der Chemie Und Pharm. 1861;118(3):325–330. [Google Scholar]
- 44.Rybtchinski B., Konstantinovsky L., Shimon L.J., Vigalok A., Milstein D. Solvent-stabilized alkylrhodium(III) hydride complexes: a special mode of reversible C-H bond elimination involving an agostic intermediate. Chemistry. 2000;6(17):3287–3292. doi: 10.1002/1521-3765(20000901)6:17<3287::aid-chem3287>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi S., Baba T. A novel strategy for biomass upgrade: cascade approach to the synthesis of useful compounds via C-C bond formation using biomass-derived sugars as carbon nucleophiles. Molecules. 2016;21(7) doi: 10.3390/molecules21070937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cergol K.M., Jensen P., Turner P., Coster M.J. Reversibility in the boron-mediated ketone-ketone aldol reaction. Chem. Commun. (Camb.) 2007;(13):1363–1365. doi: 10.1039/b617094c. [DOI] [PubMed] [Google Scholar]
- 47.Civis S., Szabla R., Szyja B.M., Smykowski D., Ivanek O., Knizek A., Kubelik P., Sponer J., Ferus M., Sponer J.E. TiO2-catalyzed synthesis of sugars from formaldehyde in extraterrestrial impacts on the early Earth. Sci. Rep. 2016;6 doi: 10.1038/srep23199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro R. Prebiotic ribose synthesis: a critical analysis. Orig. Life Evol. Biosph. 1988;18(1-2):71–85. doi: 10.1007/BF01808782. [DOI] [PubMed] [Google Scholar]
- 49.Gross M., Reiter S., Zollner N. Metabolism of D-ribose administered continuously to healthy persons and to patients with myoadenylate deaminase deficiency. Klin. Wochenschr. 1989;67(23):1205–1213. doi: 10.1007/BF01716208. [DOI] [PubMed] [Google Scholar]
- 50.Bayram M., St Cyr J.A., Abraham W.T. D-ribose aids heart failure patients with preserved ejection fraction and diastolic dysfunction: a pilot study. Ther. Adv. Cardiovasc. Dis. 2015;9(3):56–65. doi: 10.1177/1753944715572752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Zhou J., Mo W.C., He Y.G., Wei Y., He R.Q., Yi F.P. Accumulation of simulated pathological level of formaldehyde decreases cell viability and adhesive morphology in neuronal cells. Prog. Biochem. Biophys. 2017;44(7):601–614. [Google Scholar]
- 52.Chen X., Su T., Chen Y., He Y., Liu Y., Xu Y., Wei Y., Li J., He R. d-Ribose as a Contributor to Glycated Haemoglobin. EBioMedicine. 2017;25:143–153. doi: 10.1016/j.ebiom.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dassarma B., Nandi D.K., Gangopadhyay S., Samanta S. Hepatoprotective effect of food preservatives (butylated hydroxyanisole, butylated hydroxytoluene) on carbon tetrachloride-induced hepatotoxicity in rat. Toxicol. Rep. 2018;5:31–37. doi: 10.1016/j.toxrep.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sevim C., Comakli S., Taghizadehghalehjoughi A., Ozkaraca M., Mesnage R., Kovatsi L., Burykina T.I., Kalogeraki A., Antoniou M.N., Tsatsakis A. An imazamox-based herbicide causes apoptotic changes in rat liver and pancreas. Toxicol. Rep. 2019;6:42–50. doi: 10.1016/j.toxrep.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su T., He R. Formaldehyde and Cognition. Springer Publisher; 2017. Methods in determination of formaldehyde; pp. 271–295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.